Ni(NH3)2(NO3)2—A 3-D Network through Bridging Nitrate Units Isolated from the Thermal Decomposition of Nickel Hexammine Dinitrate
Abstract
1. Introduction
2. Results and Discussion
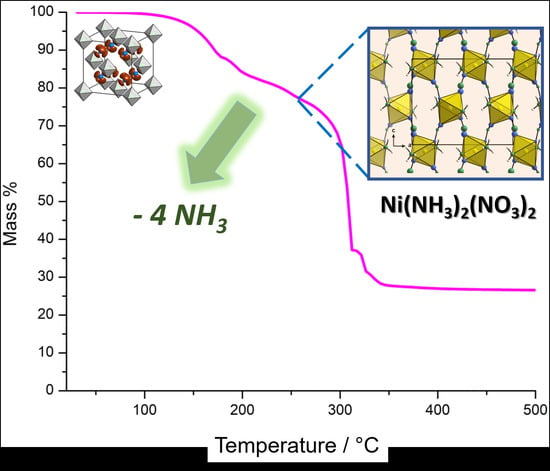
2.1. Thermal Decomposition of [Ni(NH3)6](NO3)2
2.2. Crystal Structure Solution of Ni(NH3)2(NO3)2
2.3. Crystal Structure of Ni(NH3)2(NO3)2
2.4. IR Spectroscopy of Ni(NH3)2(NO3)2
3. Materials and Methods
3.1. Synthesis
3.2. Thermal Analysis
3.3. Fourier Transform Infrared (FTIR) Spectroscopy
3.4. Powder X-ray Diffraction
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zamfirescu, C.; Dincer, I. Using ammonia as sustainable fuel. J. Power Sources 2008, 185, 459–465. [Google Scholar] [CrossRef]
- Sørensen, R.Z.; Hummelshøj, J.S.; Klerke, A.; Reves, J.B.; Vegge, T.; Nørskov, J.K.; Christensen, C.H. Indirect, Reversible High-Density Hydrogen Storage in Compact Metal Ammine Salts. J. Am. Chem. Soc. 2008, 130, 8660–8668. [Google Scholar] [CrossRef] [PubMed]
- Reardon, H.; Hanlon, J.M.; Grant, M.; Fullbrook, I.; Gregory, D.H. Ammonia Uptake and Release in the MnX2–NH3 (X = Cl, Br) Systems and Structure of the Mn(NH3)nX2 (n = 6, 2) Ammines. Crystals 2012, 2, 193–212. [Google Scholar] [CrossRef]
- Breternitz, J.; Vilk, Y.E.; Giraud, E.; Reardon, H.; Hoang, T.K.A.; Godula-Jopek, A.; Gregory, D.H. Facile Uptake and Release of Ammonia by Nickel Halide Ammines. ChemSusChem 2016, 9, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Breternitz, J.; Farrugia, L.J.; Godula-Jopek, A.; Saremi-Yaramahdi, S.; Malka, I.E.; Hoang, T.K.A.; Gregory, D.H. Reaction of [Ni(H2O)6](NO3)2 with gaseous NH3; crystal growth via in-situ solvation. J. Cryst. Growth 2015, 412, 1–6. [Google Scholar] [CrossRef]
- Migdał-Mikuli, A.; Mikuli, E.; Dziembaj, R.; Majda, D.; Hetmańczyk, Ł. Thermal decomposition of [Mg(NH3)6](NO3)2, [Ni(NH3)6](NO3)2 and [Ni(ND3)6](NO3)2. Thermochim. Acta 2004, 419, 223–229. [Google Scholar] [CrossRef]
- Mikuli, E.; Migdał-Mikuli, A.; Majda, D. Thermal decomposition of polycrystalline [Ni(NH3)6](NO3)2. J. Therm. Anal. Calorim. 2013, 112, 1191–1198. [Google Scholar] [CrossRef]
- Visser, J.W. A fully automatic program for finding the unit cell from powder data. J. Appl. Crystallogr. 1969, 2, 89–95. [Google Scholar] [CrossRef]
- Werner, P.-E.; Eriksson, L.; Westdahl, M. TREOR, a semi-exhaustive trial-and-error powder indexing program for all symmetries. J. Appl. Crystallogr. 1985, 18, 367–370. [Google Scholar] [CrossRef]
- Boultif, A.; Louër, D. Indexing of powder diffraction patterns for low-symmetry lattices by the successive dichotomy method. J. Appl. Crystallogr. 1991, 24, 987–993. [Google Scholar] [CrossRef]
- Le Bail, A. Monte carlo indexing with mcmaille. Powder Diffr. 2004, 19, 249–254. [Google Scholar] [CrossRef]
- Petříček, V.; Dušek, M.; Palatinus, L. Crystallographic Computing System JANA2006: General features. Z. Kristallogr. Cryst. Mater. 2014, 229, 345–352. [Google Scholar] [CrossRef]
- Palatinus, L.; Chapuis, G. SUPERFLIP—A computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J. Appl. Crystallogr. 2007, 40, 786–790. [Google Scholar] [CrossRef]
- Morozov, I.V.; Korenev, Y.M.; Troyanov, S.I. Synthesis and crystal structure of New Amminecopper(II) Nitrates: [Cu(NH3)2](NO3)2 and [Cu(NH3)](NO3)2. Z. Anorg. Allgem. Chem. 1996, 622, 2003–2007. [Google Scholar] [CrossRef]
- Lippert, B.; Lock, C.; Rosenberg, B.; Zvagulis, M. cis-Dinitratodiammineplatinum(II), cis-Pt(NH3)2(NO3)2. Crystalline structure and vibrational spectra. Inorg. Chem. 1977, 16, 1525–1529. [Google Scholar] [CrossRef]
- Addison, C.C.; Logan, N.; Wallwork, S.C.; Garner, C.D. Structural aspects of co-ordinated nitrate groups. Q. Rev. Chem. Soc. 1971, 25, 289–322. [Google Scholar] [CrossRef]
- Curtis, N.F.; Curtis, Y.M. Some Nitrato–Amine Nickel(II) Compounds with Monodentate and Bidentate Nitrate Ions. Inorg. Chem. 1965, 4, 804–809. [Google Scholar] [CrossRef]





| Step | Cumulative Mass Change/% | TG Temperatures/°C | DTA Peak Temperatures/°C | Gases Evolved (from MS) | |
|---|---|---|---|---|---|
| Onset | Final | ||||
| 1 | −11.8 | 107 | 179 | 173 | NH3 |
| 2 | −16.9 | 187 | 208 | 198 | NH3 |
| 3 | −23.4 | 208 | 262 | 257 | NH3 |
| 4 | −63.1 | 272 | 316 | 309 | NH3, N2, N2O, NO |
| 5 | −72.7 | 321 | 375 | 336 | H2O |
| Chemical Formula | Ni(NH3)2(NO3)2 |
|---|---|
| Formula Weight | 216.8 |
| Crystal system | Orthorhombic |
| Spacegroup | Pca21 (No. 29) |
| a/Å | 11.0628(5) |
| b/Å | 6.0454(3) |
| c/Å | 9.3526(4) |
| V/ų | 625.49(5) |
| Z | 4 |
| crystallographic density/g cm−³ | 2.31(5) |
| No of data, parameters | 4120, 68 |
| Rp; wRp | 0.044, 0.060 |
| Robs; wR2(all) | 0.033, 0.044 |
| Atom 1 | Atom 2 | Distance/Å |
|---|---|---|
| Nitrate bonds | ||
| N11 | O11 | 1.23(6) |
| N11 | O12 | 1.31(6) |
| N11 | O13 | 1.16(6) |
| N21 | O21 | 1.32(6) |
| N21 | O22 | 1.25(4) |
| N21 | O23 | 1.23(4) |
| Nickel–ligand bonds | ||
| Ni1 | N1 | 2.10(2) |
| Ni1 | N2 | 2.00(2) |
| Ni1 | O11 | 2.16(3) |
| Ni1 | O12 | 2.21(3) |
| Ni1 | O21 | 2.14(3) |
| Ni1 | O22 | 2.12(2) |
| Band | Wavenumber/cm−1 | |||
|---|---|---|---|---|
| Ni(NH3)2(NO3)2 | Ni(en)2(NO3)2 [17] | Ni(NH3)2Cl2 [4] | Ni(NH3)6(NO3)2 [5] | |
| νa(NH3) | 3375 | - | 3346 | 3364 |
| νs(NH3) | 3296 | - | 3265 | 3282 |
| 2δa(HNH) | 3195 | - | 3167 | 3167 |
| ν1 + ν3(NO3) | 2475 | 2455 | - | - |
| ν1 + ν3(NO3) | 2330 | 2320 | - | - |
| ν1 + ν4(NO3) | 1765 | 1762, 1741 | - | - |
| δa(HNH) | 1616 | - | 1607 | 1616 |
| ν3(NO3) | 1417 | 1420 | - | 1329 |
| ν3(NO3) | 1303 | 1303 | - | |
| δs(HNH) | 1250 | - | 1281 | - |
| δs(HNH) | 1229 | - | 1239 | 1202 |
| ν1(NO3) | 1048 | 1033, 1034 | - | - |
| ν2(NO3) | 812 | 818 | - | 823 |
| ν4(NO3) | 751 | 728 | - | - |
| ν4(NO3) | 721 | 708 | - | - |
| ρ(NH3) | 643 | - | 675 | 648 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Breternitz, J.; Godula-Jopek, A.; Gregory, D.H. Ni(NH3)2(NO3)2—A 3-D Network through Bridging Nitrate Units Isolated from the Thermal Decomposition of Nickel Hexammine Dinitrate. Inorganics 2018, 6, 59. https://doi.org/10.3390/inorganics6020059
Breternitz J, Godula-Jopek A, Gregory DH. Ni(NH3)2(NO3)2—A 3-D Network through Bridging Nitrate Units Isolated from the Thermal Decomposition of Nickel Hexammine Dinitrate. Inorganics. 2018; 6(2):59. https://doi.org/10.3390/inorganics6020059
Chicago/Turabian StyleBreternitz, Joachim, Agata Godula-Jopek, and Duncan H. Gregory. 2018. "Ni(NH3)2(NO3)2—A 3-D Network through Bridging Nitrate Units Isolated from the Thermal Decomposition of Nickel Hexammine Dinitrate" Inorganics 6, no. 2: 59. https://doi.org/10.3390/inorganics6020059
APA StyleBreternitz, J., Godula-Jopek, A., & Gregory, D. H. (2018). Ni(NH3)2(NO3)2—A 3-D Network through Bridging Nitrate Units Isolated from the Thermal Decomposition of Nickel Hexammine Dinitrate. Inorganics, 6(2), 59. https://doi.org/10.3390/inorganics6020059