Manganese Fluorene Phosphonates: Formation of Isolated Chains
Abstract
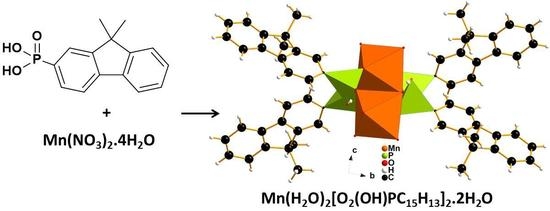
:1. Introduction
2. Results and Discussion
2.1. Structural Study
2.2. Hirshfeld Surfaces, 2D-Fingerprint Plots and Crystal Voids Properties
2.3. Magnetic Properties
3. Experimental Section
3.1. Materials and Methods
3.2. Synthesis of the Mono-Phosphonate Mn(H2O)2[O2(OH)PC15H13]2·2H2O
3.3. Thermogravimetric Analysis
3.4. Scanning Electron Microscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Clearfield, A.; Demadis, K. Metal Phosphonate Chemistry: From Synthesis to Applications; Royal Society of Chemistry: Cambridge, UK, 2011; ISBN 978-1-84973-356-4. [Google Scholar]
- Alberti, G.; Costantino, U.; Allulli, S.; Tomassini, N. Crystalline Zr(R-PO3)2 and Zr(R-OPO3)2 compounds (R = organic radical). J. Inorg. Nucl. Chem. 1978, 40, 1113–1117. [Google Scholar] [CrossRef]
- Dines, M.B.; DiGiacomo, P.M. Derivatized lamellar phosphates and phosphonates of M(IV) ions. Inorg. Chem. 1981, 20, 92–97. [Google Scholar] [CrossRef]
- Rueff, J.M.; Poienar, M.; Guesdon, A.; Martin, C.; Maignan, A.; Jaffrès, P.A. Hydrothermal synthesis for new multifunctional materials: A few examples of phosphates and phosphonate-based hybrid materials. J. Solid State Chem. 2016, 236, 236–245. [Google Scholar] [CrossRef]
- Evrard, Q.; Chaker, Z.; Roger, M.; Sevrain, C.M.; Delahaye, E.; Gallart, M.; Gilliot, P.; Leuvrey, C.; Rueff, J.M.; Rabu, P.; et al. Layered Simple Hydroxides Functionalized by Fluorene-Phosphonic Acids: Synthesis, Interface Theoretical Insights, and Magnetoelectric Effect. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef]
- Queffélec, C.; Petit, M.; Janvier, P.; Knight, D.A.; Bujoli, B. Surface Modification Using Phosphonic Acids and Esters. Chem. Rev. 2012, 112, 3777–3807. [Google Scholar] [CrossRef] [PubMed]
- Sevrain, C.M.; Berchel, M.; Couthon, H.; Jaffrès, P.A. Phosphonic acid: Preparation and applications. Beilstein J. Org. Chem. 2017, 13, 2186–2213. [Google Scholar] [CrossRef] [PubMed]
- Rueff, J.M.; Barrier, N.; Boudin, S.; Dorcet, V.; Caignaert, V.; Boullay, P.; Hix, G.B.; Jaffrès, P.A. Remarkable thermal stability of Eu(4-phosphonobenzoate): Structure investigations and luminescence properties. Dalton Trans. 2009, 10614–10620. [Google Scholar] [CrossRef] [PubMed]
- Mutelet, B.; Boudin, S.; Pérez, O.; Rueff, J.M.; Labbé, C.; Jaffrès, P.A. La1−xLnxH(O3PCH3)2 (Ln = Tb, Eu; 0 < x ≤ 1): An organic-inorganic hybrid with lanthanide chains and tunable luminescence properties. Dalton Trans. 2015, 44, 1186–1192. [Google Scholar] [PubMed]
- Maeda, K. Metal phosphonate open-framework materials. Microporous Mesoporous Mater. 2004, 73, 47–55. [Google Scholar] [CrossRef]
- Li, J.T.; Cao, D.K.; Akutagawa, T.; Zheng, L.M. Zn3(4-OOCC6H4PO3)2: A polar metal phosphonate with pillared layered structure showing SHG-activity and large dielectric anisotropy. Dalton Trans. 2010, 39, 8606–8608. [Google Scholar] [CrossRef] [PubMed]
- Clearfield, A. Metal Phosphonate Chemistry. In Progress in Inorganic Chemistry; Karlin, K.D., Ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1998; pp. 371–510. ISBN 978-0-470-16648-2. [Google Scholar]
- Benítez, I.O.; Bujoli, B.; Camus, L.J.; Lee, C.M.; Odobel, F.; Talham, D.R. Monolayers as Models for Supported Catalysts: Zirconium Phosphonate Films Containing Manganese(III) Porphyrins. J. Am. Chem. Soc. 2002, 124, 4363–4370. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Abney, C.; Lin, W. Enantioselective catalysis with homochiral metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Peng, Y.; Han, X.; Liu, Y.; Lin, X.; Cui, Y. Sixteen isostructural phosphonate metal-organic frameworks with controlled Lewis acidity and chemical stability for asymmetric catalysis. Nat. Commun. 2017, 8, 2171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallouk, T.E.; Gavin, J.A. Molecular Recognition in Lamellar Solids and Thin Films. Acc. Chem. Res. 1998, 31, 209–217. [Google Scholar] [CrossRef] [Green Version]
- Brousseau, L.C.; Mallouk, T.E. Molecular Design of Intercalation-Based Sensors. 1. Ammonia Sensing with Quartz Crystal Microbalances Modified by Copper Biphenylbis(phosphonate) Thin Films. Anal. Chem. 1997, 69, 679–687. [Google Scholar] [CrossRef]
- Clearfield, A. Recent advances in metal phosphonate chemistry II. Curr. Opin. Solid State Mater. Sci. 2002, 6, 495–506. [Google Scholar] [CrossRef]
- Berchel, M.; Gall, T.L.; Denis, C.; Hir, S.L.; Quentel, F.; Elléouet, C.; Montier, T.; Rueff, J.M.; Salaün, J.Y.; Haelters, J.P.; et al. A silver-based metal–organic framework material as a ‘reservoir’ of bactericidal metal ions. New J. Chem. 2011, 35, 1000–1003. [Google Scholar] [CrossRef]
- Rueff, J.M.; Perez, O.; Caignaert, V.; Hix, G.; Berchel, M.; Quentel, F.; Jaffrès, P.A. Silver-Based Hybrid Materials from meta- or para-Phosphonobenzoic Acid: Influence of the Topology on Silver Release in Water. Inorg. Chem. 2015, 54, 2152–2159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fanucci, G.E.; Krzystek, J.; Meisel, M.W.; Brunel, L.C.; Talham, D.R. Antiferromagnetic Resonance as a Tool for Investigating Magnetostructural Correlations: The Canted Antiferromagnetic State of KMnPO4·H2O and a Series of Manganese Phosphonates. J. Am. Chem. Soc. 1998, 120, 5469–5479. [Google Scholar] [CrossRef]
- Rueff, J.M.; Caignaert, V.; Chausson, S.; Leclaire, A.; Simon, C.; Perez, O.; Le Pluart, L.; Jaffrès, P.A. meta-Phosphonobenzoic Acid: A Rigid Heterobifunctional Precursor for the Design of Hybrid Materials. Eur. J. Inorg. Chem. 2008, 2008, 4117–4125. [Google Scholar] [CrossRef]
- Perez, O.; Bloyet, C.; Rueff, J.M.; Barrier, N.; Caignaert, V.; Jaffrès, P.A.; Raveau, B. Topochemical Route from Supramolecular to Hybrid Materials: Tetraphenylmethane-Based Tectons and Lanthanum Phosphonate Derivative. Cryst. Growth Des. 2016, 16, 6781–6789. [Google Scholar] [CrossRef]
- Rueff, J.M.; Perez, O.; Pautrat, A.; Barrier, N.; Hix, G.B.; Hernot, S.; Couthon-Gourvès, H.; Jaffrès, P.A. Structural Study of Hydrated/Dehydrated Manganese Thiophene-2,5-diphosphonate Metal Organic Frameworks, Mn2(O3P–C4H2S–PO3)·2H2O. Inorg. Chem. 2012, 51, 10251–10261. [Google Scholar] [CrossRef] [PubMed]
- Rueff, J.M.; Caignaert, V.; Leclaire, A.; Simon, C.; Haelters, J.P.; Jaffrès, P.A. m-phosphonobenzoic acid and copper(II) as precursors of helical chain and lamellar hybrid materials. CrystEngComm 2009, 11, 556–559. [Google Scholar] [CrossRef]
- Yücesan, G.; Zorlu, Y.; Stricker, M.; Beckmann, J. Metal-organic solids derived from arylphosphonic acids. Coord. Chem. Rev. 2018, 369, 105–122. [Google Scholar] [CrossRef]
- Bulut, A.; Zorlu, Y.; Topkaya, R.; Aktaş, B.; Doğan, S.; Kurt, H.; Yücesan, G. Macrocyclic Cu(II)-organophosphonate building block with room temperature magnetic ordering. Dalton Trans. 2015, 44, 12526–12529. [Google Scholar] [CrossRef] [PubMed]
- Bladek, K.J.; Reid, M.E.; Nishihara, H.; Akhtar, F.; Gelfand, B.S.; Shimizu, G.K.H. Microsphere Assemblies via Phosphonate Monoester Coordination Chemistry. Chem. Eur. J. 2018, 24, 1533–1538. [Google Scholar] [CrossRef] [PubMed]
- Hugot, N.; Roger, M.; Rueff, J.M.; Cardin, J.; Per×ez, O.; Caignaert, V.; Raveau, B.; Rogez, G.; Jaffrès, P.A. Copper–Fluorenephosphonate Cu(PO3-C13H9)·H2O: A Layered Antiferromagnetic Hybrid. Eur. J. Inorg. Chem. 2016, 2016, 266–271. [Google Scholar] [CrossRef]
- Bloyet, C.; Rueff, J.M.; Caignaert, V.; Lohier, J.-F.; Cardin, J.; Jaffrès, P.A.; Raveau, B. Fluorenyl Zinc Phosphonate Zn(H2O)PO3–C13H9·H2O: Hybrid Columnar Structure with Strong C–H···π Interactions. Z. Anorg. Allg. Chem. 2017, 643, 250–255. [Google Scholar] [CrossRef]
- Cao, G.; Lee, H.; Lynch, V.M.; Mallouk, T.E. Synthesis and Structural Characterization of a Homologous Series of Divalent-Metal Phosphonates, MII(O3PR)·H2O and MII(HO3PR). Inorg. Chem. 1988, 27, 2781–2785. [Google Scholar] [CrossRef]
- Merrill, C.A.; Cheetham, A.K. Inorganic−Organic Framework Structures; M(II) Ethylenediphosphonates (M = Co, Ni, Mn) and a Mn(II) Ethylenediphosphonato-phenanthroline. Inorg. Chem. 2007, 46, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.F.; Li, L.J.; Lv, X.X.; Wang, C.; Zhao, X.B. Tuning the structure of metal phosphonates using uncoordinating methyl group: Syntheses, structures and properties of a series of metal diphosphonates. CrystEngComm 2014, 16, 7043–7052. [Google Scholar] [CrossRef]
- Brown, I.D.; Altermatt, D. Bond-valence parameters obtained from a systematic analysis of the Inorganic Crystal Structure Database. Acta Crystallogr. Sect. B Struct. Sci. 1985, 41, 244–247. [Google Scholar] [CrossRef] [Green Version]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Spackman, M.A.; Mitchell, A.S. Novel tools for visualizing and exploring intermolecular interactions in molecular crystals. Acta Crystallogr. Sect. B Struct. Sci. 2004, 60, 627–668. [Google Scholar] [CrossRef] [PubMed]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting intermolecular interactions in molecular crystals. CrystEngComm 2002, 4, 378–392. [Google Scholar] [CrossRef]
- Wolff, S.K.; Grimwood, D.J.; McKinnon, J.J.; Turner, M.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer; University of Western Australia: Crawley, WA, Australia, 2012. [Google Scholar]
- Demadis, K.D.; Anagnostou, Z.; Panera, A.; Mezei, G.; Kirillova, M.V.; Kirillov, A.M. Three-component 1D and 2D metal phosphonates: Structural variability, topological analysis and catalytic hydrocarboxylation of alkanes. RSC Adv. 2017, 7, 17788–17799. [Google Scholar] [CrossRef]
- Carlin, R.L. Magnetochemistry; Springer: Berlin/Heidelberg, Germany, 1986; ISBN 978-3-642-70735-3. [Google Scholar]







| Formula | Mn(H2O)2[O2(OH)PC15H13]2·2H2O |
|---|---|
| FW | 673.486406 |
| Space group | P21/c |
| a (Å) | 9.3882(4) |
| b (Å) | 37.3410(16) |
| c (Å) | 9.2398(4) |
| α (°) | 90.000 |
| β (°) | 90.000 |
| γ (°) | 90.000 |
| Z | 4 |
| V (Å3) | 3239.15(24) |
| dcalc (g/cm3) | 1.30498 |
| µ (mm−1) | 0.551 |
| radiation source λ (Å) | Mo Kα 0.71073 |
| Pattern range 2Ѳ (°) | 4.86–61.02 |
| no. of reflexions | 42391 |
| no. of soft constraints | 3 |
| R[F2 > 2σ(F2)] | 0.0452 |
| Rint (internal R-value) | 0.0308 |
| S (Goodness of the fit) | 1.051 |
| Mn(H2O)2[O2(OH)PC15H13]2·2H2O | ||
|---|---|---|
| Contact types (%) | Orientation I | Orientation II |
| P···O | 0.1 | 0.1 |
| O···O | 8.4 | 8.7 |
| O···C | 0.2 | 0.2 |
| O···H | 19.8 | 13.8 |
| C···C | 0 | 1.2 |
| C···H | 19.5 | 24.5 |
| H···H | 41.8 | 42.2 |
| Sum | 89.8 | 90.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bloyet, C.; Rueff, J.-M.; Caignaert, V.; Raveau, B.; Lohier, J.-F.; Roger, M.; Rogez, G.; Jaffrès, P.-A. Manganese Fluorene Phosphonates: Formation of Isolated Chains. Inorganics 2018, 6, 92. https://doi.org/10.3390/inorganics6030092
Bloyet C, Rueff J-M, Caignaert V, Raveau B, Lohier J-F, Roger M, Rogez G, Jaffrès P-A. Manganese Fluorene Phosphonates: Formation of Isolated Chains. Inorganics. 2018; 6(3):92. https://doi.org/10.3390/inorganics6030092
Chicago/Turabian StyleBloyet, Clarisse, Jean-Michel Rueff, Vincent Caignaert, Bernard Raveau, Jean-François Lohier, Mélissa Roger, Guillaume Rogez, and Paul-Alain Jaffrès. 2018. "Manganese Fluorene Phosphonates: Formation of Isolated Chains" Inorganics 6, no. 3: 92. https://doi.org/10.3390/inorganics6030092
APA StyleBloyet, C., Rueff, J.-M., Caignaert, V., Raveau, B., Lohier, J.-F., Roger, M., Rogez, G., & Jaffrès, P.-A. (2018). Manganese Fluorene Phosphonates: Formation of Isolated Chains. Inorganics, 6(3), 92. https://doi.org/10.3390/inorganics6030092