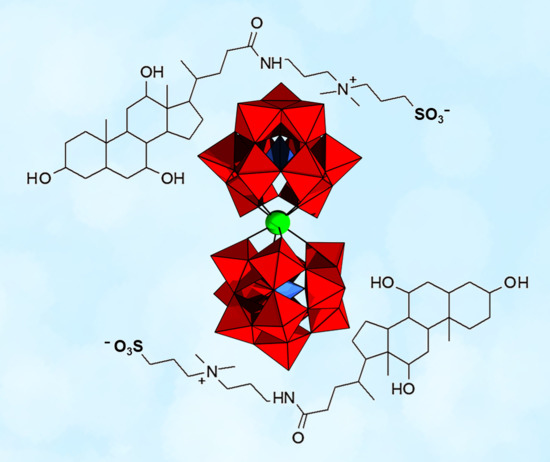
Effect of [Zr(α-PW11O39)2]10− Polyoxometalate on the Self-Assembly of Surfactant Molecules in Water Studied by Fluorescence and DOSY NMR Spectroscopy
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Interaction between Micelles and 1 Studied Using the Pyrene Fluorescence Method
2.2. The Interaction between Micelles and 1 Studied by DOSY
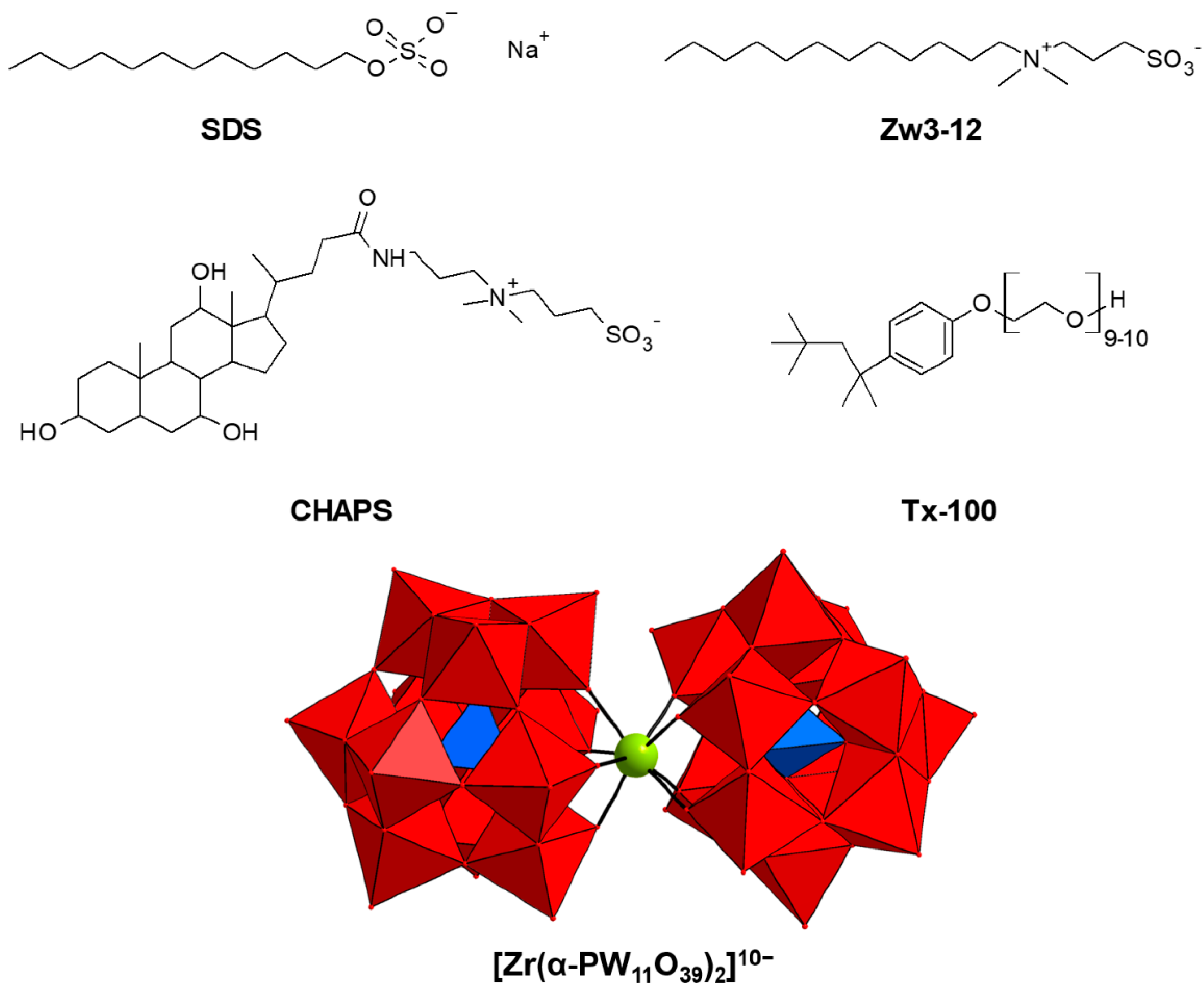
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pope, M.T. Heteropoly and Isopoly Oxometalates; Springer: Berlin/Heidelberg, Germany, 1983. [Google Scholar]
- Kozhevnikov, I. Sustainable heterogeneous acid catalysis by heteropoly acids. In Green Chemistry—Green Catalysis: Heterogeneous Catalysis; Anastas, P.T., Crabtree, R.H., Eds.; Wiley-VCH: New York, NY, USA, 2009; Volume 2, pp. 153–174. [Google Scholar]
- Wang, S.S.; Yang, G. Recent advances in polyoxometalate-catalyzed reactions. Chem. Rev. 2015, 115, 4893–4962. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Juan, J.M.; Coronado, E.; Gaita-Arino, A. Magnetic polyoxometalates: From molecular magnetism to molecular spintronics and quantum computing. Chem. Soc. Rev. 2012, 41, 7464–7478. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.; Peters, F.; Pope, M.T.; Gatteschi, D. Polyoxometalates: Very large clusters—Nanoscale magnets. Chem. Rev. 1998, 98, 239–271. [Google Scholar] [CrossRef] [PubMed]
- Absillis, G.; Cartuyvels, E.; Van Deun, R.; Parac-Vogt, T.N. Hydrolytic cleavage of an RNA-model phosphodiester catalyzed by a highly negatively charged polyoxomolybdate [Mo7O24]6− cluster. J. Am. Chem. Soc. 2008, 130, 17400–17408. [Google Scholar] [CrossRef] [PubMed]
- Ly, H.G.; Absillis, G.; Janssens, R.; Proost, P.; Parac-Vogt, T.N. Highly amino acid selective hydrolysis of myoglobin at aspartate residues as promoted by zirconium(IV)-substituted polyoxometalates. Angew. Chem. Int. Ed. 2015, 54, 7391–7394. [Google Scholar] [CrossRef] [PubMed]
- Luong, T.K.N.; Govaerts, I.; Robben, J.; Shestakova, P.; Parac-Vogt, T.N. Polyoxometalates as artificial nucleases: Hydrolytic cleavage of DNA promoted by a highly negatively charged ZrIV-substituted Keggin polyanion. Chem. Commun. 2017, 53, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Van Rompuy, L.; Parac-Vogt, T.N. Polyoxometalates as sialidase mimics: Selective and non-destructive removal of sialic acid from a glycoprotein promoted by phosphotungstic acids. Chem. Commun. 2017, 53, 10600–10603. [Google Scholar] [CrossRef] [PubMed]
- Sap, A.; Vandebroek, L.; Goovaerts, V.; Martens, E.; Proost, P.; Parac-Vogt, T.N. Highly Selective and Tunable Protein Hydrolysis by a Polyoxometalate Complex in Surfactant Solutions: A Step toward the Development of Artificial Metalloproteases for Membrane Proteins. ACS Omega 2017, 2, 2026–2033. [Google Scholar] [CrossRef] [PubMed]
- Sap, A.; van Tichelen, L.; Mortier, A.; Proost, P.; Parac-Vogt, T.N. Tuning the Selectivity and Reactivity of Metal-Substituted Polyoxometalates as Artificial Proteases by Varying the Nature of the Embedded Lewis Acid Metal Ion. Eur. J. Inorg. Chem. 2016, 32, 5098–5105. [Google Scholar] [CrossRef]
- Sap, A.; De Zitter, E.; van Meervelt, L.; Parac-Vogt, T.N. Structural Characterization of the Complex between Hen Egg-White Lysozyme and ZrIV-Substituted Keggin Polyoxometalate as Artificial Protease. Chem. Eur. J. 2015, 21, 11692–11695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quanten, T.; de Maeyer, T.; Shestakova, P.; Parac-Vogt, T.N. Selectivity and reactivity of ZrIV and CeIV substituted Keggin type polyoxometalates towards cytochrome c in surfactants solutions. Front. Chem. 2018, 6, 372. [Google Scholar] [CrossRef] [PubMed]
- Quanten, T.; Shestakova, P.; Van Den Bulck, D.; Kirschhock, C.; Parac-Vogt, T.N. Interaction Study and Reactivity of ZrIV-Substituted Wells–Dawson Polyoxometalate towards Hydrolysis of Peptide Bonds in Surfactant Solutions. Chem. Eur. J. 2016, 22, 3775–3784. [Google Scholar] [CrossRef] [PubMed]
- Tanford, C. The hydrophobic effect and the organization of living matter. Science 1978, 200, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Kurth, D.G.; Lehmann, P.; Volkmer, D.; Cölfen, H.; Koop, M.J.; Müller, A.; Du Chesne, A. Surfactant-Encapsulated Clusters (SECs): (DODA)20(NH4)[H3Mo57V6(NO)6O183(H2O)18], a Case Study. Chem. Eur. J. 2000, 6, 385–393. [Google Scholar] [CrossRef]
- Kurth, D.G.; Lehmann, P.; Volkmer, D.; Müller, A.; Schwahn, D.J. Biologically inspired polyoxometalate–surfactant composite materials. Investigations on the structures of discrete, surfactant-encapsulated clusters, monolayers, and Langmuir–Blodgett films of (DODA)40(NH4)2[(H2O)n⊂Mo132O372(CH3CO2)30(H2O)72]. Chem. Soc. Dalton Trans. 2000, 3989–3998. [Google Scholar] [CrossRef]
- Bu, W.; Fan, H.; Wu, L.; Hou, X.; Hu, C.; Zhang, G.; Zhang, X. Surfactant-Encapsulated Polyoxoanion: Structural Characterization of Its Langmuir Films and Langmuir−Blodgett Films. Langmuir 2002, 18, 6398–6403. [Google Scholar] [CrossRef]
- Zhang, B.; Yin, P.; Haso, F.; Hu, L.; Liu, T. Soft Matter Approaches for Enhancing the Catalytic Capabilities of Polyoxometalate Clusters. J. Clust. Sci. 2014, 25, 695–710. [Google Scholar] [CrossRef]
- Nisar, A.; Wang, X. Surfactant-encapsulated polyoxometalate building blocks: Controlled assembly and their catalytic properties. Dalton Trans. 2012, 41, 9832–9845. [Google Scholar] [CrossRef] [PubMed]
- Van Lokeren, L.; Cartuyvels, E.; Absillis, G.; Willem, R.; Parac-Vogt, T.N. Phosphoesterase activity of polyoxomolybdates: Diffusion ordered NMR spectroscopy as a tool for obtaining insights into the reactivity of polyoxometalate clusters. Chem. Commun. 2008, 0, 2774–2776. [Google Scholar] [CrossRef] [PubMed]
- Shestakova, P.; Absillis, G.; Martinez, F.J.; De Proft, F.; Willem, R.; Parac-Vogt, T.N. Integrating 31P DOSY NMR Spectroscopy and Molecular Mechanics as a Powerful Tool for Unraveling the Chemical Structures of Polyoxomolybdate-Based Amphiphilic Nanohybrids in Aqueous Solution. Chem. Eur. J. 2014, 20, 5258–5270. [Google Scholar] [CrossRef] [PubMed]
- Luong, T.K.N.; Shestakova, P.; Absillis, G.; Parac-Vogt, T.N. Detailed Mechanism of Phosphoanhydride Bond Hydrolysis Promoted by a Binuclear ZrIV-Substituted Keggin Polyoxometalate Elucidated by a Combination of 31P, 31P DOSY, and 31P EXSY NMR Spectroscopy. Inorg. Chem. 2016, 55, 4864–4873. [Google Scholar] [CrossRef] [PubMed]
- Kalyanasundaram, K.; Thomas, J.K. Environmental effects on vibronic band intensities in pyrene monomer fluorescence and their application in studies of micellar systems. J. Am. Chem. Soc. 1977, 99, 2039–2044. [Google Scholar] [CrossRef]
- Kalyanasundaram, K. Chapter 1—Introduction. In Photochemistry in Microheterogeneous Systems; Kalyanasundaram, K., Ed.; Academic Press Inc.: Orlando, FL, USA, 1987; pp. 36–91. ISBN 978-0-12-394995-0. [Google Scholar]
- Liu, X.-F.; Dong, L.-L.; Fang, Y. Synthesis and Self-Aggregation of a Hydroxyl-Functionalized Imidazolium-Based Ionic Liquid Surfactant in Aqueous Solution. J. Surf. Deterg. 2010, 14, 203–210. [Google Scholar] [CrossRef]
- Quagliotto, P.; Barbero, N.; Barolo, C.; Costabello, K.; Marchese, L.; Coluccia, S.; Kalyanasundaram, K.; Viscardi, G. Characterization of monomeric and gemini cationic amphiphilic molecules by fluorescence intensity and anisotropy. Dyes Pigments 2009, 82, 124–129. [Google Scholar] [CrossRef] [Green Version]
- Matsuoka, K.; Suzuki, M.; Honda, C.; Endo, K.; Moroi, Y. Micellization of conjugated chenodeoxy- and ursodeoxycholates and solubilization of cholesterol into their micelles: Comparison with other four conjugated bile salts species. Chem. Phys. Lipids 2006, 139, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Howell, S.C.; Mittal, R.; Huang, L.; Travis, B.; Breyer, R.M.; Sanders, C.R. CHOBIMALT: A Cholesterol-Based Detergent. Biochemistry 2010, 49, 9572–9583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hierrezuelo, J.M.; Aguiar, J.; Carnero Ruiz, C. Synergism in mixtures of zwitterionic and ionic surfactants. J. Colloid Interface Sci. 2006, 294, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Sabate, R.; Estelrich, J. Evidence of the Existence of Micelles in the Fibrillogenesis of β-Amyloid Peptide. J. Phys. Chem. B 2005, 109, 11027–11032. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, V.M.; Goncalves, C.; de Melo-Diogo, D.; Costa, E.C.; Queiroz, J.A.; Pichon, C.; Sousa, F.; Correia, I.J. Poly(2-ethyl-2-oxazoline)-PLA-g-PEI amphiphilic triblock micelles for co-delivery of minicircle DNA and chemotherapeutics. J. Control. Release 2014, 189, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Mok, M.M.; Thiagarajan, R.; Flores, M.; Morse, D.C.; Lodge, T.P. Apparent Critical Micelle Concentrations in Block Copolymer/Ionic Liquid Solutions: Remarkably Weak Dependence on Solvophobic Block Molecular Weight. Macromolecules 2012, 45, 4818–4829. [Google Scholar] [CrossRef]
- Piogé, S.; Fontaine, L.; Gaillard, C.; Nicol, E.; Pascual, S. Self-Assembling Properties of Well-Defined Poly(ethylene oxide)-b-poly(ethyl acrylate) Diblock Copolymers. Macromolecules 2009, 42, 4262–4272. [Google Scholar] [CrossRef]
- Feitosa, E.; Brown, W.; Vasilescu, M.; Swanson-Vethamuthu, M. Effect of Temperature on the Interaction between the Nonionic Surfactant C12E5 and Poly(ethylene oxide) Investigated by Dynamic Light Scattering and Fluorescence Methods. Macromolecules 1996, 29, 6837–6846. [Google Scholar] [CrossRef]
- Evertsson, H.; Nilsson, S.; Holmberg, C.; Sundelöf, L.-O. Temperature Effects on the Interactions between EHEC and SDS in Dilute Aqueous Solutions. Steady-State Fluorescence Quenching and Equilibrium Dialysis Investigations. Langmuir 1996, 12, 5781–5789. [Google Scholar] [CrossRef]
- Aguiar, J.; Carpena, P.; Molina-Bolı́var, J.A.; Carnero Ruiz, C. On the determination of the critical micelle concentration by the pyrene 1:3 ratio method. J. Colloid Interface Sci. 2003, 258, 116–122. [Google Scholar] [CrossRef]
- Anthony, O.; Zana, R. Fluorescence Investigation of the Binding of Pyrene to Hydrophobic Microdomains in Aqueous Solutions of Polysoaps. Macromolecules 1994, 27, 3885–3891. [Google Scholar] [CrossRef]
- Regev, O.; Zana, R. Aggregation Behavior of Tyloxapol, a Nonionic Surfactant Oligomer, in Aqueous Solution. J. Colloid Interface Sci. 1999, 210, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Tiller, G.E.; Mueller, T.J.; Dockter, M.E.; Struve, W.G. Hydrogenation of Triton X-100 eliminates its fluorescence and ultraviolet light absorption while preserving its detergent properties. Anal. Biochem. 1984, 141, 262–266. [Google Scholar] [CrossRef]
- Brown, W.; Rymden, R.; Van Stam, J.; Almgren, M.; Svensk, G. Static and dynamic properties of nonionic amphiphile micelles: Triton X-100 in aqueous solution. J. Phys. Chem. 1989, 93, 2512–2519. [Google Scholar] [CrossRef]
- Denkova, P.S.; Lokeren, L.V.; Verbruggen, I.; Willem, R. Self-Aggregation and Supramolecular Structure Investigations of Triton X-100 and SDP2S by NOESY and Diffusion Ordered NMR Spectroscopy. J. Phys. Chem. B 2008, 112, 10935–10941. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, A.; London, E. Fluorimetric determination of critical micelle concentration avoiding interference from detergent charge. Anal. Biochem. 1984, 139, 408–412. [Google Scholar] [CrossRef]
- Giacomelli, C.E.; Vermeer, A.W.P.; Norde, W. Micellization and Adsorption Characteristics of CHAPS. Langmuir 2000, 16, 4853–4858. [Google Scholar] [CrossRef]
- Qin, X.; Liu, M.; Yang, D.; Zhang, X. Concentration-Dependent Aggregation of CHAPS Investigated by NMR Spectroscopy. J. Phys. Chem. B 2010, 114, 3863–3868. [Google Scholar] [CrossRef] [PubMed]
- Stark, R.E.; Leff, P.D.; Milheim, S.G.; Kropf, A. Physical studies of CHAPS, a new detergent for the study of visual pigments. J. Phys. Chem. 1984, 88, 6063–6067. [Google Scholar] [CrossRef]
- Schuerholz, T.; Kehne, J.; Gieselmann, A.; Neumann, E. Functional reconstitution of the nicotinic acetylcholine receptor by CHAPS dialysis depends on the concentrations of salt, lipid, and protein. Biochemistry 2002, 31, 5067–5077. [Google Scholar] [CrossRef]
- Hjelmeland, L.M.; Nebert, D.W.; Osborne, J.C. Sulfobetaine derivatives of bile acids: Nondenaturing surfactants for membrane biochemistry. Anal. Biochem. 1983, 130, 72–82. [Google Scholar] [CrossRef]
- Vulliezlenormand, B.; Eisele, J.L. Determination of Detergent Critical Micellar Concentration by Solubilization of a Colored Dye. Anal. Biochem. 1993, 208, 241–243. [Google Scholar] [CrossRef]
- Neugebauer, J.M. Detergents: An overview. Meth. Enzymology 1990, 182, 239–253. [Google Scholar]
- Samsonoff, C.; Daily, J.; Almog, R.; Berns, D.S. The use of Coomassie brilliant blue for critical micelle concentration determination of detergents. J. Colloid Interface Sci. 1986, 109, 325–329. [Google Scholar] [CrossRef]
- Johnson, C.S., Jr. Diffusion ordered nuclear magnetic resonance spectroscopy: principles and applications. Prog. Nucl. Magn. Reson. Spectrosc. 1999, 34, 203–256. [Google Scholar] [CrossRef]
- Sokolov, M.N.; Chubarova, E.V.; Peresypkina, E.V.; Virovets, A.V.; Fedin, V.P. Complexes of ZrIV and HfIV with monolacunary Keggin- and Dawson-type anions. Russ. Chem. Bull. 2007, 56, 220–224. [Google Scholar] [CrossRef]
- Kato, C.N.; Shinohara, A.; Hayashi, K.; Nomiya, K. Syntheses and X-ray Crystal Structures of Zirconium(IV) and Hafnium(IV) Complexes Containing Monovacant Wells–Dawson and Keggin Polyoxotungstates. Inorg. Chem. 2006, 45, 8108–8119. [Google Scholar] [CrossRef] [PubMed]
- Kondinski, A.; Parac-Vogt, T.N. Keggin Structure, Quō Vādis? Front. Chem. 2018, 6, 364. [Google Scholar] [CrossRef] [PubMed]
- Solé-Daura, A.; Goovaerts, V.; Stroobants, K.; Absillis, G.; Jiménez-Lozano, P.; Poblet, J.M.; Hirst, J.D.; Parac-Vogt, T.N.; Carbó, J.J. Probing Polyoxometalate–Protein Interactions Using Molecular Dynamics Simulations. Chem. Eur. J. 2016, 22, 15280–15289. [Google Scholar] [CrossRef] [PubMed]


| SDS | TX-100 | CHAPS | Zw3-12 | |
|---|---|---|---|---|
| a, b | 4.16 ± 0.01 | 0.41 ± 0.08 | 5.80 ± 0.1 | 2.28 ± 0.03 |
| 16 mM Et2NH2Cl a | 1.44 ± 0.01 | 0.26 ± 0.04 | 5.55 ± 0.05 | 2.30 ± 0.03 |
| 1% 1 a | 1.40 ± 0.01 | 0.23 ± 0.05 | 2.0 ± 0.4 | 1.10 ± 0.05 c |
| SDS | TX-100 | CHAPS | Zw3-12 | |
|---|---|---|---|---|
| b, c | 1.66 ± 0.01 | 1.97 ± 0.01 | 1.24 ± 0.02 | 2.03 ± 0.01 |
| 16 mM Et2NH2Cl b | 1.67 ± 0.01 | 2.00 ± 0.01 | 1.28 ± 0.01 | 2.01 ± 0.01 |
| 1% 1 b | 1.64 ± 0.01 | 1.83 ± 0.01 | 1.17 ± 0.02 | d |
| Sample | Compound | D × 10−11 (m2·s−1) |
|---|---|---|
| Et2NH2Cl a | Et2NH2+ | 70.5 |
| Et2NH2+ + 1 b | Et2NH2+ | 51.1 |
| 1 | 20.9 d | |
| CHAPS | CHAPS | 14.5 |
| CHAPS + 9 mM 1 | CHAPS | 8.78 |
| Et2NH2+ | 43.2 | |
| 1 | 8.21 d | |
| CHAPS + Et2NH2Cl c | CHAPS | 14.1 |
| Et2NH2+ | 63.9 | |
| Zw3-12 | Zw3-12 | 11.5 |
| Zw3-12 + 9 mM 1 | Zw3-12 | 6.12 |
| Et2NH2+ | 40.4 | |
| 1 | 9.24 d | |
| Zw3-12 + Et2NH2Cl c | Zw3-12 | 10.8 |
| Et2NH2+ | 65.8 | |
| TX-100 | TX-100 | 4.55 |
| TX-100 + 9 mM 1 | TX-100 | 4.46 |
| Et2NH2+ | 45.9 | |
| 1 | 16.9 d | |
| TX-100 + Et2NH2Cl c | TX-100 | 4.63 |
| Et2NH2+ | 65.6 | |
| SDS | SDS | 9.49 |
| SDS + 9 mM 1 | SDS | 6.76 |
| Et2NH2+ | 39.9 | |
| 1 | 19.5 d | |
| SDS + Et2NH2Cl c | SDS | 8.22 |
| Et2NH2+ | 26.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quanten, T.; Shestakova, P.; Kondinski, A.; Parac-Vogt, T.N. Effect of [Zr(α-PW11O39)2]10− Polyoxometalate on the Self-Assembly of Surfactant Molecules in Water Studied by Fluorescence and DOSY NMR Spectroscopy. Inorganics 2018, 6, 112. https://doi.org/10.3390/inorganics6040112
Quanten T, Shestakova P, Kondinski A, Parac-Vogt TN. Effect of [Zr(α-PW11O39)2]10− Polyoxometalate on the Self-Assembly of Surfactant Molecules in Water Studied by Fluorescence and DOSY NMR Spectroscopy. Inorganics. 2018; 6(4):112. https://doi.org/10.3390/inorganics6040112
Chicago/Turabian StyleQuanten, Thomas, Pavletta Shestakova, Aleksandar Kondinski, and Tatjana N. Parac-Vogt. 2018. "Effect of [Zr(α-PW11O39)2]10− Polyoxometalate on the Self-Assembly of Surfactant Molecules in Water Studied by Fluorescence and DOSY NMR Spectroscopy" Inorganics 6, no. 4: 112. https://doi.org/10.3390/inorganics6040112