Excited-State Relaxation in Luminescent Molybdenum(0) Complexes with Isocyanide Chelate Ligands
Abstract
:1. Introduction
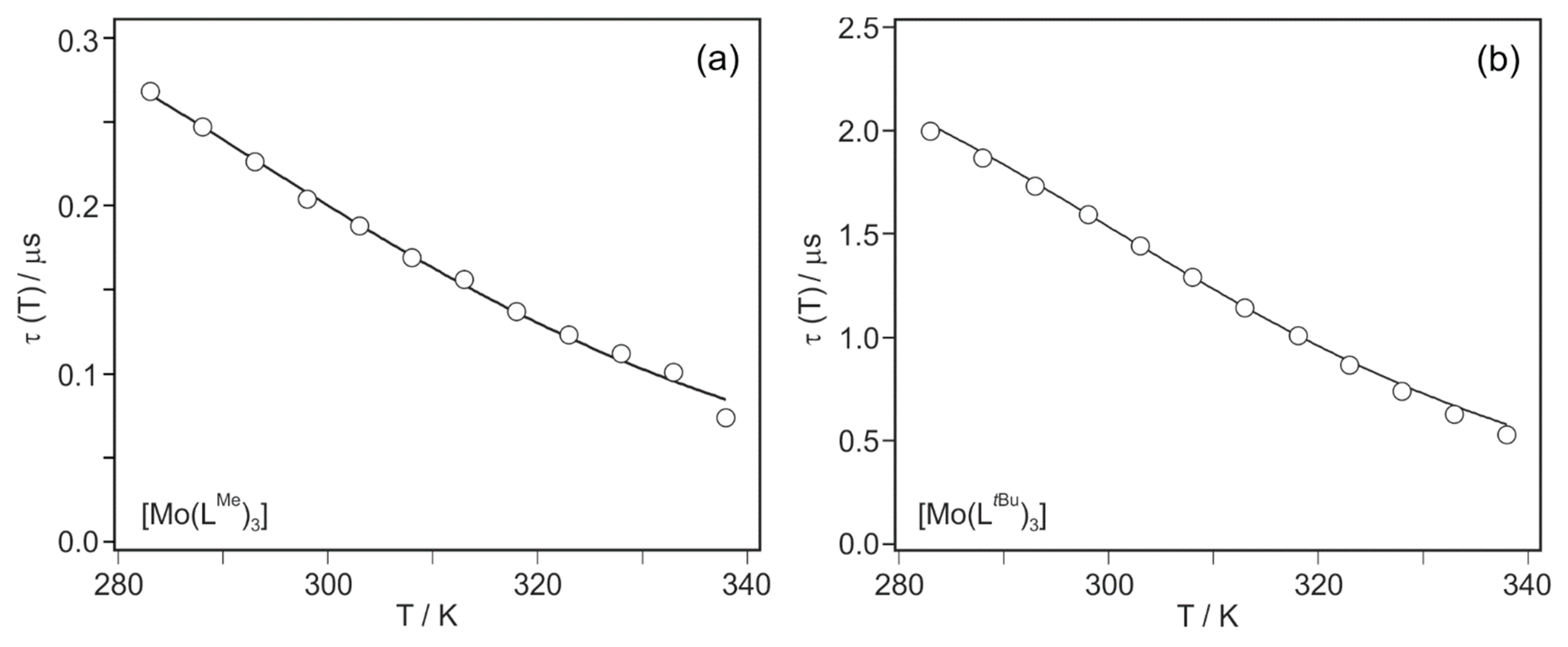
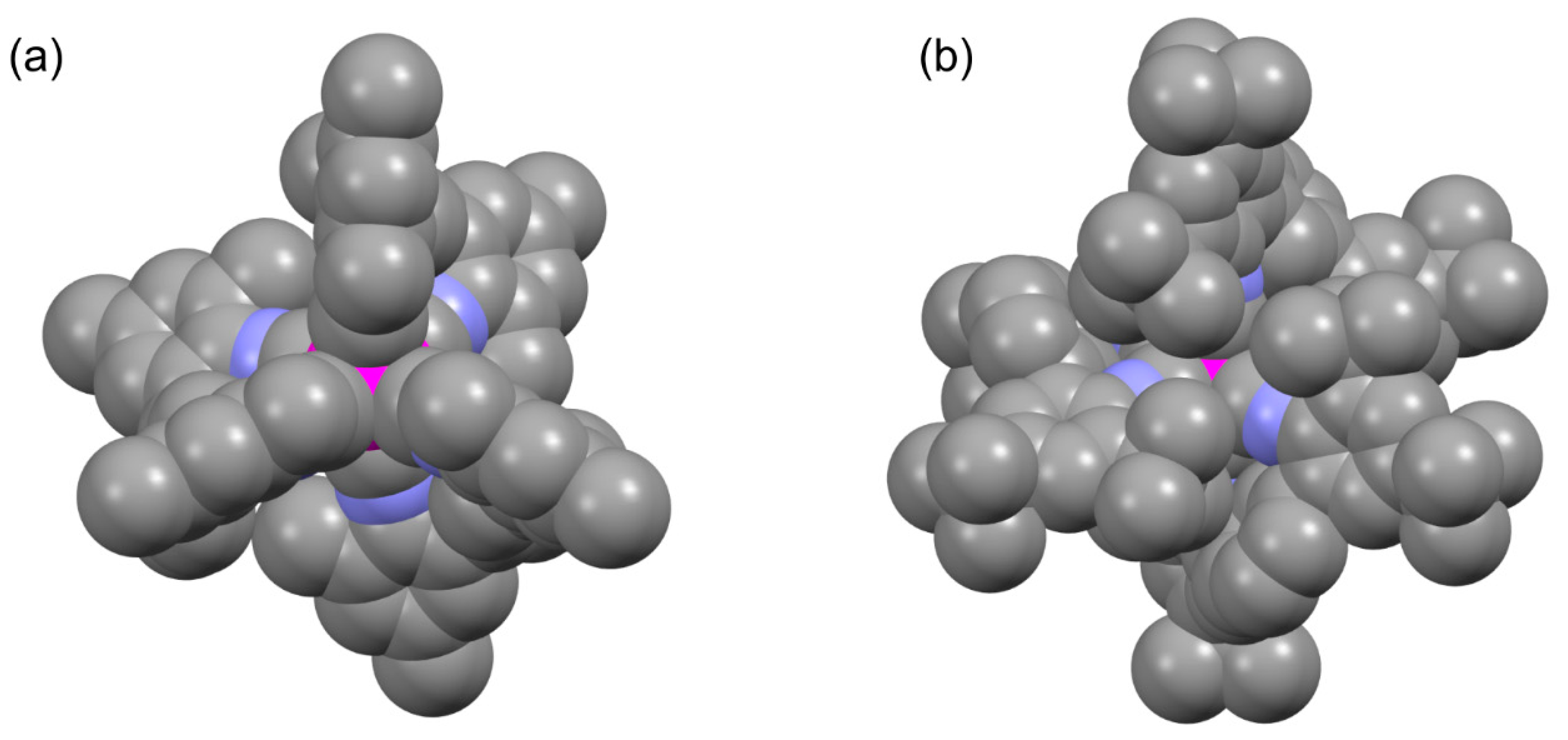
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mann, K.R.; Cimolino, M.; Geoffroy, G.L.; Hammond, G.S.; Orio, A.A.; Albertin, G.; Gray, H.B. Electronic Structures and Spectra of Hexakisphenylisocyanide Complexes of Cr0, Mo0, W0, MnI, and MnII. Inorg. Chim. Acta 1976, 16, 97–101. [Google Scholar] [CrossRef]
- Mann, K.R.; Gray, H.B.; Hammond, G.S. Excited-State Reactivity Patterns of Hexakisarylisocyano Complexes of Chromium(0), Molybdenum(0), and Tungsten(0). J. Am. Chem. Soc. 1977, 99, 306–307. [Google Scholar] [CrossRef]
- King, R.B.; Saran, M.S. Isocyanide-Metal Complexes. II. CO and CN Stretching Modes in tert-Butyl Isocyanide Derivatives of Octahedral Metal-Carbonyls. Inorg. Chem. 1974, 13, 74–78. [Google Scholar] [CrossRef]
- Coville, N.J.; Albers, M.O. A Synthetic Route to M(CO)6−n(RNC)n (M = Cr, Mo, W; n = 1–6) from M(CO)6 and Isonitriles. Inorg. Chim. Acta 1982, 65, L7–L8. [Google Scholar] [CrossRef]
- Lyons, L.J.; Pitz, S.L.; Boyd, D.C. Electrochemical and IR Spectroelectrochemical Investigations of the Series Mo(CO)6−n(CNR)n (n = 1–6) (R = 2,8-Dimethylphenyl): In-situ Observation of fac-mer and cis-trans Isomerizations. Inorg. Chem. 1995, 34, 316–322. [Google Scholar] [CrossRef]
- Angelici, R.J.; Quick, M.H.; Kraus, G.A.; Plummer, D.T. Synthesis of Chelating Bidentate Isocyano and Cyano Ligands and Their Metal Complexes. Inorg. Chem. 1982, 21, 2178–2184. [Google Scholar] [CrossRef] [Green Version]
- Treichel, P.M.; Firsich, D.W.; Essenmacher, G.P. Manganese(I) and Chromium(0) Complexes of Phenyl Isocyanide. Inorg. Chem. 1979, 18, 2405–2409. [Google Scholar] [CrossRef]
- Sattler, W.; Henling, L.M.; Winkler, J.R.; Gray, H.B. Bespoke Photoreductants: Tungsten Arylisocyanides. J. Am. Chem. Soc. 2015, 137, 1198–1205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sattler, W.; Ener, M.E.; Blakemore, J.D.; Rachford, A.A.; LaBeaume, P.J.; Thackeray, J.W.; Cameron, J.F.; Winkler, J.R.; Gray, H.B. Generation of Powerful Tungsten Reductants by Visible Light Excitation. J. Am. Chem. Soc. 2013, 135, 10614–10617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kvapilova, H.; Sattler, W.; Sattler, A.; Sazanovich, I.V.; Clark, I.P.; Towrie, M.; Gray, H.B.; Zalis, S.; Vlcek, A. Electronic Excited States of Tungsten(0) Arylisocyanides. Inorg. Chem. 2015, 54, 8518–8528. [Google Scholar] [CrossRef] [PubMed]
- Margulieux, G.W.; Weidemann, N.; Lacy, D.C.; Moore, C.E.; Rheingold, A.L.; Figueroa, J.S. Isocyano Analogues of [Co(CO)]4n: A Tetraisocyanide of Cobalt Isolated in Three States of Charge. J. Am. Chem. Soc. 2010, 132, 5033–5035. [Google Scholar] [CrossRef] [PubMed]
- Monti, F.; Baschieri, A.; Matteucci, E.; Mazzanti, A.; Sambri, L.; Barbieri, A.; Armaroli, N. A Chelating Diisocyanide Ligand for Cyclometalated Ir(III) Complexes with Strong and Tunable Luminescence. Faraday Discuss. 2015, 185, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Knorn, M.; Rawner, T.; Czerwieniec, R.; Reiser, O. Copper(phenanthroline)(bisisonitrile)+-Complexes for the Visible-Light-Mediated Atom Transfer Radical Addition and Allylation Reactions. ACS Catal. 2015, 5, 5186–5193. [Google Scholar] [CrossRef]
- Drance, M.J.; Sears, J.D.; Mrse, A.M.; Moore, C.E.; Rheingold, A.L.; Neidig, M.L.; Figueroa, J.S. Terminal Coordination of Diatomic Boron Monofluoride to Iron. Science 2019, 363, 1203–1205. [Google Scholar] [CrossRef] [PubMed]
- Cañada, L.M.; Kölling, J.; Teets, T.S. Blue-Phosphorescent Bis-Cyclometalated Iridium Complexes with Arylisocyanide Ancillary Ligands. Polyhedron 2020, 178, 114332. [Google Scholar] [CrossRef]
- Hahn, F.E. The Coordination Chemistry of Multidentate Isocyanide Ligands. Angew. Chem. Int. Ed. 1993, 32, 650–665. [Google Scholar] [CrossRef]
- Büldt, L.A.; Wenger, O.S. Chromium(0), Molybdenum(0), and Tungsten(0) Isocyanide Complexes as Luminophores and Photosensitizers with Long-Lived Excited States. Angew. Chem. Int. Ed. 2017, 56, 5676–5682. [Google Scholar] [CrossRef] [Green Version]
- Braun, J.D.; Lozada, I.B.; Kolodziej, C.; Burda, C.; Newman, K.M.E.; van Lierop, J.; Davis, R.L.; Herbert, D.E. Iron(II) Coordination Complexes with Panchromatic Absorption and Nanosecond Charge-Transfer Excited State Lifetimes. Nat. Chem. 2019, 11, 1144–1150. [Google Scholar] [CrossRef]
- Chábera, P.; Kjaer, K.S.; Prakash, O.; Honarfar, A.; Liu, Y.Z.; Fredin, L.A.; Harlang, T.C.B.; Lidin, S.; Uhlig, J.; Sundström, V.; et al. Fe-II Hexa N-Heterocyclic Carbene Complex with a 528 ps Metal-to-Ligand Charge-Transfer Excited-State Lifetime. J. Phys. Chem. Lett. 2018, 9, 459–463. [Google Scholar] [CrossRef]
- Steube, J.; Burkhardt, L.; Päpcke, A.; Moll, J.; Zimmer, P.; Schoch, R.; Wölper, C.; Heinze, K.; Lochbrunner, S.; Bauer, M. Excited-State Kinetics of an Air-Stable Cyclometalated Iron(II) Complex. Chem. Eur. J. 2019, 25, 11826–11830. [Google Scholar] [CrossRef]
- Duchanois, T.; Liu, L.; Pastore, M.; Monari, A.; Cebrian, C.; Trolez, Y.; Darari, M.; Magra, K.; Frances-Monerris, A.; Domenichini, E.; et al. NHC-Based Iron Sensitizers for DSSCs. Inorganics 2018, 6, 63. [Google Scholar] [CrossRef] [Green Version]
- Carey, M.C.; Adelman, S.L.; McCusker, J.K. Insights into the Excited State Dynamics of Fe(II) Polypyridyl Complexes from Variable-Temperature Ultrafast Spectroscopy. Chem. Sci. 2019, 10, 134–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wenger, O.S. Is Iron the New Ruthenium? Chem. Eur. J. 2019, 25, 6043–6052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Büldt, L.A.; Guo, X.; Vogel, R.; Prescimone, A.; Wenger, O.S. A Tris(diisocyanide)chromium(0) Complex Is a Luminescent Analog of Fe(2,2’-Bipyridine)32+. J. Am. Chem. Soc. 2017, 139, 985–992. [Google Scholar] [CrossRef] [Green Version]
- Büldt, L.A.; Guo, X.; Prescimone, A.; Wenger, O.S. A Molybdenum(0) Isocyanide Analogue of Ru(2,2’-Bipyridine)32+: A Strong Reductant for Photoredox Catalysis. Angew. Chem. Int. Ed. 2016, 55, 11247–11250. [Google Scholar] [CrossRef]
- Büldt, L.A.; Wenger, O.S. Luminescent Complexes Made from Chelating Isocyanide Ligands and Earth-Abundant Metals. Dalton Trans. 2017, 46, 15175–15177. [Google Scholar] [CrossRef] [Green Version]
- Herr, P.; Glaser, F.; Büldt, L.A.; Larsen, C.B.; Wenger, O.S. Long-Lived, Strongly Emissive, and Highly Reducing Excited States in Mo(0) Complexes with Chelating Isocyanides. J. Am. Chem. Soc. 2019, 141, 14394–14402. [Google Scholar] [CrossRef]
- Damrauer, N.H.; Boussie, T.R.; Devenney, M.; McCusker, J.K. Effects of Intraligand Electron Delocalization, Steric Tuning, and Excited-State Vibronic Coupling on the Photophysics of Aryl-Substituted Bipyridyl Complexes of Ru(II). J. Am. Chem. Soc. 1997, 119, 8253–8268. [Google Scholar] [CrossRef]
- Caspar, J.V.; Meyer, T.J. Application of the Energy-Gap Law to Nonradiative Excited-State Decay. J. Phys. Chem. 1983, 87, 952–957. [Google Scholar] [CrossRef]
- Kober, E.M.; Caspar, J.V.; Lumpkin, R.S.; Meyer, T.J. Application of the Energy-Gap Law to Excited-State Decay of Osmium(II) Polypyridine Complexes: Calculation of Relative Nonradiative Decay-Rates from Emission Spectral Profiles. J. Phys. Chem. 1986, 90, 3722–3734. [Google Scholar] [CrossRef]
- Hauser, A.; Reber, C. Spectroscopy and Chemical Bonding in Transition Metal Complexes. Struct. Bond. 2016, 172, 291–312. [Google Scholar]
- Claude, J.P.; Meyer, T.J. Temperature-Dependence of Nonradiative Decay. J. Phys. Chem. 1995, 99, 51–54. [Google Scholar] [CrossRef]
- Wenger, O.S.; Güdel, H.U. Optical Spectroscopy of CrCl63− Doped Cs2NaScCl6: Broadband Near-Infrared Luminescence and Jahn-Teller Effect. J. Chem. Phys. 2001, 114, 5832–5841. [Google Scholar] [CrossRef]
- Treadway, J.A.; Strouse, G.F.; Ruminski, R.R.; Meyer, T.J. Long-Lived Near-Infrared MLCT Emitters. Inorg. Chem. 2001, 40, 4508–4509. [Google Scholar] [CrossRef]
- Ashford, D.L.; Glasson, C.R.K.; Norris, M.R.; Concepcion, J.J.; Keinan, S.; Brennaman, M.K.; Templeton, J.L.; Meyer, T.J. Controlling Ground and Excited State Properties through Ligand Changes in Ruthenium Polypyridyl Complexes. Inorg. Chem. 2014, 53, 5637–5646. [Google Scholar] [CrossRef]
- Roundhill, D.M. Photochemistry and Photophysics of Metal Complexes; Plenum Press: New York, NY, USA, 1994. [Google Scholar]
- Medlycott, E.A.; Hanan, G.S. Designing Tridentate Ligands for Ruthenium(II) Complexes with Prolonged Room Temperature Luminescence Lifetimes. Chem. Soc. Rev. 2005, 34, 133–142. [Google Scholar] [CrossRef]
- Dixon, I.M.; Heully, J.L.; Alary, F.; Elliott, P.I.P. Theoretical Illumination of Highly Original Photoreactive 3MC States and the Mechanism of the Photochemistry of Ru(II) Tris(Bidentate) Complexes. Phys. Chem. Chem. Phys. 2017, 19, 27765–27778. [Google Scholar] [CrossRef]
- Sun, Q.C.; Dereka, B.; Vauthey, E.; Daku, L.M.L.; Hauser, A. Ultrafast Transient IR Spectroscopy and DFT Calculations of Ruthenium(II) Polypyridyl Complexes. Chem. Sci. 2017, 8, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Ponce, A.; Gray, H.B.; Winkler, J.R. Electron Tunneling Through Water: Oxidative Quenching of Electronically Excited Ru(tpy)22+ (tpy = 2,2′:6,2″-terpyridine) by Ferric Ions in Aqueous Glasses at 77 K. J. Am. Chem. Soc. 2000, 122, 8187–8191. [Google Scholar] [CrossRef] [Green Version]
- Abrahamsson, M.; Jäger, M.; Osterman, T.; Eriksson, L.; Persson, P.; Becker, H.C.; Johansson, O.; Hammarström, L. A 3.0 ms Room Temperature Excited State Lifetime of a Bistridentate RuII-polypyridine Complex for Rod-Like Molecular Arrays. J. Am. Chem. Soc. 2006, 128, 12616–12617. [Google Scholar] [CrossRef]
- Van Houten, J.; Watts, R.J. Temperature-Dependence of Photophysical and Photochemical Properties of Tris(2,2’-bipyridyl)ruthenium(II) Ion in Aqueous-Solution. J. Am. Chem. Soc. 1976, 98, 4853–4858. [Google Scholar] [CrossRef]
- Parker, C.A.; Rees, W.T. Correction of Fluorescence Spectra and Measurement of Fluorescence Quantum Efficiency. Analyst 1960, 85, 587–600. [Google Scholar] [CrossRef]
- Wenger, O.S. Photoactive Complexes with Earth-Abundant Metals. J. Am. Chem. Soc. 2018, 140, 13522–13533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Förster, C.; Heinze, K. Photophysics and Photochemistry with Earth-abundant Metals—Fundamentals and Concepts. Chem. Soc. Rev. 2020. [Google Scholar] [CrossRef]



| Compound | λmax/nm | φem | τ/ns |
|---|---|---|---|
| [Mo(LMe)3] | 607 | 0.023 | 166 |
| [Mo(LtBu)3] | 585 | 0.203 | 1110 (85%)/2330 (15%) 1 |
| Compound | kr/105 s−1 | knr/105 s−1 |
|---|---|---|
| [Mo(LMe)3] 1 | 1.39 | 58.9 |
| [Mo(LtBu)3] 1,2 | 1.78 | 5.95 |
| [Ru(dmb)3]2+ 3,4 | 0.83 | 10.6 |
| Compound | E0/cm−1 | E00/cm−1 | ħ·ωM/cm−1 | SM | Δν1/2/cm−1 |
|---|---|---|---|---|---|
| [Mo(LMe)3] 1 | 16,700 | 18,100 | 1650 | 0.23 | 1800 |
| [Mo(LtBu)3] 1 | 17,150 | 18,400 | 1600 | 0.15 | 1700 |
| [Ru(dmb)3]2+ 2,3 | 15,980 | 17,310 | 1330 | 1.05 | 1750 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herr, P.; Wenger, O.S. Excited-State Relaxation in Luminescent Molybdenum(0) Complexes with Isocyanide Chelate Ligands. Inorganics 2020, 8, 14. https://doi.org/10.3390/inorganics8020014
Herr P, Wenger OS. Excited-State Relaxation in Luminescent Molybdenum(0) Complexes with Isocyanide Chelate Ligands. Inorganics. 2020; 8(2):14. https://doi.org/10.3390/inorganics8020014
Chicago/Turabian StyleHerr, Patrick, and Oliver S. Wenger. 2020. "Excited-State Relaxation in Luminescent Molybdenum(0) Complexes with Isocyanide Chelate Ligands" Inorganics 8, no. 2: 14. https://doi.org/10.3390/inorganics8020014





