Temporomandibular Joint Ankylosis in a Girl Child: Immunochemical Evaluation of Tissue Material Obtained from Repeated Arthroplasty Surgeries
Abstract
:1. Introduction
2. Case Presentation
3. Immunohistochemical Analysis
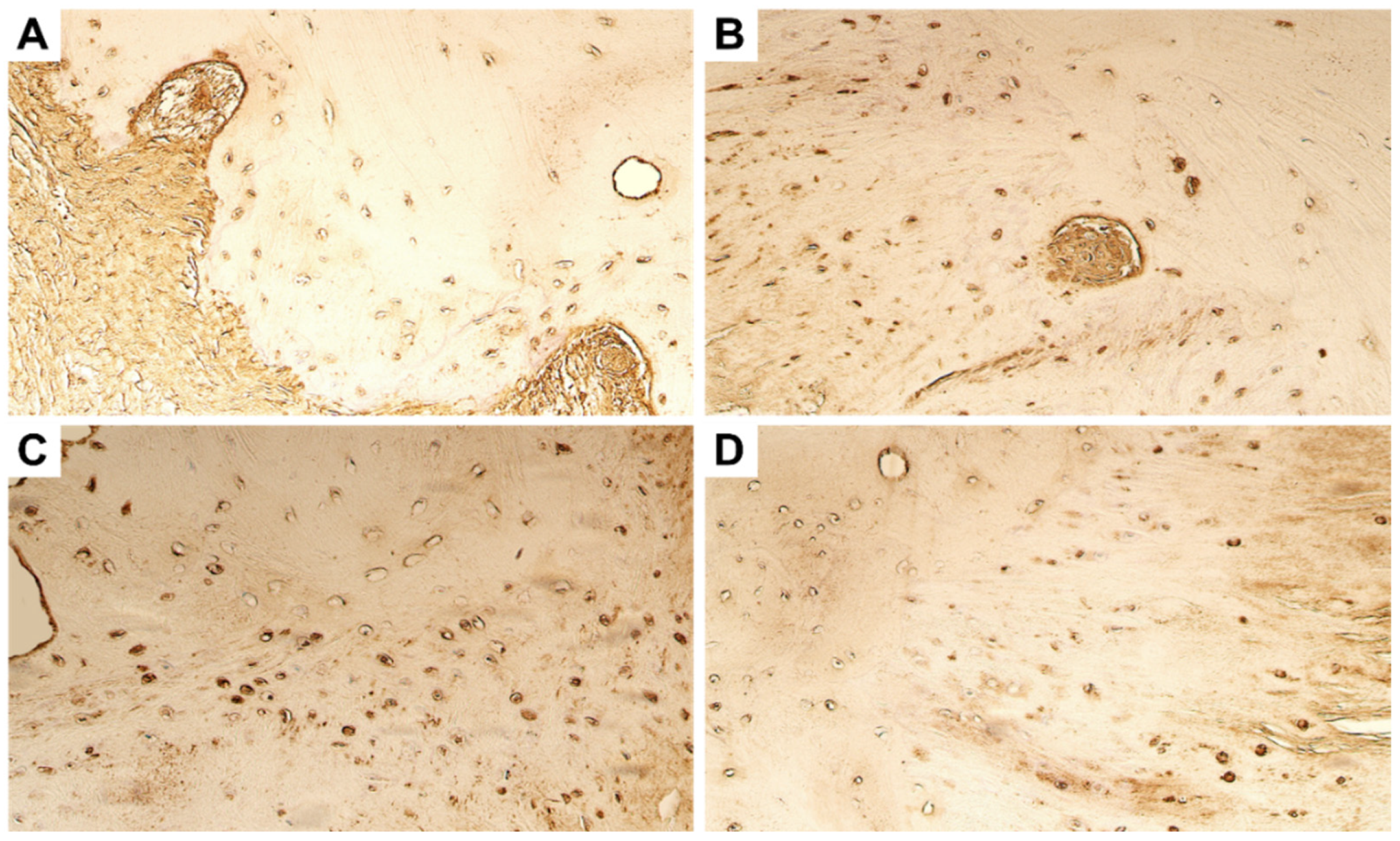
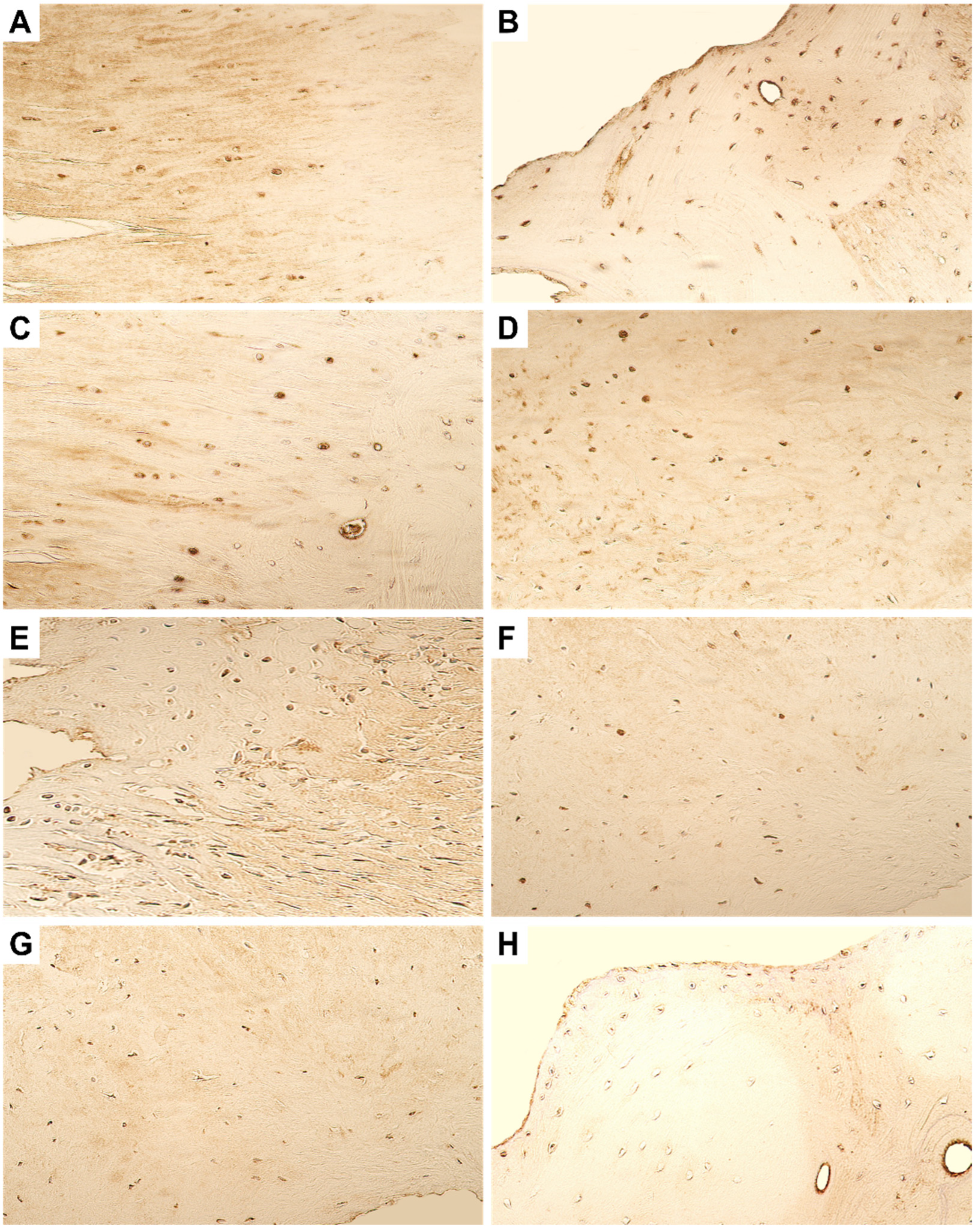
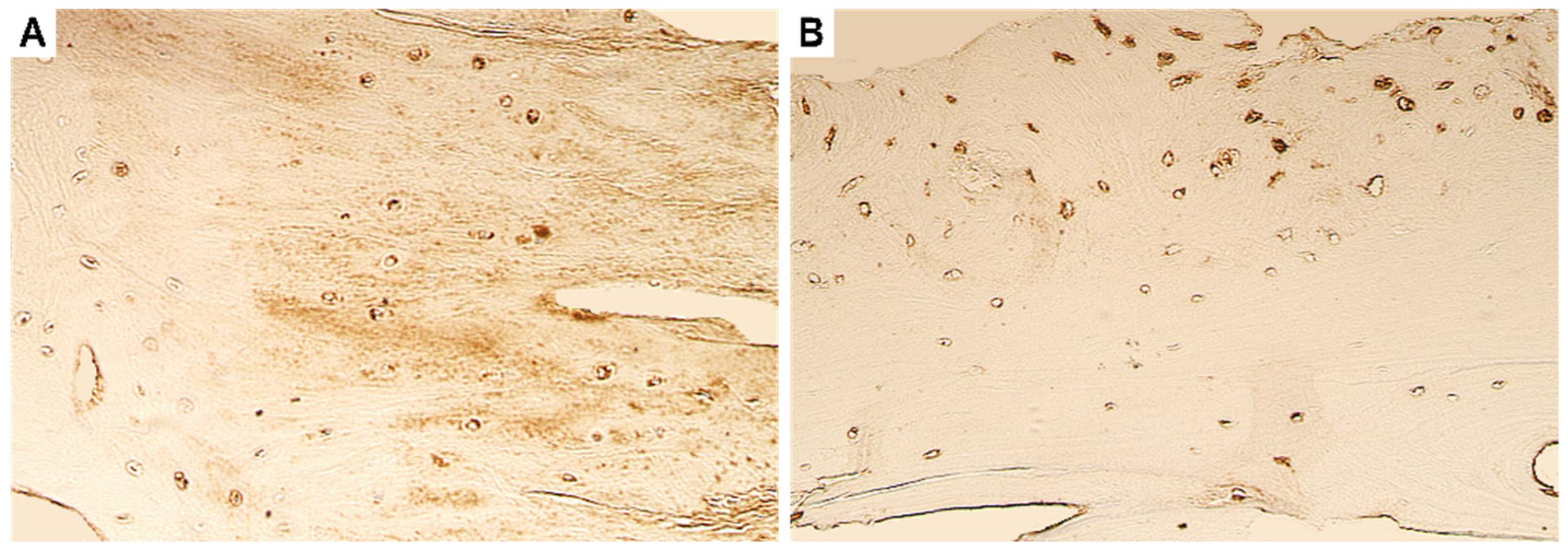
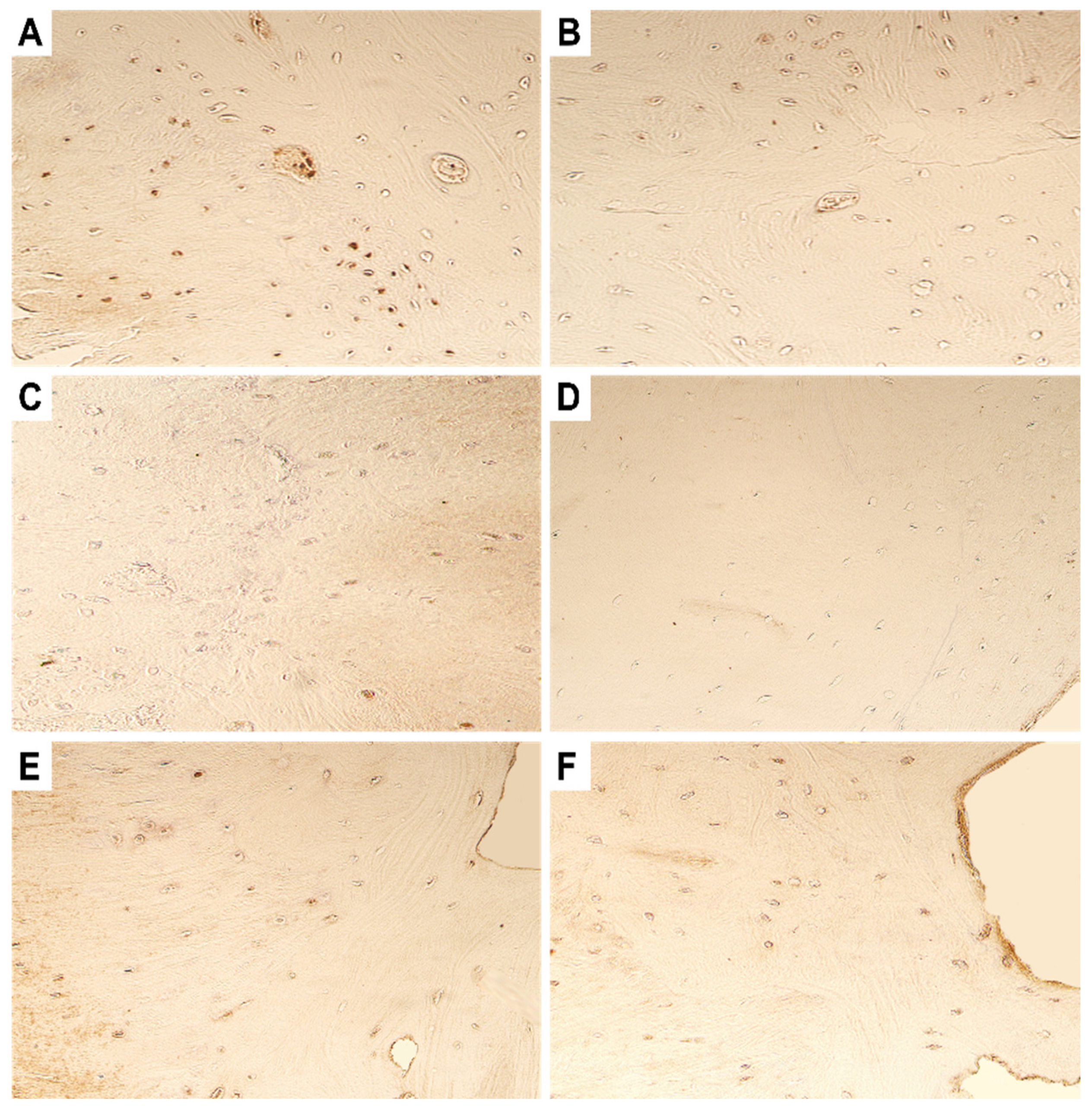
4. Immunohistochemistry Results
4.1. Evaluation of Expression of Tissue Remodelling Factors
4.2. Evaluation of Expression of Growth Factors and Cytokines
4.3. Evaluation of Expression of Antimicrobial Peptide
4.4. Evaluation of Expression of Transcription Factors
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hossain, M.A.; Shah, S.A.A.; Biswas, R.S.R. Frequency of Temporomandibular Joint Ankylosis in Various Age Groups with Reference to Etiology. Chattagram Maa-O-Shishu Hosp. Med. Coll. J. 2014, 13, 17–20. [Google Scholar] [CrossRef] [Green Version]
- Saeed, N.R.; Mcleod, N.; Hensher, R. Temporomandibular joint replacement in rheumatoid-induced disease. Br. J. Oral Maxillofac. Surg. 2001, 39, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Salins, P.C. New perspectives in the management of cranio-mandibular ankylosis. Int. J. Oral Maxillofac. Surg. 2000, 29, 337–340. [Google Scholar] [CrossRef]
- Ma, J.; Liang, L.; Jiang, H.; Gu, B. Gap Arthroplasty versus Interpositional Arthroplasty for Temporomandibular Joint Ankylosis: A Meta-Analysis. PLoS ONE 2015, 10, e0127652. [Google Scholar] [CrossRef] [Green Version]
- Mishra, N.; Sharma, N.K.; Dhiman, N.K.; Jaiswara, C.; Tiwari, P.; Singh, A.K. Temporomandibular joint ankylosis: A tertiary center-based epidemiological study. Natl. J. Maxillofac. Surg. 2021, 12, 392–396. [Google Scholar] [CrossRef]
- Shah, A.A. Silastics as interpositional gap arthroplasty in TMJ ankylosis. Ann KE Med. Coll. 2004, 10, 84–85. [Google Scholar]
- Upadya, V.H.; Bhat, H.K.; Rao, B.H.S.; Reddy, S.G. Classification and surgical management of temporomandibular joint ankylosis: A review. J. Korean Assoc. Oral Maxillofac. Surg. 2021, 47, 239–248. [Google Scholar] [CrossRef]
- Sharma, H.; Chowdhury, S.; Navaneetham, A.; Upadhyay, S.; Alam, S. Costochondral Graft as Interpositional material for TMJ Ankylosis in Children: A Clinical Study. J. Maxillofac. Oral Surg. 2015, 14, 565–572. [Google Scholar] [CrossRef] [Green Version]
- Al-Rawee, R.Y.; Al-Khayat, A.M.S.; Saeed, S.S. True bony TMJ ankylosis in children: Case report. Int. J. Surg. Case Rep. 2019, 61, 67–72. [Google Scholar] [CrossRef]
- Li, J.M.; An, J.G.; Wang, X.; Yan, Y.B.; Xiao, E.; He, Y.; Zhang, Y. Imaging and histologic features of traumatic temporomandibular joint ankylosis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 118, 330–337. [Google Scholar] [CrossRef]
- Duan, D.; Li, J.; Xiao, E.; He, L.; Yan, Y.; Chen, Y.; Zhang, Y. Histopathological features of hypertrophic bone mass of temporomandibular joint ankylosis (TMJA): An explanation of pathogenesis of TMJA. J. Craniomaxillofac. Surg. 2015, 43, 926–933. [Google Scholar] [CrossRef]
- Pilmane, M.; Skagers, A. Growth factors, genes, bone proteins and apoptosis in the temporomandibular joint (TMJ) of children with ankylosis and during disease recurrence. Stomatologija 2011, 13, 96–101. [Google Scholar]
- Córdova, L.A.; Reyes, M.; Soto, R.; Hernández, M.; Cortés, J.E. Dysregulated healing response participates in the pathophysiology of temporomandibular joint ankylosis. J. Craniomaxillofac. Surg. 2021, 49, 592–597. [Google Scholar] [CrossRef]
- Ohno, S.; Murakami, K.; Tanimoto, K.; Sugiyama, H.; Makihira, S.; Shibata, T.; Yoneno, K.; Kato, Y.; Tanne, K. Immunohistochemical study of matrilin-1 in arthritic articular cartilage of the mandibular condyle. J. Oral Pathol. Med. 2003, 32, 237–242. [Google Scholar] [CrossRef]
- Hsu, S.M.; Raine, L.; Fanger, H. The use of antiavidin antibody and avidin-biotin-peroxidase complex in immunoperoxidase technics. Am. J. Clin. Pathol. 1981, 75, 816–821. [Google Scholar] [CrossRef]
- Pilmane, M.; Jain, N.; Vitenberga-Verza, Z. Expression Analysis of FGF/FGFR and FOX Family Proteins in Mucosal Tissue Obtained from Orofacial Cleft-Affected Children. Biology 2021, 10, 423. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Liang, H.; Xu, J.; Xue, M.; Jackson, C. Matrix metalloproteinases in bone development and pathology: Current knowledge and potential clinical utility. Met. Med. 2016, 3, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Ortega, N.; Behonick, D.J.; Werb, Z. Matrix remodeling during endochondral ossification. Trends Cell Biol. 2004, 14, 86–93. [Google Scholar] [CrossRef] [Green Version]
- Ortega, N.; Behonick, D.J.; Colnot, C.; Cooper, D.N.; Werb, Z. Galectin-3 is a downstream regulator of matrix metalloproteinase-9 function during endochondral bone formation. Mol. Biol. Cell 2005, 16, 3028–3039. [Google Scholar] [CrossRef]
- Vu, T.H.; Shipley, J.M.; Bergers, G.; Berger, J.E.; Helms, J.A.; Hanahan, D.; Shapiro, S.D.; Senior, R.M.; Werb, Z. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell 1998, 93, 411–422. [Google Scholar] [CrossRef] [Green Version]
- Nyman, J.S.; Lynch, C.C.; Perrien, D.S.; Thiolloy, S.; O’Quinn, E.C.; Patil, C.A.; Bi, X.; Pharr, G.M.; Mahadevan-Jansen, A.; Mundy, G.R. Differential effects between the loss of MMP-2 and MMP-9 on structural and tissue-level properties of bone. J. Bone Min. Res. 2011, 26, 1252–1260. [Google Scholar] [CrossRef]
- Johansson, N.; Saarialho-Kere, U.; Airola, K.; Herva, R.; Nissinen, L.; Westermarck, J.; Vuorio, E.; Heino, J.; Kähäri, V.M. Collagenase-3 (MMP-13) is expressed by hypertrophic chondrocytes, periosteal cells, and osteoblasts during human fetal bone development. Dev. Dyn. 1997, 208, 387–397. [Google Scholar] [CrossRef]
- Mitchell, P.G.; Magna, H.A.; Reeves, L.M.; Lopresti-Morrow, L.L.; Yocum, S.A.; Rosner, P.J.; Geoghegan, K.F.; Hambor, J.E. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J. Clin. Investig. 1996, 97, 761–768. [Google Scholar] [CrossRef] [Green Version]
- García, S.; Forteza, J.; López-Otin, C.; Gómez-Reino, J.J.; González, A.; Conde, C. Matrix metalloproteinase-8 deficiency increases joint inflammation and bone erosion in the K/BxN serum-transfer arthritis model. Arthritis Res. Ther. 2010, 12, R224. [Google Scholar] [CrossRef] [Green Version]
- Ratajczak-Wielgomas, K.; Gosk, J.; Rabczyński, J.; Augoff, K.; Podhorska-Okołów, M.; Gamian, A.; Rutowski, R. Expression of MMP-2, TIMP-2, TGF-β1, and decorin in Dupuytren’s contracture. Connect. Tissue Res. 2012, 53, 469–477. [Google Scholar] [CrossRef]
- Corbel, M.; Theret, N.; Caulet-Maugendre, S.; Germain, N.; Lagente, V.; Clement, B.; Boichot, E. Repeated endotoxin exposure induces interstitial fibrosis associated with enhanced gelatinase (MMP-2 and MMP-9) activity. Inflamm. Res. 2001, 50, 129–135. [Google Scholar] [CrossRef]
- Takawale, A.; Fan, D.; Basu, R.; Shen, M.; Parajuli, N.; Wang, W.; Wang, X.; Oudit, G.Y.; Kassiri, Z. Myocardial recovery from ischemia-reperfusion is compromised in the absence of tissue inhibitor of metalloproteinase 4. Circ. Heart Fail. 2014, 7, 652–662. [Google Scholar] [CrossRef] [Green Version]
- Chia, S.L.; Sawaji, Y.; Burleigh, A.; McLean, C.; Inglis, J.; Saklatvala, J.; Vincent, T. Fibroblast growth factor 2 is an intrinsic chondroprotective agent that suppresses ADAMTS-5 and delays cartilage degradation in murine osteoarthritis. Arthritis Rheum. 2009, 60, 2019–2027. [Google Scholar] [CrossRef]
- Detamore, M.S.; Athanasiou, K.A. Effects of growth factors on temporomandibular joint disc cells. Arch. Oral Biol. 2004, 49, 577–583. [Google Scholar] [CrossRef]
- Purcell, P.; Joo, B.W.; Hu, J.K.; Tran, P.V.; Calicchio, M.L.; O’Connell, D.J.; Maas, R.L.; Tabin, C.J. Temporomandibular joint formation requires two distinct hedgehog-dependent steps. Proc. Natl. Acad. Sci. USA 2009, 106, 18297–18302. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Huang, J.; Zhou, S.; Luo, F.; Tan, Q.; Sun, X.; Ni, Z.; Chen, H.; Du, X.; Xie, Y.; et al. Loss of Fgfr1 in chondrocytes inhibits osteoarthritis by promoting autophagic activity in temporomandibular joint. J. Biol. Chem. 2018, 293, 8761–8774. [Google Scholar] [CrossRef]
- Kristensen, K.D.; Alstergren, P.; Stoustrup, P.; Küseler, A.; Herlin, T.; Pedersen, T.K. Cytokines in healthy temporomandibular joint synovial fluid. J. Oral Rehabil. 2014, 41, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Kim, S.G.; Kim, B.S.; Lee, J.Y.; Yun, P.Y.; Bae, J.H.; Oh, J.S.; Ahn, J.M.; Kim, J.S.; Lee, S.Y. Analysis of the cytokine profiles of the synovial fluid in a normal temporomandibular joint: Preliminary study. J. Craniomaxillofac. Surg. 2012, 40, e337–e341. [Google Scholar] [CrossRef] [PubMed]
- Joosten, L.A.; Helsen, M.M.; Saxne, T.; van De Loo, F.A.; Heinegard, D.; van Den Berg, W.B. IL-1 alpha beta blockade prevents cartilage and bone destruction in murine type II collagen-induced arthritis, whereas TNF-alpha blockade only ameliorates joint inflammation. J. Immunol. 1999, 163, 5049–5055. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Takahashi, N.; Jimi, E.; Udagawa, N.; Takami, M.; Kotake, S.; Nakagawa, N.; Kinosaki, M.; Yamaguchi, K.; Shima, N.; et al. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J. Exp. Med. 2000, 191, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Varoga, D.; Pufe, T.; Mentlein, R.; Kohrs, S.; Grohmann, S.; Tillmann, B.; Hassenpflug, J.; Paulsen, F. Expression and regulation of antimicrobial peptides in articular joints. Ann. Anat. 2005, 187, 499–508. [Google Scholar] [CrossRef]
- Leonardi, R.; Crimi, S.; De Ponte, F.; Loreto, C.; Belli, E.; Musumeci, G. β-defensin-3 and β-defensin-4 in synovial fluids from temporomandibular joints with osteoarthrosis. J. Oral Maxillofac. Surg. Med. Pathol. 2015, 27, 263–266. [Google Scholar] [CrossRef]
- Liao, L.; Zhang, S.; Zhou, G.Q.; Ye, L.; Huang, J.; Zhao, L.; Chen, D. Deletion of Runx2 in condylar chondrocytes disrupts TMJ tissue homeostasis. J. Cell Physiol. 2019, 234, 3436–3444. [Google Scholar] [CrossRef]
- Yan, Y.B.; Li, J.M.; Xiao, E.; An, J.G.; Gan, Y.H.; Zhang, Y. A pilot trial on the molecular pathophysiology of traumatic temporomandibular joint bony ankylosis in a sheep model. Part I: Expression of Wnt signaling. J. Craniomaxillofac. Surg. 2014, 42, e15–e22. [Google Scholar] [CrossRef]
- Güven, O. Formation of Condyle-Like Structure after Treatment of Temporomandibular Joint Ankylosis: Literature Review and Long-Term Follow-Up of Two Patients. Case Rep. Med. 2017, 2017, 9060174. [Google Scholar] [CrossRef] [PubMed]


| Primary Antibody | Full Name | Function | Dilution | Manufacturer |
|---|---|---|---|---|
| MMP-2 | Matrix metallopeptidase 2 | Tissue remodeling | 1:400 | Biorbyt Limited (Cambridge, UK) |
| MMP-8 | Matrix metallopeptidase 8 | Tissue remodeling | 1:100 | Biorbyt Limited (Cambridge, UK) |
| MMP-9 | Matrix metallopeptidase 9 | Tissue remodeling | 1:100 | Biorbyt Limited (Cambridge, UK) |
| MMP-13 | Matrix metallopeptidase 13 | Tissue remodeling | 1:100 | Santa Cruz (Dallas, TX, USA) |
| TIMP-2 | TIMP metallopeptidase inhibitor 2 | Tissue remodeling | 1:50 | Santa Cruz (Dallas, TX, USA) |
| TIMP-4 | TIMP metallopeptidase inhibitor 4 | Tissue remodeling | 1:100 | Abcam (Cambridge, UK) |
| bFGF | Basic fibroblast growth factor (FGF2) | Growth factor | 1:200 | Abcam (Cambridge, UK) |
| FGFR-1 | Fibroblast growth factor receptor 1 | Growth factor | 1:100 | Biorbyt Limited (Cambridge, UK) |
| IL-1α | Interleukin-1α | Cytokine | 1:100 | Biorbyt Limited (Cambridge, UK) |
| TNF-α | Tumor necrosis factor alpha | Cytokine | 1:200 | Abcam (Cambridge, UK) |
| ΒD-2 | Beta defensin-2 | Antimicrobial peptide | 1:100 | Santa Cruz (Dallas, TX, USA) |
| RUNX-2 | RUNX family transcription factor 2 | Transcription factor | 1:250 | Abcam (Cambridge, UK) |
| WNT-1 | Wnt family member 1 | Transcription factor | 1:100 | Abcam (Cambridge, UK) |
| WNT-3a | Wnt family member 3a | Transcription factor | 1:800 | Abcam (Cambridge, UK) |
| Tissue Material | MMP-2 | MMP-8 | MMP-9 | MMP-13 | TIMP-2 | TIMP-4 | bFGF | FGFR-1 | IL-1α | TNF-α | BD-2 | RUNX-2 | WNT-1 | WNT-3a |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary surgery tissue | ||||||||||||||
| Bone | +++ | +++ | ++ | + | +++ | +++ | ++ | ++ | ++ | +++ | ++ | ++ | + | + |
| Fibrous cartilage | +++ | +++ | ++ | + | +++ | ++ | + | ++ | ++ | ++ | ++ | ++ | 0 | + |
| Repeated surgery tissue | ||||||||||||||
| Bone | + | +++ | + | 0 | +++ | +++ | ++ | ++ | ++ | + | ++ | + | + | + |
| Fibrous cartilage | + | +++ | + | 0 | +++ | ++ | ++ | ++ | ++ | + | ++ | + | 0 | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jain, N.; Pilmane, M.; Skagers, A.; Jain, S.; Fedirko, P. Temporomandibular Joint Ankylosis in a Girl Child: Immunochemical Evaluation of Tissue Material Obtained from Repeated Arthroplasty Surgeries. Dent. J. 2023, 11, 16. https://doi.org/10.3390/dj11010016
Jain N, Pilmane M, Skagers A, Jain S, Fedirko P. Temporomandibular Joint Ankylosis in a Girl Child: Immunochemical Evaluation of Tissue Material Obtained from Repeated Arthroplasty Surgeries. Dentistry Journal. 2023; 11(1):16. https://doi.org/10.3390/dj11010016
Chicago/Turabian StyleJain, Nityanand, Mara Pilmane, Andrejs Skagers, Shivani Jain, and Pavlo Fedirko. 2023. "Temporomandibular Joint Ankylosis in a Girl Child: Immunochemical Evaluation of Tissue Material Obtained from Repeated Arthroplasty Surgeries" Dentistry Journal 11, no. 1: 16. https://doi.org/10.3390/dj11010016






