Osteonecrosis of the Jaw
Abstract
:1. Introduction
2. Types of Osteonecrosis
2.1. Medication Related Osteonecrosis of the Jaw
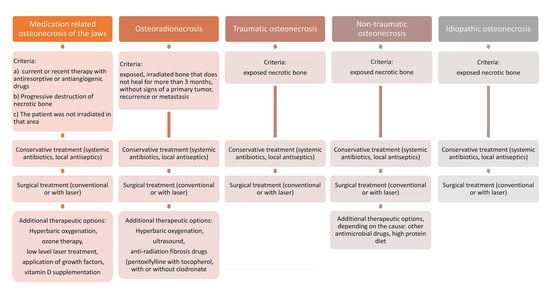
- current or previous therapy with antiresorptive or antiangiogenic drugs
- exposed or necrotic bone in the maxillofacial region that persists for more than eight weeks
- the patient was not irradiated in the jaw area.
2.1.1. Pathophysiology
2.1.2. Clinical Status
2.1.3. Classification
2.1.4. Prevention
2.1.5. Medication That Can Cause MRONJ
2.1.6. Risk Assessment
2.1.7. Therapy
2.2. Osteoradionecrosis
2.2.1. Pathophysiology
2.2.2. Clinical Status
2.2.3. Classification
2.2.4. Prevention
2.2.5. Risk Assessment
2.2.6. Therapy
2.3. Other Causes of Osteonecrosis
2.3.1. Trauma
2.3.2. Infection
2.3.3. Acquired and Congenital Diseases
2.3.4. Neoplasms
2.3.5. Narcotics
2.3.6. Spontaneous Osteonecrosis
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pavelka, K. Osteonecrosis. Baillieres Best Pract. Res. Clin. Rheumatol. 2000, 14, 399–414. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.R.; Cundy, T. Osteonecrosis of the jaw. Skeletal Radiol. 2009, 38, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Rice, N.; Polyzois, I.; Ekanayake, K.; Omer, O.; Stassen, L. The management of osteoradionecrosis of the jaws—A review. Surg. 2015, 13, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Henien, M.; Patel, V.; Sproat, C.; McGurk, M. Spontaneous osteonecrosis of the maxilla. Dent. Updat. 2016, 43, 563–566. [Google Scholar] [CrossRef]
- Lončar Brzak, B.; Vučičević Boras, V.; Kotarac Knežević, A.; Sušić, M.; Seiwerth, S.; Gabrić, D. Idiopathic Exposed Bone Lesions of the Jaw. Dent. J. 2019, 7, 55. [Google Scholar] [CrossRef] [Green Version]
- Ruggiero, S.L.; Dodson, T.B.; Fantasia, J.; Goodday, R.; Aghaloo, T.; Mehrotra, B.; O’Ryan, F.; American Association of Oral and Max-illofacial Surgeons. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw—2014 update. J. Oral. Maxillofac. Surg. 2014, 72, 1938–1956. [Google Scholar] [CrossRef]
- Schiodt, M.; Otto, S.; Fedele, S.; Bedogni, A.; Nicolatou-Galitis, O.; Guggenberger, R.; Herlofson, B.B.; Ristow, O.; Kofod, T. Workshop of European task force on medication-related osteonecrosis of the jaw—Current challenges. Oral Dis. 2019, 25, 1815–1821. [Google Scholar] [CrossRef] [Green Version]
- Campisi, G.; Mauceri, R.; Bertoldo, F.; Bettini, G.; Biasotto, M.; Colella, G.; Consolo, U.; Di Fede, O.; Favia, G.; Fusco, V.; et al. Medication-Related Osteonecrosis of Jaws (MRONJ) Prevention and Diagnosis: Italian Consensus Update 2020. Int. J. Environ. Res. Public Health 2020, 17, 5998. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Insight into bisphosphonate-associated osteomyelitis of the jaw: Pathophysiology, mechanisms and clinical management. Expert Opin. Drug Saf. 2008, 7, 491–512. [Google Scholar] [CrossRef]
- Marx, R.E.; Sawatari, Y.; Fortin, M.; Broumand, V. Bisphosphonate-Induced Exposed Bone (Osteonecrosis/Osteopetrosis) of the Jaws: Risk Factors, Recognition, Prevention, and Treatment. J. Oral Maxillofac. Surg. 2005, 63, 1567–1575. [Google Scholar] [CrossRef]
- Kün-Darbois, J.D.; Fauvel, F. Medication-related osteonecrosis and osteoradionecrosis of the jaws: Update and current man-agement. Morphologie 2021, 105, 170–187. [Google Scholar] [CrossRef]
- Landesberg, R.; Woo, V.; Cremers, S.; Cozin, M.; Marolt, D.; Vunjak-Novakovic, G.; Kousteni, S.; Raghavan, S. Potential pathophysiological mechanisms in osteonecrosis of the jaw. Ann. N. Y. Acad. Sci. 2011, 1218, 62–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basso, F.G.; Pansani, T.N.; Soares, D.; Cardoso, L.M.; Hebling, J.; Costa, C.A.D.S. Influence of bisphosphonates on the adherence and metabolism of epithelial cells and gingival fibroblasts to titanium surfaces. Clin. Oral Investig. 2017, 22, 893–900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruggiero, S.L.; Dodson, T.B.; Aghaloo, T.; Carlson, E.R.; Ward, B.B.; Kademani, D. American Association of Oral and Maxillofacial Surgeons’ Position Paper on Medication-Related Osteonecrosis of the Jaws—2022 Update. J. Oral Maxillofac. Surg. 2022, 80, 920–943. [Google Scholar] [CrossRef] [PubMed]
- Valente, N.A.; Chatelain, S.; Alfonsi, F.; Mortellaro, C.; Barone, A. Medication-Related Osteonecrosis of the Jaw: The Use of Leu-kocyte-Platelet-Rich Fibrin as an Adjunct in the Treatment. J. Craniofacial Surg. 2019, 30, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Otto, S.; Schreyer, C.; Hafner, S.; Mast, G.; Ehrenfeld, M.; Sturzenbaum, S.; Pautke, C. Bisphosphonate-related osteonecrosis of the jaws–characteristics, risk factors, clinical features, localization and impact on oncological treatment. J. Craniomaxillofac. Surg. 2012, 40, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Assaf, A.T.; Zrnc, T.A.; Riecke, B.; Winker, J.; Zustin, J.; Friedrich, R.E.; Heiland, M.; Smeets, R.; Gröbe, A. Intraoperative efficiency of fluorescence imaging by Visually Surg. J. Craniomaxillofac. Surg. 2013, 42, 3157–3164. [Google Scholar]
- Kim, J.-E.; Yoo, S.; Choi, S.-C. Several issues regarding the diagnostic imaging of medication-related osteonecrosis of the jaw. Imaging Sci. Dent. 2020, 50, 273–279. [Google Scholar] [CrossRef]
- Elad, S.; Gomori, M.J.; Ben-Ami, N.; Friedlander-Barenboim, S.; Regev, E.; Lazarovici, T.S.; Yarom, N. Bisphosphonate-related osteonecrosis of the jaw: Clinical correlations with computerized tomography presentation. Clin. Oral Investig. 2010, 14, 43–50. [Google Scholar] [CrossRef]
- Migliorati, C.A.; Brennan, M.T.; Peterson, D.E. Medication-Related Osteonecrosis of the Jaws. J. Natl. Cancer Inst. Monogr. 2019, 2019, lgz009. [Google Scholar] [CrossRef]
- Hutchinson, M.; O’Ryan, F.; Chavez, V.; Lathon, P.V.; Sanchez, G.; Hatcher, D.C.; Indresano, A.T.; Lo, J.C. Radiographic Findings in Bisphosphonate-Treated Patients With Stage 0 Disease in the Absence of Bone Exposure. J. Oral Maxillofac. Surg. 2010, 68, 2232–2240. [Google Scholar] [CrossRef] [PubMed]
- Aghaloo, T.L.; Dry, S.M.; Mallya, S.; Tetradis, S. Stage 0 Osteonecrosis of the Jaw in a Patient on Denosumab. J. Oral Maxillofac. Surg. 2014, 72, 702–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farias, D.S.; Filho, E.V.Z.; de Oliveira, T.F.L.; Tinôco-Araújo, J.E.; Sampieri, M.B.D.S.; Antunes, H.; Santos, P.S.D.S. Clinical and Image Findings in Bisphosphonate-Related Osteonecrosis of the Jaws. J. Craniofacial Surg. 2012, 24, 1248–1251. [Google Scholar] [CrossRef]
- Yamashita, J.; McCauley, L.K. Antiresorptives and osteonecrosis of the jaw. J. Evid. Based Dent. Pract. 2012, 12, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Diel, I.J.; Bergner, R.; Grotz, K.A. Adverse effects of bisphopshonates: Current issues. J. Support. Oncol. 2007, 5, 475–482. [Google Scholar] [PubMed]
- Nicolatou-Galitis, O.; Schiødt, M.; Mendes, R.A.; Ripamonti, C.; Hope, S.; Drudge-Coates, L.; Niepel, D.; Van den Wyngaert, T. Medication-related osteonecrosis of the jaw: Definition and best practice for prevention, diagnosis, and treatment. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 127, 117–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troeltzsch, M.; Woodlock, T.; Kriegelstein, S.; Steiner, T.; Messlinger, K.; Troeltzsch, M. Physiology and pharmacology of nonbisphosphonate drugs implicated in osteonecrosis of the jaw. J. Canadian Dent. Assoc. 2012, 78, c85. [Google Scholar]
- Ortega, J.; Vigil, C.E.; Chodkiewicz, C. Current Progress in Targeted Therapy for Colorectal Cancer. Cancer Control. 2010, 17, 7–15. [Google Scholar] [CrossRef]
- Estilo, C.L.; Fornier, M.; Farooki, A.; Carlson, D.; Bohle, G., 3rd; Huryn, J.M. Osteonecrosis of the jaw related to bevacizumab. J. Clin. Oncol. 2008, 26, 4037–4038. [Google Scholar] [CrossRef]
- Fedele, S.; Bedogni, G.; Scoletta, M.; Favia, G.; Colella, G.; Agrillo, A.; Bettini, G.; Di Fede, O.; Oteri, G.; Fusco, V.; et al. Up to a quarter of patients with osteonecrosis of the jaw asso-ciated with antiresorptive agents remain undiagnosed. Br. J. Oral Maxillofac. Surg. 2015, 53, 13–17. [Google Scholar] [CrossRef]
- Otto, S.; Schuler, K.; Ihrler, S.; Ehrenfeld, M.; Mast, G. Osteonecrosis or Metastases of the Jaw or Both? Case Report and Review of the Literature. J. Oral Maxillofac. Surg. 2010, 68, 1185–1188. [Google Scholar] [CrossRef] [PubMed]
- Lesclous, P.; Grabar, S.; Najm, S.A.; Carrel, J.-P.; Lombardi, T.; Saffar, J.-L.; Samson, J. Relevance of surgical management of patients affected by bisphosphonate-associated osteonecrosis of the jaws. A prospective clinical and radiological study. Clin. Oral Investig. 2014, 18, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Atalay, B.; Yalcin, S.; Emes, Y.; Aktas, I.; Aybar, B.; Issever, H.; Mandel, N.M.; Cetin, O.; Oncu, B. Bisphosphonate-related osteonecrosis: Laser-assisted surgical treatment or conventional surgery? Lasers Med. Sci. 2011, 26, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Angiero, F.; Sannino, C.; Borloni, R.; Crippa, R.; Benedicenti, S.; Romanos, G.E. Osteonecrosis of the jaws caused by bisphosphonates: Evaluation of a new therapeutic approach using the Er:YAG laser. Lasers Med. Sci. 2009, 24, 849–856. [Google Scholar] [CrossRef]
- Lemound, J.; Eckardt, A.; Kokemüller, H.; Von See, C.; Voss, P.J.; Tavassol, F.; Rücker, M.; Rana, M.; Gellrich, N.-C. Bisphosphonate-associated osteonecrosis of the mandible: Reliable soft tissue reconstruction using a local myofascial flap. Clin. Oral Investig. 2012, 16, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Gallego, L.; Junquera, L.; Pelaz, A.; Hernando, J.; Megias, J. The use of pedicled buccal fat pad combined with sequestrectomy in bisphosphonate-related osteonecrosis of the maxilla. Med. Oral Patol. Oral Cir. Bucal. 2012, 17, e236–e241. [Google Scholar] [CrossRef]
- Karasneh, J.A.; Al-Eryani, K.; Clark, G.T.; Sedghizadeh, P.P. Modified protocol including topical minocycline in orabase to manage medication-related osteonecrosis of the jaw cases. J. Oral Pathol. Med. 2016, 45, 718–720. [Google Scholar] [CrossRef]
- Curi, M.M.; Cossolin, G.S.I.; Koga, D.H.; Zardetto, C.; Christianini, S.; Feher, O.; Cardoso, C.L.; dos Santos, M.O. Bisphosphonate-Related Osteonecrosis of the Jaws—An Initial Case Series Report of Treatment Combining Partial Bone Resection and Autologous Platelet-Rich Plasma. J. Oral Maxillofac. Surg. 2011, 69, 2465–2472. [Google Scholar] [CrossRef]
- Cheung, A.; Seeman, E. Teriparatide Therapy for Alendronate-Associated Osteonecrosis of the Jaw. N. Engl. J. Med. 2010, 363, 2473–2474. [Google Scholar] [CrossRef]
- Freiberger, J.J.; Padilla-Burgos, R.; Chhoeu, A.H.; Kraft, K.H.; Boneta, O.; Moon, R.E.; Piantadosi, C. Hyperbaric Oxygen Treatment and Bisphosphonate-Induced Osteonecrosis of the Jaw: A Case Series. J. Oral Maxillofac. Surg. 2007, 65, 1321–1327. [Google Scholar] [CrossRef]
- Merigo, E.; Cella, L.; Oppici, A.; Arbasi, M.C.; Clini, F.; Fontana, M.; Fornaini, C. Combined Approach to Treat Medication-Related Osteonecrosis of the Jaws. J. Lasers Med. Sci. 2018, 9, 92–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vescovi, P.; Merigo, E.; Manfredi, M.; Meleti, M.; Fornaini, C.; Bonanini, M.; Rocca, J.P.; Nammour, S. Nd:YAG Laser Biostimulation in the Treatment of Bisphosphonate-Associated Osteonecrosis of the Jaw: Clinical Experience in 28 Cases. Photomed. Laser Surg. 2008, 26, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Favia, G.; Tempesta, A.; Limongelli, L.; Crincoli, V.; Maiorano, E. Medication-related osteonecrosis of the jaw: Surgical or non-surgical treatment? Oral Dis. 2018, 24, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Pouso, A.I.; Pérez-Sayáns, M.; García, A.; Carballo, J. Vitamin D supplementation: Hypothetical effect on medication-related osteonecrosis of the jaw. Med. Hypotheses 2018, 116, 79–83. [Google Scholar] [CrossRef]
- Heim, N.; Warwas, F.B.; Wilms, C.T.; Reich, R.H.; Martini, M. Vitamin D (25-OHD) deficiency may increase the prevalence of medication-related osteonecrosis of the jaw. J. Cranio-Maxillofacial Surg. 2017, 45, 2068–2074. [Google Scholar] [CrossRef]
- Demircan, S.; Isler, S.C. Changes in serological bone turnover markers in bisphosphonate induced osteonecrosis of the jaws: A case control study. Niger J. Clin. Pract. 2020, 23, 154–158. [Google Scholar]
- Bedogni, A.; Bettini, G.; Bedogni, G.; Basso, D.; Gatti, D.; Valisena, S.; Brunello, A.; Sorio, M.; Berno, T.; Giannini, S.; et al. Is vitamin D deficiency a risk factor for osteonecrosis of the jaw in patients with cancer? A matched case–control study. J. Cranio-Maxillofacial Surg. 2019, 47, 1203–1208. [Google Scholar] [CrossRef]
- Nabil, S.; Samman, N. Incidence and prevention of osteoradionecrosis after dental extraction in irradiated patients: A systematic review. Int. J. Oral Maxillofac. Surg. 2011, 40, 229–243. [Google Scholar] [CrossRef]
- Marx, R.E. Osteoradionecrosis: A new concept of its pathophysiology. J. Oral Maxillofac. Surg. 1983, 41, 283–288. [Google Scholar] [CrossRef]
- Lyons, A.; Ghazali, N. Osteoradionecrosis of the jaws: Current understanding of its pathophysiology and treatment. Br. J. Oral Maxillofac. Surg. 2008, 46, 653–660. [Google Scholar] [CrossRef]
- Delanian, S.; Lefaix, J.L. The radiation-induced fibroatrophic process:therapeutic perspective via the antioxidant pathway. Radiother. Oncol. 2004, 73, 119–131. [Google Scholar] [CrossRef]
- Frankart, A.J.; Frankart, M.J.; Cervenka, B.; Tang, A.L.; Krishnan, D.G.; Takiar, V. Osteoradionecrosis: Exposing the Evidence Not the Bone. Int. J. Radiat. Oncol. 2021, 109, 1206–1218. [Google Scholar] [CrossRef] [PubMed]
- Chronopoulos, A.; Zarra, T.; Ehrenfeld, M.; Otto, S. Osteoradionecrosis of the jaws: Definition, epidemiology, staging and clinical and radiological findings. A concise review. Int. Dent. J. 2018, 68, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Morton, M.; Simpson, W. The management of osteoradionecrosis of the jaws. Br. J. Oral Maxillofac. Surg. 1986, 24, 332–341. [Google Scholar] [CrossRef]
- Cheriex, K.; Nijhuis, T.; Mureau, M. Osteoradionecrosis of the Jaws: A Review of Conservative and Surgical Treatment Options. J. Reconstr. Microsurg. 2012, 29, 69–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muraki, Y.; Akashi, M.; Ejima, Y.; Hasegawa, T.; Miyawaki, D.; Shinomiya, H.; Nishii, M.; Otsuki, N.; Sasaki, R.; Nibu, K.-I.; et al. Dental intervention against osteoradionecrosis of the jaws in irradiated patients with head and neck malignancy: A single-arm prospective study. Oral Maxillofac. Surg. 2019, 23, 297–305. [Google Scholar] [CrossRef]
- Dumoulin, S.; van Maanen, A.; Magremanne, M. Dental prevention of maxillo-mandibular osteoradionecrosis: A ten-year retrospective study. J. Stomatol. Oral Maxillofac. Surg. 2020, 122, 127–134. [Google Scholar] [CrossRef]
- Tong, A.C.; Leung, A.C.; Cheng, J.C.; Sham, J. Incidence of complicated healing and osteoradionecrosis following tooth extraction in patients receiving radiotherapy for treatment of nasopharyngeal carcinoma. Aust. Dent. J. 1999, 44, 187–194. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhu, X.; Qu, S. Incidence of osteoradionecrosis in patients who have undergone dental extraction prior to radio-therapy: A systematic review and meta-analysis. J. Oral Maxillofac. Surg. Med. Pathol. 2014, 26, 269–275. [Google Scholar] [CrossRef]
- Friedman, R.B. Osteoradionecrosis: Causes and prevention. NCI Monogr. 1990, 9, 145–149. [Google Scholar]
- Schwartz, H.C.; Kagan, A.R. Osteoradionecrosis of the mandible: Scientific basis forclinical staging. Am. J. Clin. Oncol. 2002, 25, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Palma, L.F.; Marcucci, M.; Remondes, C.M.; Chambrone, L. Antibiotic therapy for the prevention of osteoradionecrosis following tooth extraction in head-and-neck cancer patients postradiotherapy: An 11-year retrospective study. Natl. J. Maxillofac. Surg. 2021, 12, 333. [Google Scholar] [CrossRef] [PubMed]
- Little, J.; Falace, D.; Miller, C.; Rhodus, N. Dental Management of the Medically Compromised Patient, 8th ed.; Mosby: St. Louis, MO, USA, 2012. [Google Scholar]
- Haroun, K.; Coblens, O.M. Reconstruction of the mandible for osteoradionecrosis. Curr. Opin. Otolaryngol. Head Neck Surg. 2019, 27, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Maxymiw, W.; Wood, R.; Liu, F.-F. Postradiation dental extractions without hyperbaric oxygen. Oral Surgery Oral Med. Oral Pathol. 1991, 72, 270–274. [Google Scholar] [CrossRef] [PubMed]
- El-Rabbany, M.; Duchnay, M.; Raziee, H.R.; Zych, M.; Tenenbaum, H.; Shah, P.S.; Azarpazhooh, A. Interventions for preventing osteoradionecrosis of the jaws in adults receiving head and neck radiotherapy. Cochrane Database Syst. Rev. 2019, 2019, CD011559. [Google Scholar] [CrossRef] [PubMed]
- Martos-Fernández, M.; Saez-Barba, M.; López-López, J.; Estrugo-Devesa, A.; Balibrea-Del-Castillo, J.M.; Bescós-Atín, C. Pentoxifylline, tocopherol, and clodronate for the treatment of mandibular osteoradionecrosis: A systematic review. Oral Surgery Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Nabil, S.; Samman, N. Risk factors for osteoradionecrosis after head and neck radiation: A systematic review. Oral Surgery Oral Med. Oral Pathol. Oral Radiol. 2012, 113, 54–69. [Google Scholar] [CrossRef]
- Kubota, H.; Miyawaki, D.; Mukumoto, N.; Ishihara, T.; Matsumura, M.; Hasegawa, T.; Akashi, M.; Kiyota, N.; Shinomiya, H.; Teshima, M.; et al. Risk factors for osteoradionecrosis of the jaw in patients with head and neck squamous cell carcinoma. Radiat. Oncol. 2021, 16, 1. [Google Scholar] [CrossRef]
- Wang, T.-H.; Liu, C.-J.; Chao, T.-F.; Chen, T.-J.; Hu, Y.-W. Risk factors for and the role of dental extractions in osteoradionecrosis of the jaws: A national-based cohort study. Head Neck 2017, 39, 1313–1321. [Google Scholar] [CrossRef]
- Jacobson, A.S.; Buchbinder, D.; Hu, K.; Urken, M.L. Paradigma shifts in the management of osteoradionecrosis of the mandible. Oral Oncol. 2010, 46, 795–801. [Google Scholar] [CrossRef]
- Gadiwalla, Y.; Patel, V. Osteonecrosis of the jaw unrelated to medication or radiotherapy. Oral Surgery Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Lanigan, D.T.; Hey, J.H.; West, R.A. Aseptic necrosis following maxillary osteotomies: Report of 36 cases. J. Oral Maxillofac. Surg. 1990, 48, 142–156. [Google Scholar] [CrossRef] [PubMed]
- Alalawi, W.A.; Almajed, E. Unilateral Hard Palate Necrosis After Ascending Palatine Artery Embolization. J. Craniofacial Surg. 2018, 29, e437–e438. [Google Scholar] [CrossRef] [PubMed]
- Boffano, P.; Kommers, S.C.; Karagozoglu, K.H.; Forouzanfar, T. Aetiology of maxillofacial fractures: A review of published studies during the last 30 years. Br. J. Oral Maxillofac. Surg. 2014, 52, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Almazrooa, S.A.; Chen, K.; Nascimben, L.; Woo, S.-B.; Treister, N. Case report: Osteonecrosis of the Mandible After Laryngoscopy and Endotracheal Tube Placement. Obstet. Anesthesia Dig. 2010, 111, 437–441. [Google Scholar] [CrossRef]
- Orebaugh, S.L.; Eutsey, R.; Chung, W. Osteonecrosis of Bilateral Mandibular Tori After Direct Laryngoscopy. Anesthesia Prog. 2021, 68, 26–28. [Google Scholar] [CrossRef]
- Chen, G.; Sung, P.-T. Gingival and localized alveolar bone necrosis related to the use of arsenic trioxide paste—Two case reports. J. Formos. Med. Assoc. 2014, 113, 187–190. [Google Scholar] [CrossRef] [Green Version]
- Bataineh, A.B.D.; Al-Omari, M.A.O.; Owais, A.I. Arsenical necrosis of the jaws. Int. Endod. J. 1997, 30, 283–287. [Google Scholar] [CrossRef]
- Wang, X.-X.; Zhang, J.; Liu, M.; Wei, F.-C. Aseptic necrosis of the maxilla after devitalisation of the teeth with arsenic trioxide. Br. J. Oral Maxillofac. Surg. 2008, 46, 81. [Google Scholar] [CrossRef]
- Pontes, F.; Pontes, H.; Adachi, P.; Rodini, C.; Almeida, D.; Pinto, D. Gingival and bone necrosis caused by accidental sodium hypochlorite injection instead of anaesthetic solution. Int. Endod. J. 2008, 41, 267–270. [Google Scholar] [CrossRef]
- Faras, F.; Abo-Alhassan, F.; Sadeq, A.; Burezq, H. Complication of improper management of sodium hypochlorite accident during root canal treatment. J. Int. Soc. Prev. Community Dent. 2016, 6, 493–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akman, A.C.; Demiralp, B.; Güncü, G.N.; Kiremitçi, A.; Sengün, D. Necrosis of gingiva and alveolar bone caused by acid etching and its treatment with subepithelial connective tissue graft. J. Can. Dent. Assoc. 2005, 71, 477–479. [Google Scholar] [PubMed]
- Baltensperger, M.; Grätz, K.; Bruder, E.; Lebeda, R.; Makek, M.; Eyrich, G. Is primary chronic osteomyelitis a uniform disease? Proposal of a classification based on a retrospective analysis of patients treated in the past 30 years. J. Cranio-Maxillofacial Surg. 2004, 32, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Ashok, N.; Tarakji, B.; Darwish, S.; Rodrigues, J.C.; Altamimi, M.A. A Review on Noma: A Recent Update. Glob. J. Health Sci. 2015, 8, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Khullar, S.; Katebi, N.; Herlofson, B.; Tvedt, D.; Olsen, B. Evidence to support the hypothesis of tuberculosis as a cause of extreme osteonecrosis and osteomyelitis of the mandible in a West African population. Int. J. Oral Maxillofac. Surg. 2016, 45, 1600–1606. [Google Scholar] [CrossRef] [PubMed]
- Murthy, V.; Vaithilingam, Y.; Livingstone, D.; Pillai, A. Prosthetic rehabilitation of palatal perforation in a patient with ‘syphilis: The great imitator’. BMJ Case Rep. 2014, 2014, bcr2014204259. [Google Scholar] [CrossRef] [Green Version]
- Crossman, T.; Herold, J. Actinomycosis of the maxilla—A case report of a rare oral infection presenting in general dental practice. Br. Dent. J. 2009, 206, 201–202. [Google Scholar] [CrossRef] [Green Version]
- Gannepalli, A.; Ayinampudi, B.K.; Baghirath, P.V.; Reddy, G.V. Actinomycotic Osteomyelitis of Maxilla Presenting as Oroantral Fistula: A Rare Case Report. Case Rep. Dent. 2015, 2015, 689240. [Google Scholar] [CrossRef] [Green Version]
- Song, J.M.; Seo, J.S.; Lee, J.Y. Mandibular osteonecrosis following herpes zoster infection in the mandibular branch of the trigeminal nerve: A case report and literature review. J. Korean Assoc Oral Maxillofac. Surg. 2015, 41, 357–360. [Google Scholar] [CrossRef] [Green Version]
- Shah, R.J.; Katyayan, P.A.; Katyayan, M.K.; Chauhan, V. Prosthetic Rehabilitation of Acquired Maxillary Defects Secondary to Mucormycosis: Clinical Cases. J. Contemp. Dent. Pract. 2014, 15, 242–249. [Google Scholar] [CrossRef]
- Suresh, A.; Joshi, A.; Desai, A.K.; Juturu, U.; Kurian, D.J.; Jain, P.; Kulkarni, R.D.; Kumar, N. COVID-19-associated fungal osteomyelitis of jaws and sinuses: An experience-driven management protocol. Med Mycol. 2022, 60, myab082. [Google Scholar] [CrossRef] [PubMed]
- Gabrić, D.; Seiwerth, S.; Baraba, A.; Boras, V.V.; Vučićević, V. Mandibular Osteonecrosis due to the Pulpal-Periodontal Syndrome: A Case Report and Review of the Literature. Acta Stomatol. Croat. 2017, 51, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.; Uppadhyay, M.; Rahangdale, T. Prosthetic rehabilitation of necrotic maxilla sequelae of faulty denture: Report of a rare case. J. Maxillofac. Oral Surg. 2009, 8, 77–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, E.; Heggie, A. Avascular necrosis of the midface secondary to disseminated intravascular coagulation. Int. J. Oral Maxillofac. Surg. 2014, 43, 1441–1444. [Google Scholar] [CrossRef] [PubMed]
- Badescu, M.C.; Rezus, E.; Ciocoiu, M.; Badulescu, O.V.; Butnariu, L.I.; Popescu, D.; Bratoiu, I.; Rezus, C. Osteonecrosis of the Jaws in Patients with Hereditary Thrombophilia/Hypofibrinolysis—From Pathophysiology to Therapeutic Implications. Int. J. Mol. Sci. 2022, 23, 640. [Google Scholar] [CrossRef] [PubMed]
- Oliver-Puigdomènech, C.; González-Navarro, B.; Polis-Yanes, C.; Estrugo-Devesa, A.; Jané-Salas, E.; López-López, J. Incidence rate of metastases in the oral cavity: A review of all metastatic lesions in the oral cavity. Med. Oral Patol. Oral Cir. Bucal. 2021, 26, e619–e625. [Google Scholar] [CrossRef]
- Trimarchi, M.; Rampi, A.; Vinciguerra, A.; Polizzi, E.; Policaro, N.S.; Gastaldi, G. Palatal prosthetic rehabilitation in patients affected by cocaine-induced midline destructive lesions. J. Biol. Regul. Homeost. Agents 2021, 34, 57–66. [Google Scholar]
- Hakobyan, K.; Poghosyan, Y. “Krokodil” drug—Related osteonecrosis of midface: A case series. J. Cranio-Maxillofacial Surg. 2019, 47, 1918–1921. [Google Scholar] [CrossRef] [PubMed]
- Lidhar, T.; Ethunandan, A.; Ethunandan, M. Spontaneous oral ulceration with bone sequestration: Its relevance in current clinical practice. Br. J. Oral Maxillofac. Surg. 2020, 58, e75–e79. [Google Scholar] [CrossRef]
- Thermos, G.; Kalogirou, E.-M.; Tosios, K.I.; Sklavounou, A. Oral ulceration with bone sequestration: Retrospective study of eight cases and literature review. Oral Dis. 2019, 25, 515–522. [Google Scholar] [CrossRef]
- Sonnier, K.E.; Horning, G.M. Spontaneous Bony Exposure: A Report of 4 Cases of Idiopathic Exposure and Sequestration of Alveolar Bone. J. Periodontol. 1997, 68, 758–762. [Google Scholar] [CrossRef] [PubMed]






| Type | Cause |
|---|---|
| Medication related osteonecrosis of the jaw | Antiresorptive (bisphosphonates and denosumab) and antiangiogenic drugs (antineoplastics, e.g., bevacizumab, sunitinib); treatment with corticosteroids presents additional risk |
| Osteoradionecrosis | Radiation treatment in patients with head and neck cancer |
| Traumatic osteonecrosis | Thermal, mechanical, or chemical trauma |
| Non-traumatic osteonecrosis | Infection; malignancy; acquired and congenital diseases; use of narcotics |
| Spontaneous | Idiopathic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lončar Brzak, B.; Horvat Aleksijević, L.; Vindiš, E.; Kordić, I.; Granić, M.; Vidović Juras, D.; Andabak Rogulj, A. Osteonecrosis of the Jaw. Dent. J. 2023, 11, 23. https://doi.org/10.3390/dj11010023
Lončar Brzak B, Horvat Aleksijević L, Vindiš E, Kordić I, Granić M, Vidović Juras D, Andabak Rogulj A. Osteonecrosis of the Jaw. Dentistry Journal. 2023; 11(1):23. https://doi.org/10.3390/dj11010023
Chicago/Turabian StyleLončar Brzak, Božana, Lorena Horvat Aleksijević, Ema Vindiš, Iva Kordić, Marko Granić, Danica Vidović Juras, and Ana Andabak Rogulj. 2023. "Osteonecrosis of the Jaw" Dentistry Journal 11, no. 1: 23. https://doi.org/10.3390/dj11010023