1. Introduction
Many dental materials are used in contact with each other in sandwich techniques. Liners, bases, and permanent restorative materials are placed adjacent to each other and allowed to set under the same conditions [
1]. One of the most controversial areas of restorative dentistry is the subject of liners and bases. Currently, there is no single protocol, with respect to the use of liners and bases, for clinicians to follow [
2]. Furthermore, the new emerging concept of minimally invasive dentistry requires new restorative techniques.
Adaptation of the restorative material to cavity margins and internal cavity surfaces is of great importance for the long-term performance of the restoration [
3]. One of the main factors responsible for defects at the marginal and internal interfaces of restorations is the shrinkage that accompanies polymerization of composite restorative materials [
4]. This phenomenon results in a change in the density of the material during the polymeric network formation process, generating a reduction of 1.7% to 5.7% in volume [
5]. The shrinkage stress may cause failures in the bond, generating gap formation (10–15 μm) [
6]. Such openings or gaps are considered deleterious because they allow the transit of fluids and bacteria between the dentin pulp complex and the oral environment [
7] leading to postoperative sensitivity and secondary caries formation [
8].
Various restorative techniques that reduce the level of stress caused by composite polymerization shrinkage have been suggested. It has been recommended the placement of a cavity liner or base of low-viscosity/low-elastic modulus materials to create a stress-absorbing layer, such as resin modified glass ionomers (RMGIs), filled adhesives, and flowable composites. This layer increases the strain capacity [
9] and reduces the stresses at the adhesive interface [
10]. In an attempt to provide volumetric reduction of composite resins, RMGIs were recommended as cavity base. Nevertheless, recent studies found [
11] that RMGIs have polymerization shrinkage similar to that of composite resins. Thus, the benefit of these techniques to reduce polymerization shrinkage and stress-relieving remains controversial [
12].
The aim of this in vitro study was to evaluate the interfacial microgaps generating between different materials and between materials and dentin after polymerization of the composite resin in Class V restorations, using Scanning Electron Microscope (SEM). The first null hypothesis of the study was that there is no difference in microgap width at the interface between the materials tested and the dentin substrate. The second null hypothesis of the study was that there is no difference in microgap width at the interface between the different materials tested.
2. Materials and Methods
The materials investigated in the present study were a nanohybrid composite resin (Clearfil Majesty), a one-step self-etch adhesive system (Clearfil Tri-S Bond), a resin-modified glass ionomer cement (Vitrebond), and a calcium hydroxide (Dycal), which are listed in
Table 1. Thirty intact third molars freshly extracted for orthodontic reasons were selected, cleaned and stored in a solution of 0.5% chloramines at 4°C until used. To ensure that the teeth were free of cracks, defects or caries they were examined under ×10 magnification by means of optical microscope.
Table 1.
The materials tested in the present study.
Table 1.
The materials tested in the present study.
| Material | Manufacturer | Type |
|---|
| Dycal | Dentsply, Culk, USA | Calcium hydroxide liner |
| Vitrebond | 3Μ ESPE, St. Paul, MN, USA | Resin-modified glass ionomer cement |
| Clearfil Tri-S Bond | Kuraray, Japan | One-step self-etch adhesive system |
| Clearfil Majesty | Kuraray, Japan | Nanohybrid composite resin |
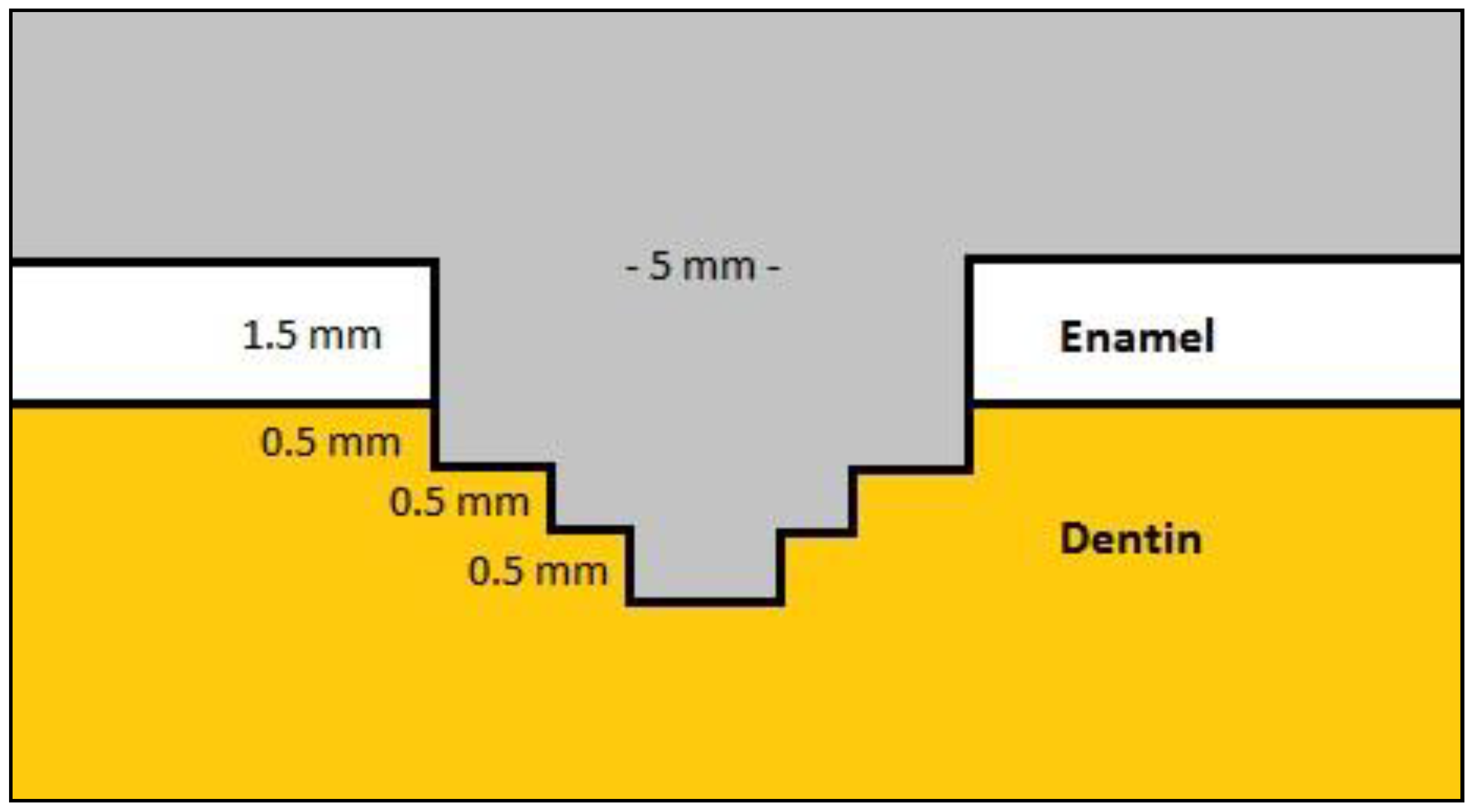
Two standardized class V cavity preparations for each tooth (total 60 cavities) were made with a No245 carbide bur on the buccal and lingual surfaces using a high-speed handpiece with water coolant. The preparations were circular (5 mm in diameter and 3 mm in depth) and the bur’s active tip was previously limited to 2 mm and 0.5 mm with an acrylic resin stop to allow for three different depths to the pulpal floor (
Figure 1), simulating the differences in depth that occur in clinical conditions. The gingival margins of the preparations were located 1 mm below the dentino-enamel junction in dentin. All cavosurface margins were prepared as butt-joint and all internal line angles were slightly round. The burs were replaced with new ones after every fifth preparation. The preparation dimensions were measured with a digital caliper for width and a periodontal probe for depth.
Figure 1.
The dimensions of the Class V cavity preparations.
Figure 1.
The dimensions of the Class V cavity preparations.
The teeth were randomly assigned into 6 groups and restored with a combination of the tested materials as indicated in
Table 2. The materials were handled according to the manufacturer’s instructions as follows:
Group 1: Clearfil Tri-S Bond was applied to the entire cavity walls with a disposable brush tip for 20 s, dried by blowing high-pressure air for 5 s, and light-cured for 20 s with a QTH unit (Elipar 2500, 3M ESPE, MN, St. Paul, USA) at 1400 mW/cm2. Clearfil Majesty composite resin was then inserted into the preparation in two increments and each increment was light-cured for 20 s.
Group 2: Equal amounts of Dycal base and catalyst were mixed for 10 s and applied into the deepest area (1.5 mm in diameter and 0.5 mm in depth) of the axial wall by means of a liner-placement instrument. The material was untouched for 4 min and after setting the adhesive and the composite resin were applied as previously described.
Group 3: Dycal applied as described in Group 2. A layer of Vitrebond was applied on Dycal into the deepest area of the axial wall (3 mm in diameter and 0.5 mm in depth). One scoop of Vitrebond powder was mixed with one drop of liquid on a mixing pad for 15 s and inserted into the preparation with a liner-placement instrument and light-cured for 20 s. The cavity was then restored with the composite resin and the adhesive as described in Group 1.
Group 4: A layer of Vitrebond (1 mm in thickness) was applied to the deepest area of the cavity and then the cavity was restored with the composite resin and the adhesive as described in Group 1.
Group 5: The adhesive Clearfil Tri-S Bond was applied and then the same procedures as for Group 4 were carried out.
Group 6: The same procedures as for Group 5 were carried out, but a second application of the adhesive followed the application of Vitrebond.
Table 2.
The six experimental groups of the study.
Table 2.
The six experimental groups of the study.
| Group | Materials |
|---|
| 1 | Dentin- | Clearfil Tri-S Bond + Clearfil Majesty |
| 2 | Dentin- | Dycal + Clearfil Tri-S Bond + Clearfil Majesty |
| 3 | Dentin- | Dycal + Vitrebond + Clearfil Tri-S Bond + Clearfil Majesty |
| 4 | Dentin- | Vitrebond + Clearfil Tri-S Bond + Clearfil Majesty |
| 5 | Dentin- | Clearfil Tri-S Bond + Vitrebond + Clearfil Majesty |
| 6 | Dentin- | Clearfil Tri-S Bond + Vitrebond + Clearfil Tri-S Bond + Clearfil Majesty |
All the preparations of the cavities and restorations were made by one operator. The restorations were finished after 24 h with finishing diamond burs and sequential abrasive disks (Sof-Lex, 3M ESPE), and the teeth stored in distilled water at 37°C for 7 days. Then the specimens were subjected to 800 cycles between 5°C and 55°C with a dwell time of 30 s. Utilizing a water-cooled diamond saw (Isomet 1000, Buhler Ltd, Lake Bluff, IL, USA) at 300 rpm, each tooth was sectioned mesiodistally in two halves. The sections were sequentially polished with a 600- and a 1,200-grit silicon carbide paper. Subsequently, each half was sectioned along the longitudinal axis through the center of the restorations to obtain a slice of 2 mm in thickness. In order to remove the grinding debris, the specimens ultrasonicated in saline solution for 20 s. After been slightly air-dried, impressions of the cut surfaces were taken using a vinyl polysiloxane impression material and replicas were made with self-curing epoxy resin (Epofix resin, Struers Tech A/S, Denmark), reproducing the interface between dental tissues and tested materials.
The specimens were mounted on aluminum stubs, sputter-coated with carbon to a thickness of approximately 200 Å in a vacuum evaporator (at low vacuum) and examined under Scanning Electron Microscope (JEOL Ltd, JSM-840, Tokyo, Japan) at 19 KV. The interfaces between the liners, the liners and dentin, and between the liners and the restorative composite were examined for microgaps at magnifications of up to ×1000. For each specimen three photomicrogaphs were taken of the axial restoration interfaces in the area of the largest microgap width to obtain the mean gap width of each experimental group. Ten measurements of microgap width were carried out for each experimental group, only on top of the materials and not peripheral, by two independent researchers who were unaware of the group of the tested specimens, and each other's measurements. The average values were collected and the statistical analysis of the data was done by SPSS 19.0 using a non-parametric Kruskal-Wallis test and the statistical significance was set at a: 0.05.
3. Results
The mean gap widths and standard deviations (μm) obtained from each experimental group between dentin and the materials tested are shown in
Table 3. The mean gap widths and standard deviations (μm) obtained from each experimental group between the materials tested are shown in
Table 4.
Table 3.
The mean gap widths and standard deviations (μm) obtained from each experimental group between dentin and the materials tested and the percentage of specimens with gap free interfaces.* Same letter between experimental groups indicate no significant difference (p>0.05).
Table 3.
The mean gap widths and standard deviations (μm) obtained from each experimental group between dentin and the materials tested and the percentage of specimens with gap free interfaces.* Same letter between experimental groups indicate no significant difference (p>0.05).
| Group | Mean gap width (μm) | Percentage of specimens with gap free interfaces |
|---|
| 1 | 4.6 ± 2.1Α | 30% |
| 2 | 21.3 ± 8.2 B | 0% |
| 3 | 23.6 ± 7.6 B | 10% |
| 4 | 18.4 ± 6.8 B | 20% |
| 5 | 4.3 ± 2.2 A | 40% |
| 6 | 5.1 ± 2.8 A | 30% |
Table 4.
The mean gap widths and standard deviations (μm) obtained between the materials tested. * Same letter between experimental groups indicate no significant difference (p>0.05).
The microgaps were consistently observed in particularly all experimental groups. The percentages of gap free interfaces of the specimens observed in each experimental group are presented in
Table 3. The results showed that there was not any statistically significant difference in the mean width of microgaps in the interfaces between Dycal-dentin (Groups 2 and 3) and between Vitrebond-dentin (Group 4)
(p>0.05). However, the mean width of microgaps in the interfaces between dentin-Clearfil Tri-S Bond (Groups 1, 5, and 6) was significantly smaller
(p<0.05) as presented in
Table 3.
Moreover, it was found that the use of Clearfil Tri-S Bond reduced the possibility of microgap formation between the bonded interface and the materials tested. In particularly, microgap width of interfaces between Dycal-Vitrebond (Group 3) and Vitrebond-Clearfil Magesty (Group 5) was significantly larger in comparison with interfaces between Dycal-Clearfil Tri-S Bond (Group 2) and Vitrebond-Clearfil Tri-S Bond (Groups 4 and 6)
(p<0.05), as presented in
Table 4.
Representative SEM photomicrographs showing the morphologic analysis of the interfaces for each experimental group are shown in
Figure 2,
Figure 3,
Figure 4,
Figure 5,
Figure 6 and
Figure 7. The most common finding was the presence of microgaps in most specimens. The qualitative evaluation of internal adaptation revealed that continuous interfaces were achieved in several areas (
Figure 2,
Figure 6, and
Figure 7). Furthermore, in all restorations non-continuous internal adaptations, characterized as “internal fissures,” were observed in dentin. In some cases, multiple cracks and fractures were observed, which may be formed as a consequence of specimen dehydration, SEM preparation, or during sectioning.
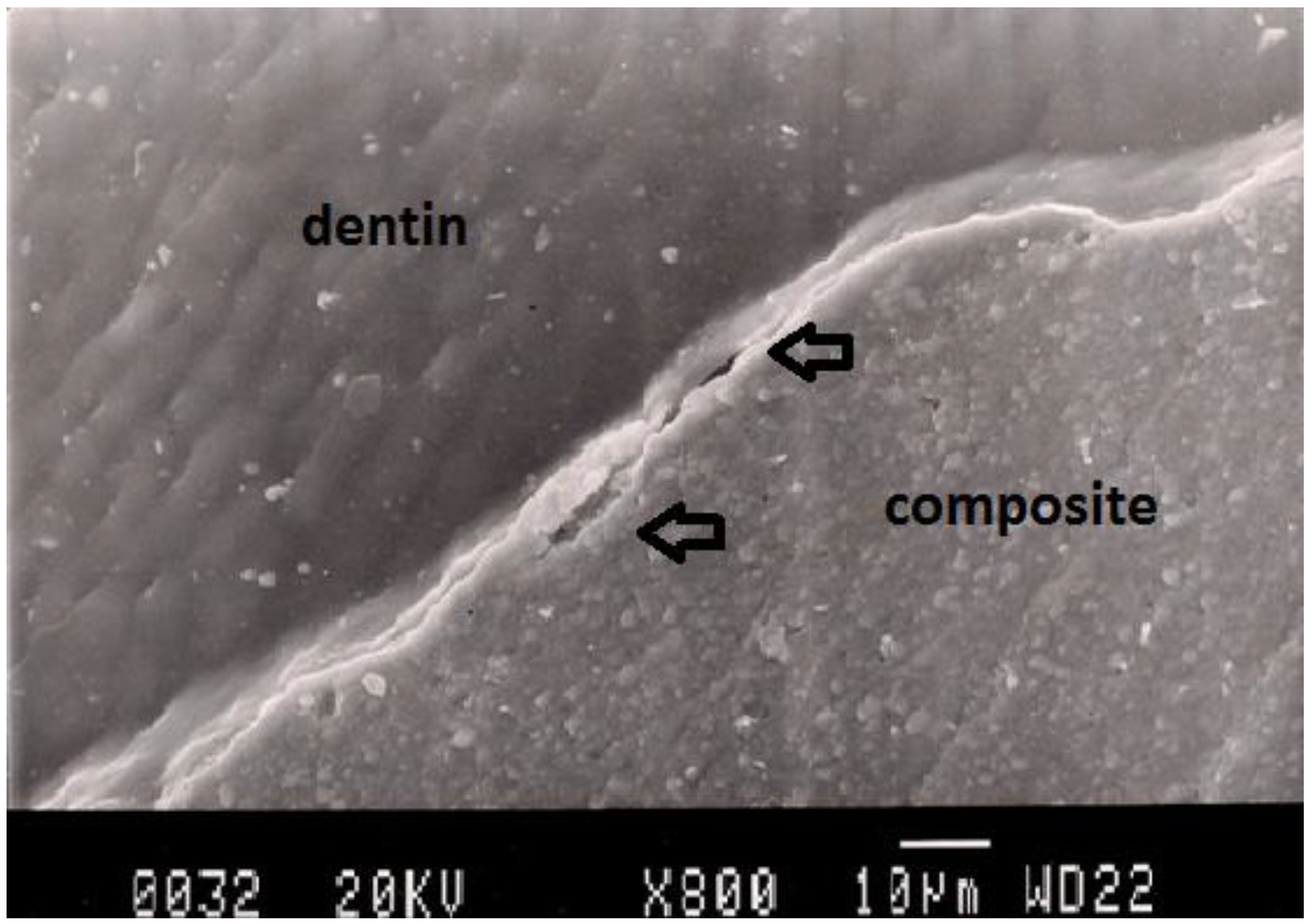
Figure 2.
Representative SEM photomicrograph of a Group 1 specimen. The arrows indicate microgap between composite restoration and dentin.
Figure 2.
Representative SEM photomicrograph of a Group 1 specimen. The arrows indicate microgap between composite restoration and dentin.
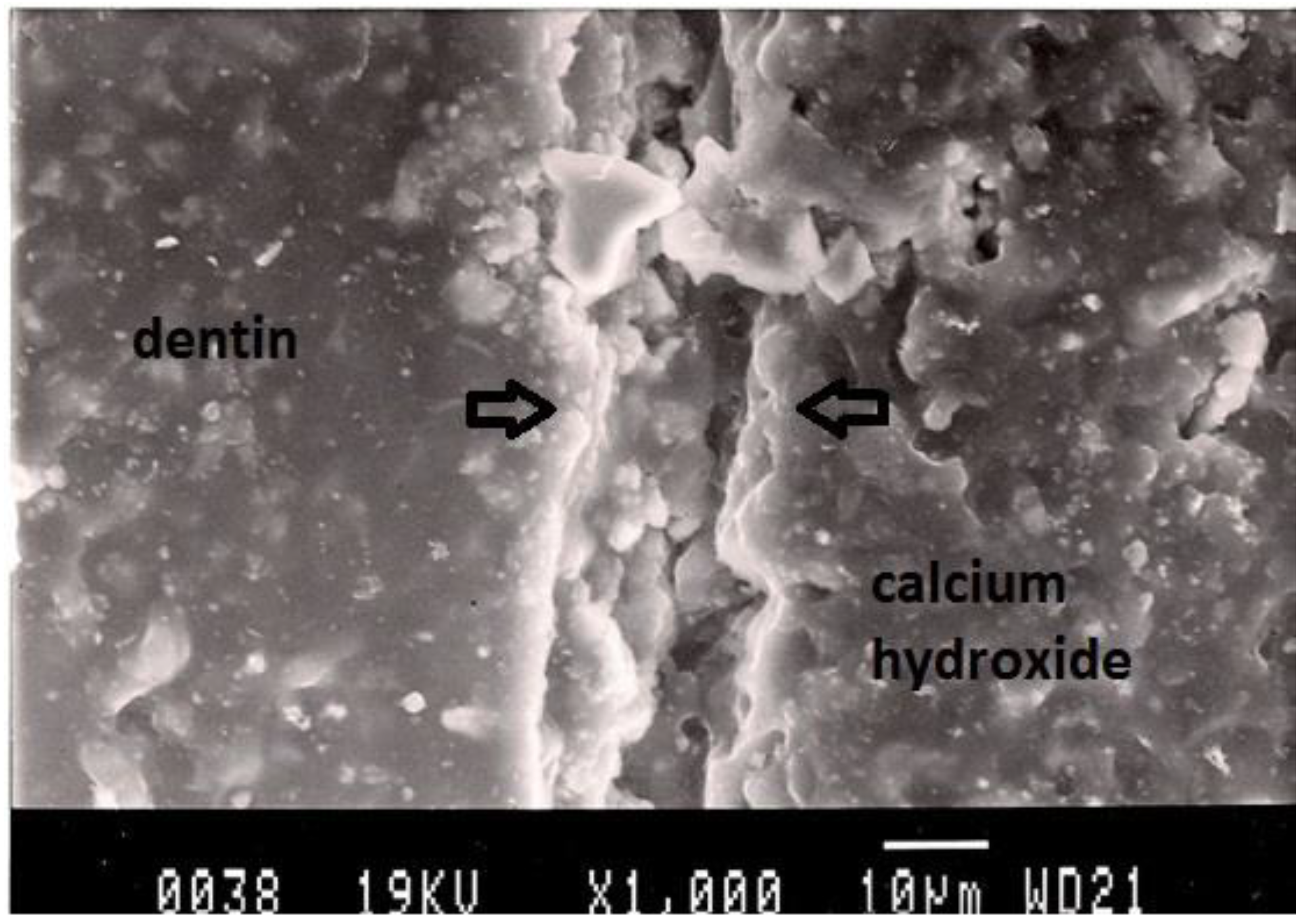
Figure 3.
Representative SEM photomicrograph of a Group 2 specimen. The arrows indicate microgap between calcium hydroxide and dentin.
Figure 3.
Representative SEM photomicrograph of a Group 2 specimen. The arrows indicate microgap between calcium hydroxide and dentin.
Figure 4.
Representative SEM photomicrograph of a Group 3 specimen. The arrows indicate microgap between calcium hydroxide (Dycal) and dentin.
Figure 4.
Representative SEM photomicrograph of a Group 3 specimen. The arrows indicate microgap between calcium hydroxide (Dycal) and dentin.
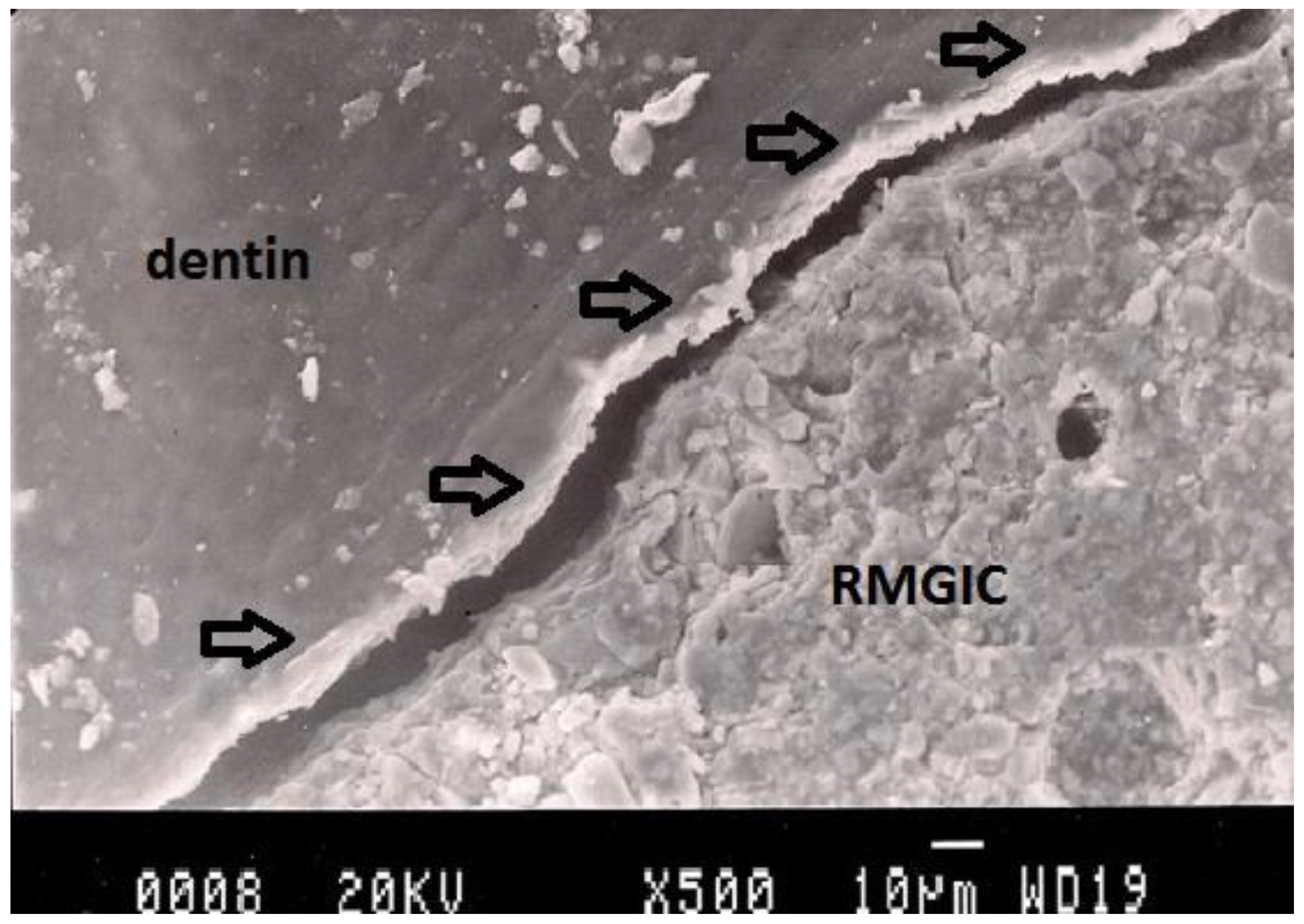
Figure 5.
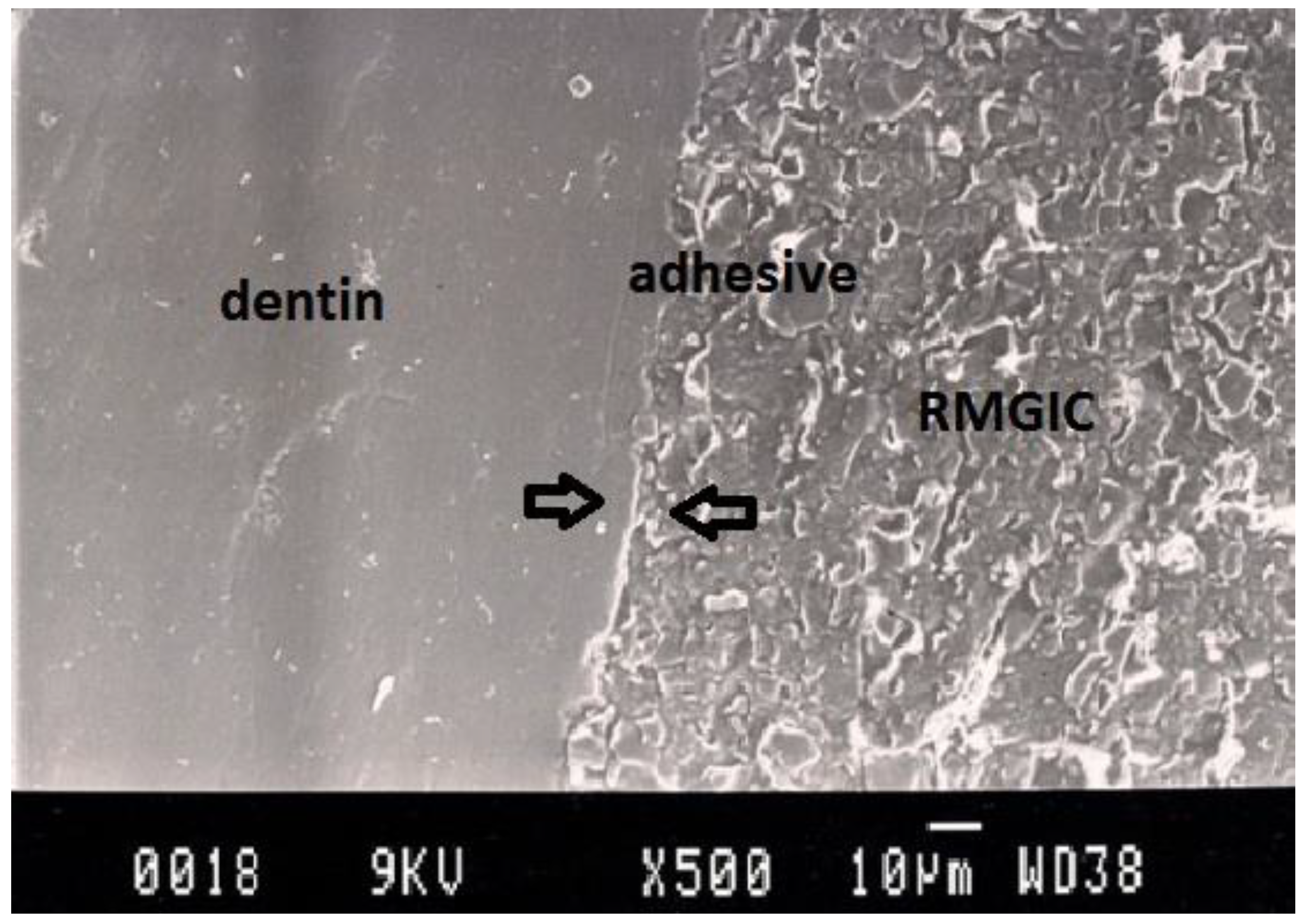
Representative SEM photomicrograph of a Group 4 specimen. The arrows indicate microgap between resin-modified glass ionomer cement (Vitremer) and dentin.
Figure 5.
Representative SEM photomicrograph of a Group 4 specimen. The arrows indicate microgap between resin-modified glass ionomer cement (Vitremer) and dentin.
Figure 6.
Representative SEM photomicrograph of a Group 5 specimen. The arrows indicate fracture within resin-modified glass ionomer cement (Vitremer), which may be due to the strong adhesion between adhesive and dentin.
Figure 6.
Representative SEM photomicrograph of a Group 5 specimen. The arrows indicate fracture within resin-modified glass ionomer cement (Vitremer), which may be due to the strong adhesion between adhesive and dentin.
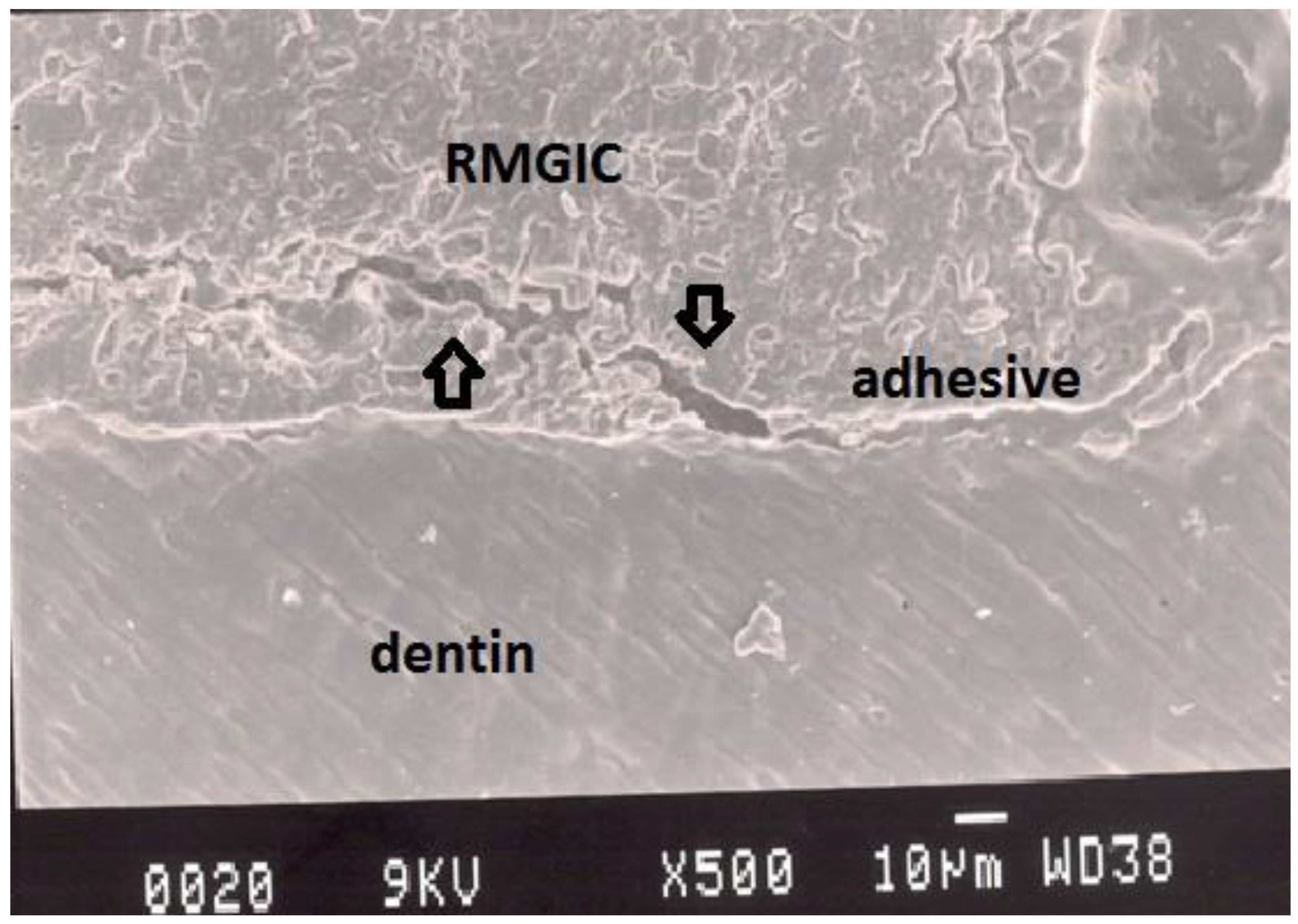
Figure 7.
Representative SEM photomicrograph of a Group 6 specimen. The arrows indicate good adaptation between resin-modified glass ionomer cement, adhesive and dentin.
Figure 7.
Representative SEM photomicrograph of a Group 6 specimen. The arrows indicate good adaptation between resin-modified glass ionomer cement, adhesive and dentin.
4. Discussion
The major concern when restoring Class V cavities with composite materials refers to the high configuration factor (C-factor) of those cavities, leading to increases in stress caused in the tooth-material interface during photopolymerization shrinkage [
8,
13]. Due to this fact, adhesive failure may occur at the restoration margins and as a result the formation of microgaps at internal cavity walls allows penetration of bacteria and fluids between the liners and dentin toward the pulp [
14]. Stavridakis
et al. [
13] found that the composition of the adhesive system used may influence the marginal and internal adaptation of the restorations, while the thickness of the layer of the adhesive does not. In a recent study [
15], the polymerization shrinkage stress and strain were found to be closely related to the internal adaptation of the composite restorations. Moreover, previous studies reported that microleakage from microgap formation at the margin of composite materials regarded as the main factor responsible for pulp irritation [
16]. In the present study, microgap formations were observed in all the experimental groups.
In the current study, the width of microgaps generating after composite photo-polymerization at dentin-Clearfil Tri-S Bond interface was significantly larger than that observed at dentin-Dycal or dentin-Vitrebond interfaces. As a consequence, the first null hypothesis of the study, which was that there is no difference in microgap width between the different materials tested and the dentin substrate, is rejected. This may be related to some inherent properties of the materials, such as hydrophilic or hydrophobic nature of the materials, setting shrinkage, water swelling, mechanism of adhesion to dentin, and sensitivity to contamination [
17,
18,
19]. The results of the study show that direct application of the adhesive to dentin exhibits better performance regarding gap formation in comparison with the use of liners or bases at dentin substrate. This is in agreement with other studies [
20,
21].
In this in vitro study, it was demonstrated that the use of Clearfil Tri-S Bond reduced the possibility of microgap formation between the bonded interface and the materials tested. Consequently, the results obtained from this study demand rejection of the second null hypothesis that there is no difference in microgap width at the interface between the different materials tested.
The use of RMGIs to replace substantial loss of dental structure and to reduce the stress caused by composite polymerization shrinkage has been recommended for many years. This concept is based on the belief that composite resins shrink more than RMGIs. Aggarval
et al. [
22] reported that placement of flowable composite liner or glass ionomer liner improved the marginal integrity in the gingival floor of Class II composite restorations. However, Oliveira
et al. [
20] found that the use of RMGIs as a stress-absorbing layer in composite restorations increases the polymerization shrinkage stresses at the adhesive interface. In the present study, the application of a RMGI to dentin increased the mean width of microgaps at the adhesive surface. Hotta and Aono [
23] reported that after application of cavity preparations with a RMGI or a conventional GI, small construction gaps at the tooth restoration interface were observed. However, the RMGI presented significantly better tensile bond strength to dentin and resin performance than the conventional GI tested. Additionally, this research revealed that there was no clear relationship between the adhesion to dentin and the adaptation to the dentin cavity floor.
Chemical adhesion between GI and dentin is accepted as being a long-term union and it has recently been shown that a mechanical union is possible between composite resin and GI. This has led to the development of the so-called ‘sandwich technique’, where GI is used as a lining under composite resin restorations particularly where the cavo-surface margin is in dentin [
24]. Titley
et al. [
25] demonstrated that the liquid component of Vitrebond reacts chemically with dentin in a manner suggestive of an effervescent chemical reaction. This reaction produces plugs in the dentinal tubules, which are resistant to dislodgement by water under pressure or by gentle washing. The findings in this study suggest that the adhesion of Vitrebond to dentin is primarily chemical in nature and that its mechanical strength is compromised if there are substantial delays in photo-polymerization. Nevertheless, in the present study the adhesion of Vitrebond to dentin seemed to be lower than that of Clearfil Tri-S Bond to dentin.
Although the placement of RMGI over calcium hydroxide liner in deep cavities has been suggested as a treatment that corrects the weak strengths of calcium hydroxide, the results of the study indicate that Dycal does not adhere to Vitrebond or dentin. This evidence has been previously reported [
26,
27]. Papadakou
et al. [
28] investigated the adaptation of two different calcium hydroxide bases under composite restorations and found that they were pulled away from the dentin floor of the cavities as a result of an apparent adhesion to the composite resin during polymerization contraction. In another
in vitro study, Peliz
et al. [
26] evaluated microgap formation in composite restorations and found that there were microgap formations in all of dentin-calcium hydroxide interfaces. These results attributed to the lack of adhesion of calcium hydroxide to the dentin surfaces.
Some authors suggested the use of flowable composite resins as an intermediate layer to improve marginal and internal adaptation of composite restorations. Li
et al. [
29] reported that composite fillings could be improved by the use of flowable materials as an intermediate layer in Class V cavities. In another study [
21], it has been postulated that the use of flowable composite liners demonstrate either similar or more cervical microleakage than do the direct composite restorations. Furthermore, experimental groups lined with RMGIs showed similar or better marginal sealing than did their resin restoration groups.
Different restorative methods may affect internal adaptation and bond strength of materials to dentin surfaces. Bakhsh
et al. [
30] investigated class-I cavity floor adaptation by swept-source optical coherence tomography (OCT) in combination with microtensile bond strength (μTBS) using different filling methods. The results of this study showed that the interaction of adhesive systems and filling techniques was significantly affecting both adaptation and μTBS. Incremental application of composite restoration was the most advantageous placement technique in terms of bond strength and internal adaptation. The lack of placement pressure with flowable composites may affect their adaptation to all-in-one adhesives; therefore, the outcome of cavity lining by flowable composite was variable. Soares
et al. [
31] reported that the number of applications of the adhesive layer had an influence on the results of μTBS; however, they did not minimize the formation of internal failures in restorations.
Souza
et al. [
32] investigated the effect of the curing method and composite volume on marginal and internal adaptation of composite restorations. The results of this study indicate that the higher the volume of composite, the greater the gap formation. In addition, modulated curing methods improved the interfacial quality of composite restorations through the reduction of internal gaps in composite restorations. On the other hand, Perreira
et al. [
33] demonstrated that the internal adaptation of Class V composite restorations were not affected by the curing method (conventional, soft-start, and pulse) or the type of composite resin tested (microfilled or hybrid).
 Clearfil Tri-S Bond + Clearfil Majesty
Clearfil Tri-S Bond + Clearfil Majesty Vitrebond + Clearfil Tri-S Bond + Clearfil Majesty
Vitrebond + Clearfil Tri-S Bond + Clearfil Majesty Clearfil Tri-S Bond + Clearfil Majesty
Clearfil Tri-S Bond + Clearfil Majesty Clearfil Majesty
Clearfil Majesty Clearfil Tri-S Bond + Clearfil Majesty
Clearfil Tri-S Bond + Clearfil Majesty