A Study for Tooth Bleaching via Carbamide Peroxide-Loaded Hollow Calcium Phosphate Spheres
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Carbamide Peroxide-Loaded HCPS and Gels Preparation
2.3. HP Release from Loaded HCPS
2.4. Tooth Staining and Bleaching
2.5. Rhodamine Degradation
2.6. Color Evaluation
2.7. Remineralization
3. Results
3.1. HCPS
3.2. Hydrogen Peroxide Release
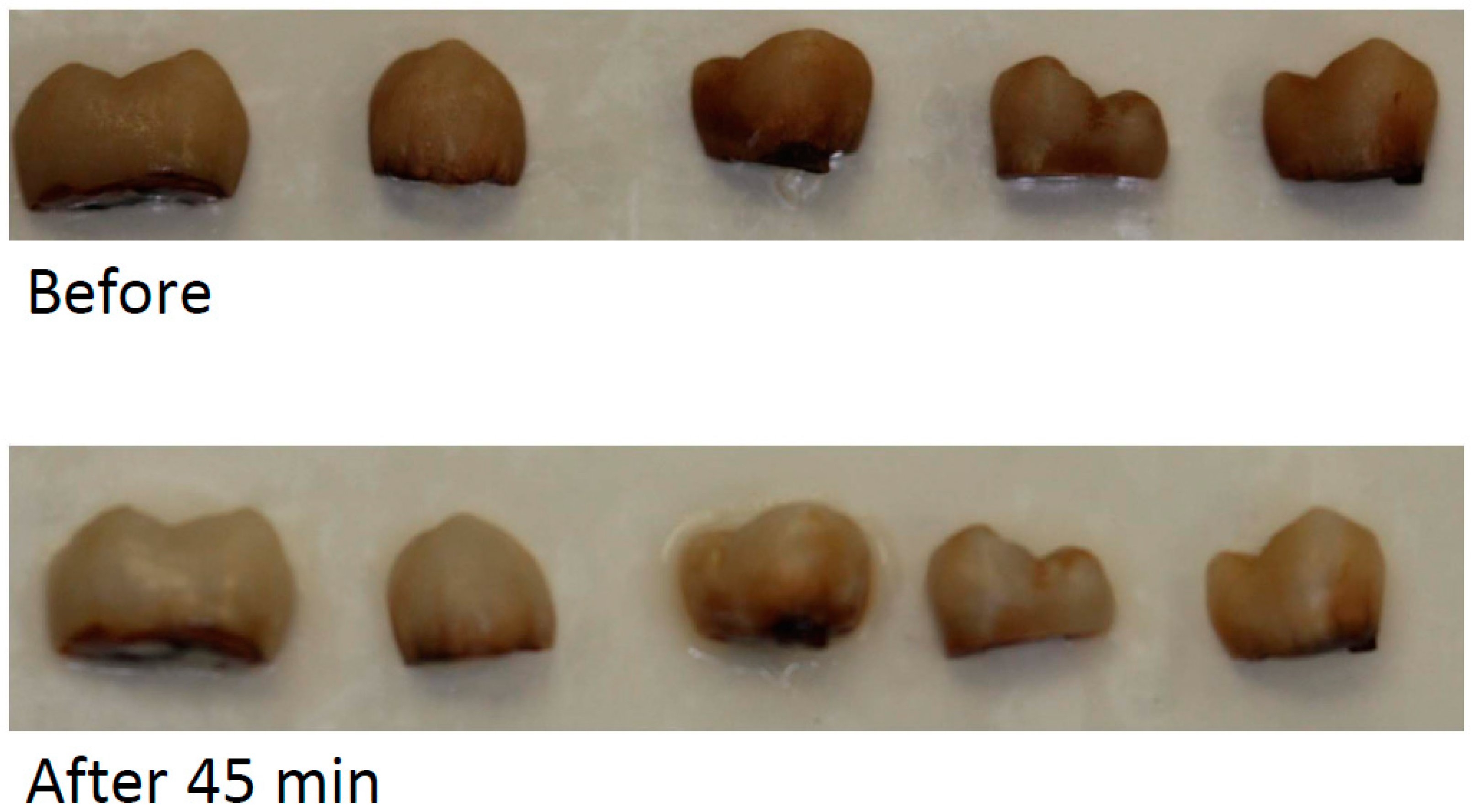
3.3. Tooth Bleaching
3.4. Rhodamine B Degradation
3.5. Tooth Remineralization
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Christensen, G.; Christensen, R. Home use bleaching study 1995. CRA Newsl. 1995, 19, 1–9. [Google Scholar]
- Cavalli, V.; Arrais, C.; Giannini, M.; Ambrosano, G. High-concentrated carbamide peroxide bleaching agents’ effects on enamel surface. J. Oral Rehabil. 2004, 31, 155–159. [Google Scholar] [CrossRef] [PubMed]
- De Abreu, D.R.; Sasaki, R.T.; Amaral, L.B.; Basting, R.T. Effect of home-use and in-office bleaching agents containing hydrogen peroxide associated with amorphous calcium phosphate on enamel microhardness and surface roughness. J. Esthet. Restor. Dent. 2011, 23, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, A.; Cunha, A.; Borges, B.; Machado, C.; dos Santos, A. Tooth whitening with hydrogen/carbamide peroxides in association with a CPP-ACP paste at different proportions. Aust Dent. J. 2012, 57, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Giniger, M.; Macdonald, J.; Ziemba, S.; Felix, H. The clinical performance of professionally dispensed bleaching gel with added amorphous calcium phosphate. J. Am. Dent. Assoc. 2005, 136, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, M.Q. Tooth-bleaching procedures and their controversial effects: A literature review. Saudi Dent. J. 2014, 26, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Haywood, V.B. Treating sensitivity during tooth whitening. Compend. Contin Educ Dent. 2005, 26, 11–20. [Google Scholar] [PubMed]
- Walters, P. Dentinal hypersensitivity: A review. J. Contemp. Dent. Pract. 2005, 6, 107–117. [Google Scholar] [PubMed]
- Fugaro, J.; Nordahl, I.; Fugaro, O. Pulp reaction to vital bleaching. Oper. Dent. 2004, 29, 363–368. [Google Scholar] [PubMed]
- Nathoo, S.; Santana, E.; Zhang, Y.P.; Lin, N.; Collins, M.; Klimpel, K.; DeVizio, W.; Giniger, M. Comparative seven-day clinical evaluation of two tooth whitening products. Compend. Contin Educ Dent. 2001, 22, 599–608. [Google Scholar] [PubMed]
- Strassler, H. Tooth whiting-now and in the future: Part 2. Contemp. Esthet. Restor. Pract. 2004, 8, 50–55. [Google Scholar]
- Leonard, R.H.; Smith, L.R.; Garland, G.E.; Caplan, D.J. Desensitizing agent efficacy during whitening in an at-risk population. J. Esthet. Restor. Dent. 2004, 16, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Walsh, L.J. Contemporary technologies for remineralization therapies: A review. Int. Dent. S. Afr. 2009, 11, 7–15. [Google Scholar]
- Cochrane, N.; Saranathan, S.; Cai, F.; Cross, K.; Reynolds, E. Enamel Subsurface Lesion Remineralisation with Casein Phosphopeptide Stabilised Solutions of Calcium, Phosphate and Fluoride. Caries Res. 2008, 42, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, N.; Cai, F.; Huq, N.; Burrow, M.; Reynolds, E. New Approaches to Enhanced Remineralization of Tooth Enamel. J. Dent. Res. 2010, 89, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Grandfield, K.; Schwenke, A.; Engqvist, H. Synthesis and release of trace elements from hollow and porous hydroxyapatite spheres. Nanotechnology 2011, 22, 305–610. [Google Scholar] [CrossRef] [PubMed]
- Sulieman, M.; Addy, M.; Rees, J.S. Development and evaluation of a method in vitro to study the effectiveness of tooth bleaching. J. Dent. 2003, 31, 415–422. [Google Scholar] [CrossRef]
- Haywood, V.; Heymann, H. Nightguard vital bleaching. Quintessence Int. 1989, 20, 173–176. [Google Scholar] [PubMed]
- Xia, W.; Qin, T.; Suska, F.; Engqvist, H. Bioactive spheres: The way of treating dentin hypersensitivity. ACS Biomater. Sci. Eng. 2016, 2, 734–740. [Google Scholar] [CrossRef]
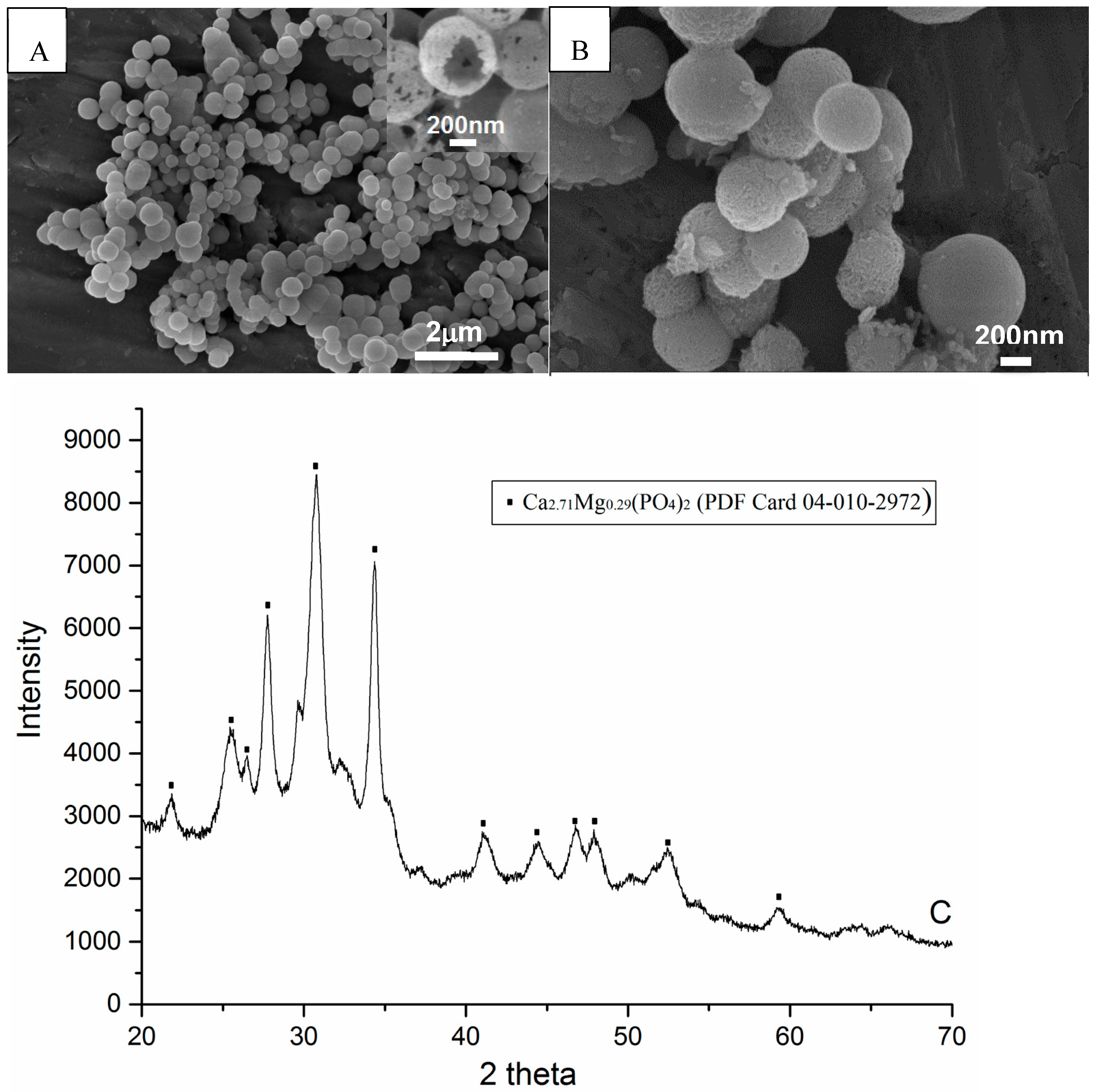
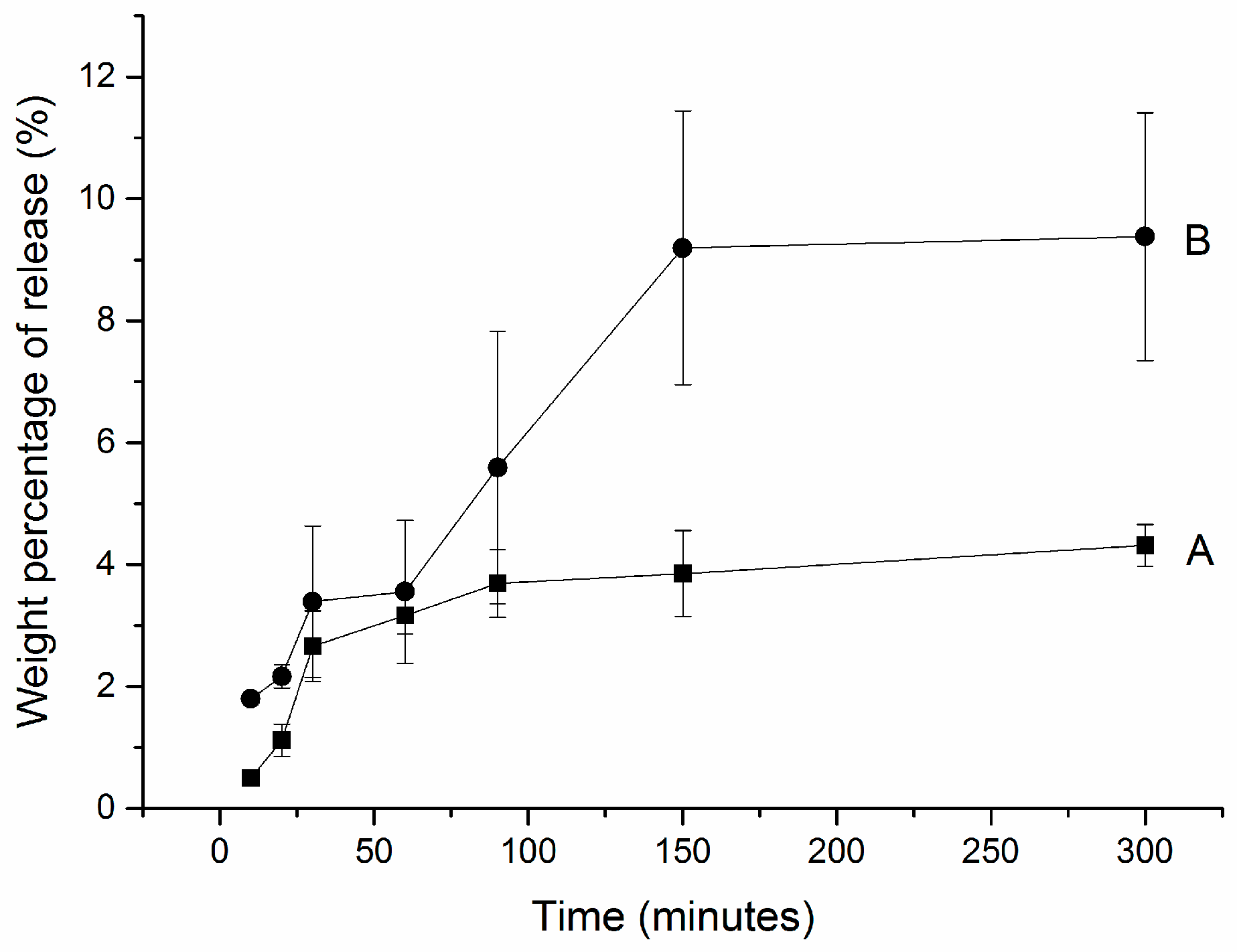



| (wt%) | CP-Loaded HCPS | Anhydrous Glycerol | Others | Estimated Maximal Whitening Agent Wt% |
|---|---|---|---|---|
| At-home gel | 50% | 50% | No | 6% HP |
| In-office gel | 50% | 50% HP(35%) | 20% HP |
| Bleaching | ∆L | ∆a | ∆b | ∆E |
|---|---|---|---|---|
| Control with 45 min bleaching | 22.30 | −10.90 | −0.70 | 24.83 |
| 45 min bleaching via in-office gel | 11.43 ± 3.32 | −8.40 ± 2.34 | −8.55 ± 2.91 | 16.56 ± 5.00 |
| 15 min after washing via in-office gel | 14.58 ± 3.26 | −7.07 ± 2.42 | −3.97 ± 3.07 | 16.68 ± 5.09 |
| 24 h after washing via in-office gel | 20.05 ± 3.63 | −5.78 ± 2.39 | 0.20 ± 1.66 | 20.87 ± 4.65 |
| 45 min bleaching via at-home gel | 9.08 ± 3.42 | −5.74 ± 1.63 | −5.10 ± 3.24 | 11.89 ± 4.48 |
| 2 h after washing via at-home gel | 8.94 ± 2.81 | −6.46 ± 1.72 | −6.26 ± 3.01 | 12.68 ± 4.47 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, T.; Mellgren, T.; Jefferies, S.; Xia, W.; Engqvist, H. A Study for Tooth Bleaching via Carbamide Peroxide-Loaded Hollow Calcium Phosphate Spheres. Dent. J. 2017, 5, 3. https://doi.org/10.3390/dj5010003
Qin T, Mellgren T, Jefferies S, Xia W, Engqvist H. A Study for Tooth Bleaching via Carbamide Peroxide-Loaded Hollow Calcium Phosphate Spheres. Dentistry Journal. 2017; 5(1):3. https://doi.org/10.3390/dj5010003
Chicago/Turabian StyleQin, Tao, Torbjörn Mellgren, Steven Jefferies, Wei Xia, and Håkan Engqvist. 2017. "A Study for Tooth Bleaching via Carbamide Peroxide-Loaded Hollow Calcium Phosphate Spheres" Dentistry Journal 5, no. 1: 3. https://doi.org/10.3390/dj5010003





