Effects of Extraoral Suction on Droplets and Aerosols for Infection Control Practices
Abstract
:1. Introduction
2. Materials and Methods
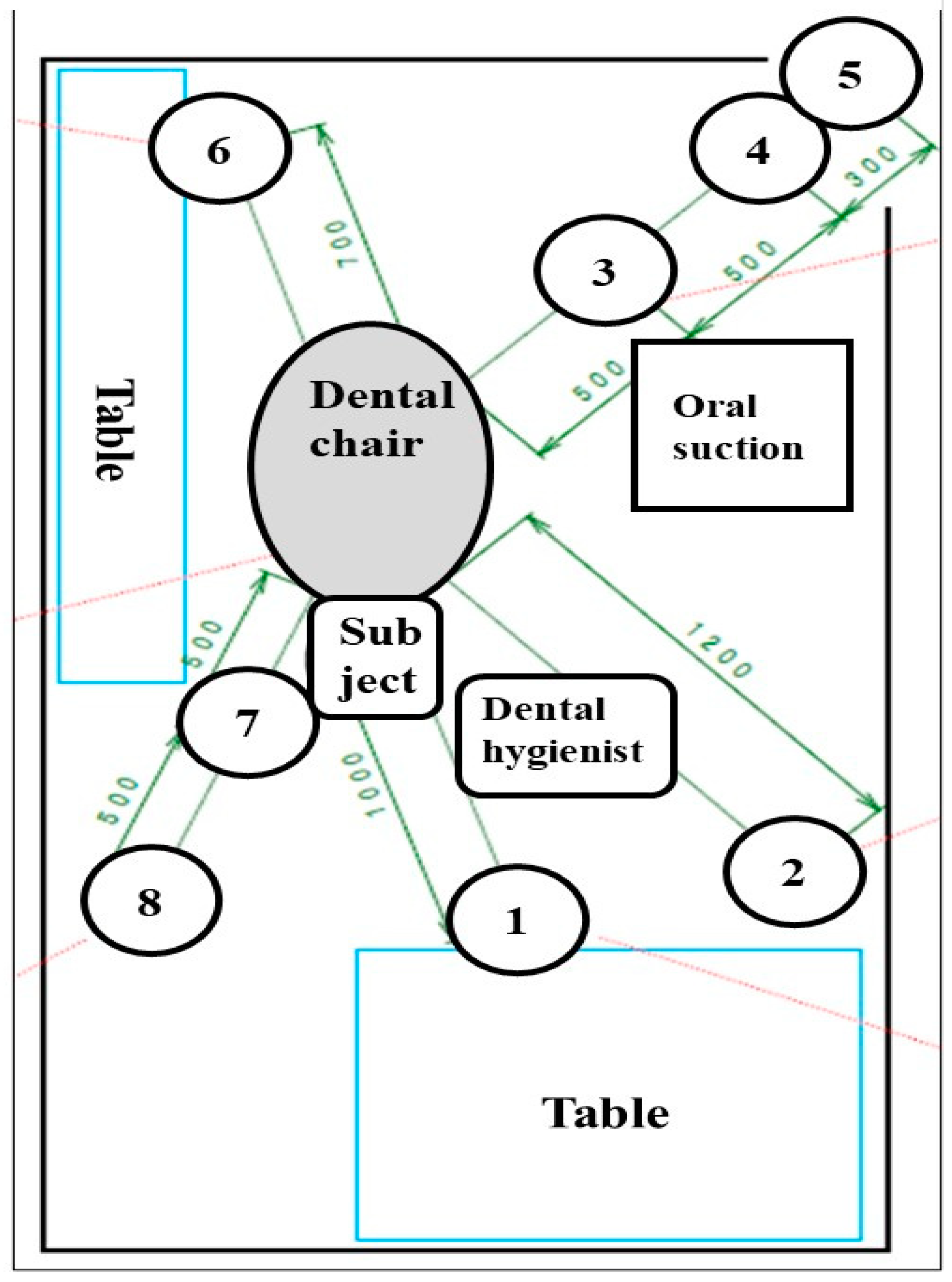
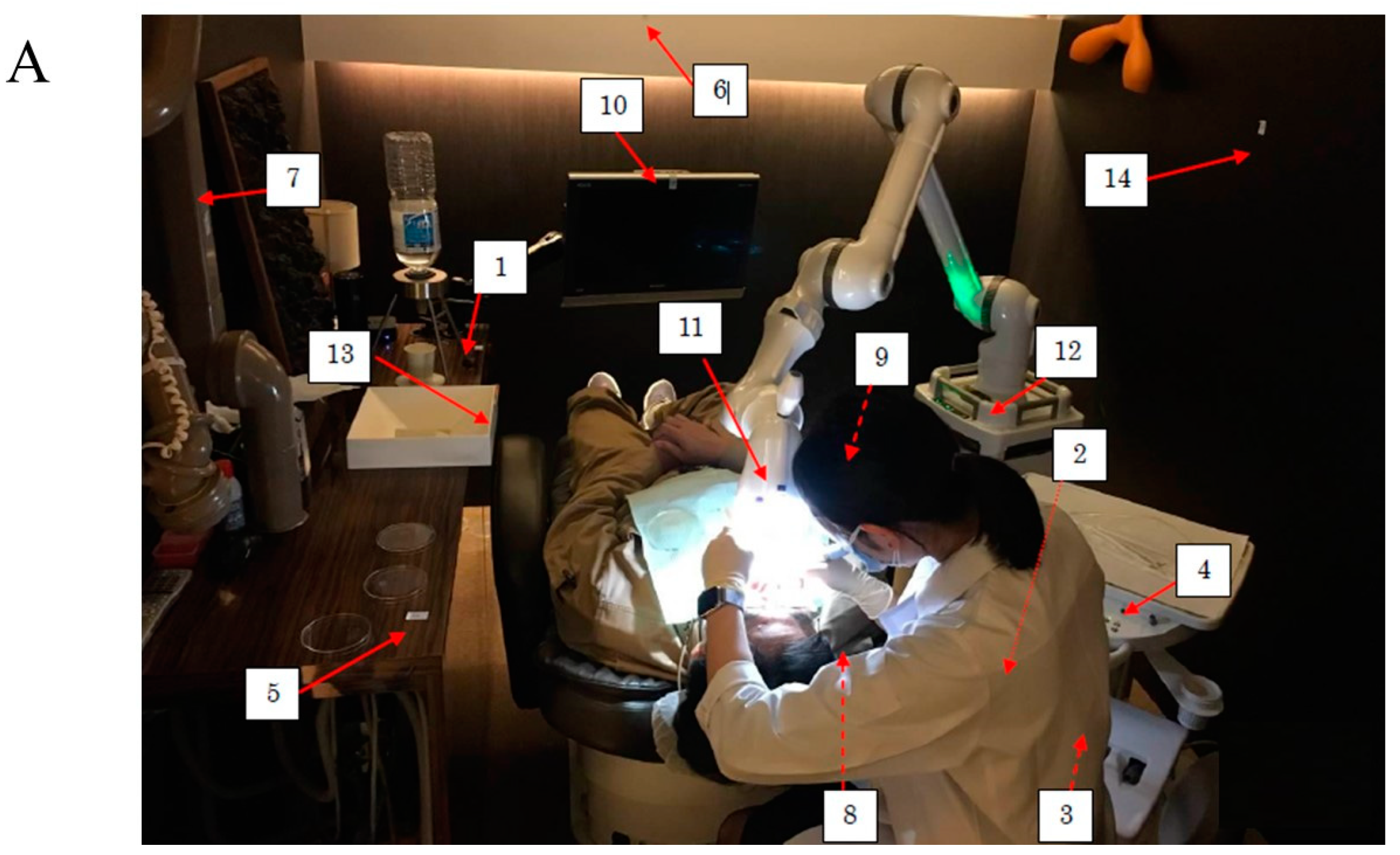
2.1. Setting of Simulated Scaling
2.2. Measurement of Bacterial Numbers in Droplets after Scaling
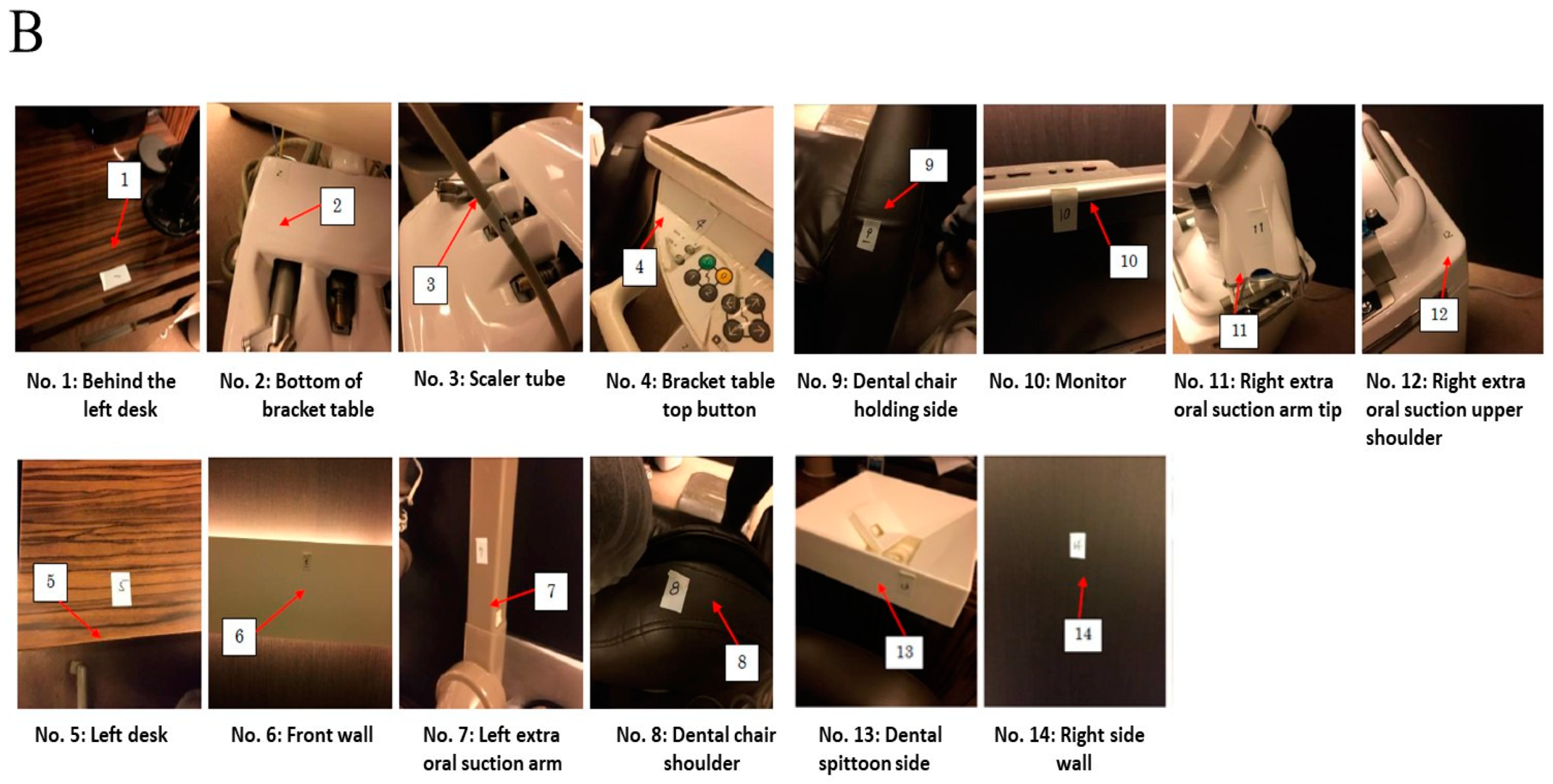
2.3. Measurements of ATP in Droplets and Aerosols after Scaling
2.4. Statistical Analysis
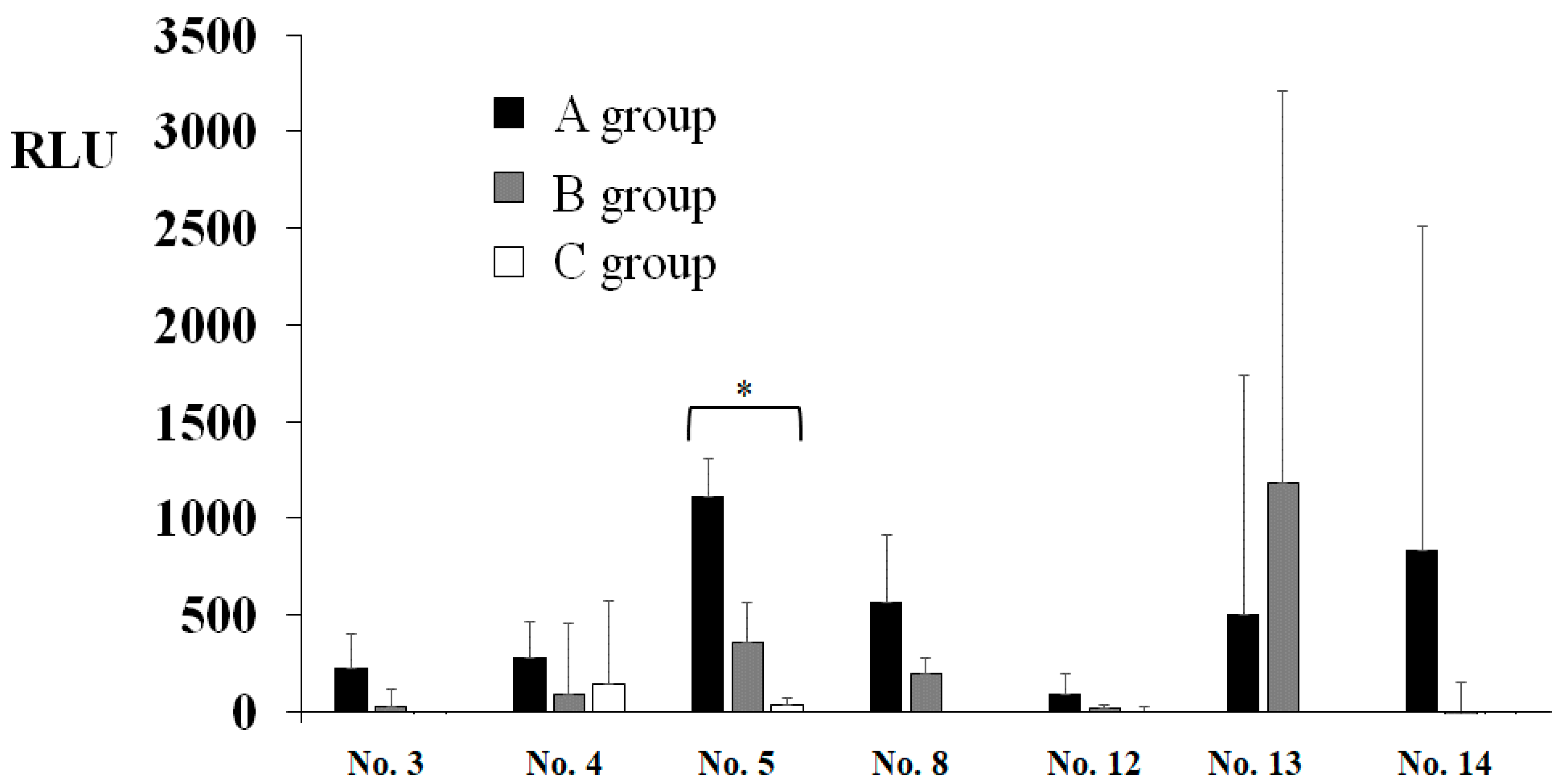
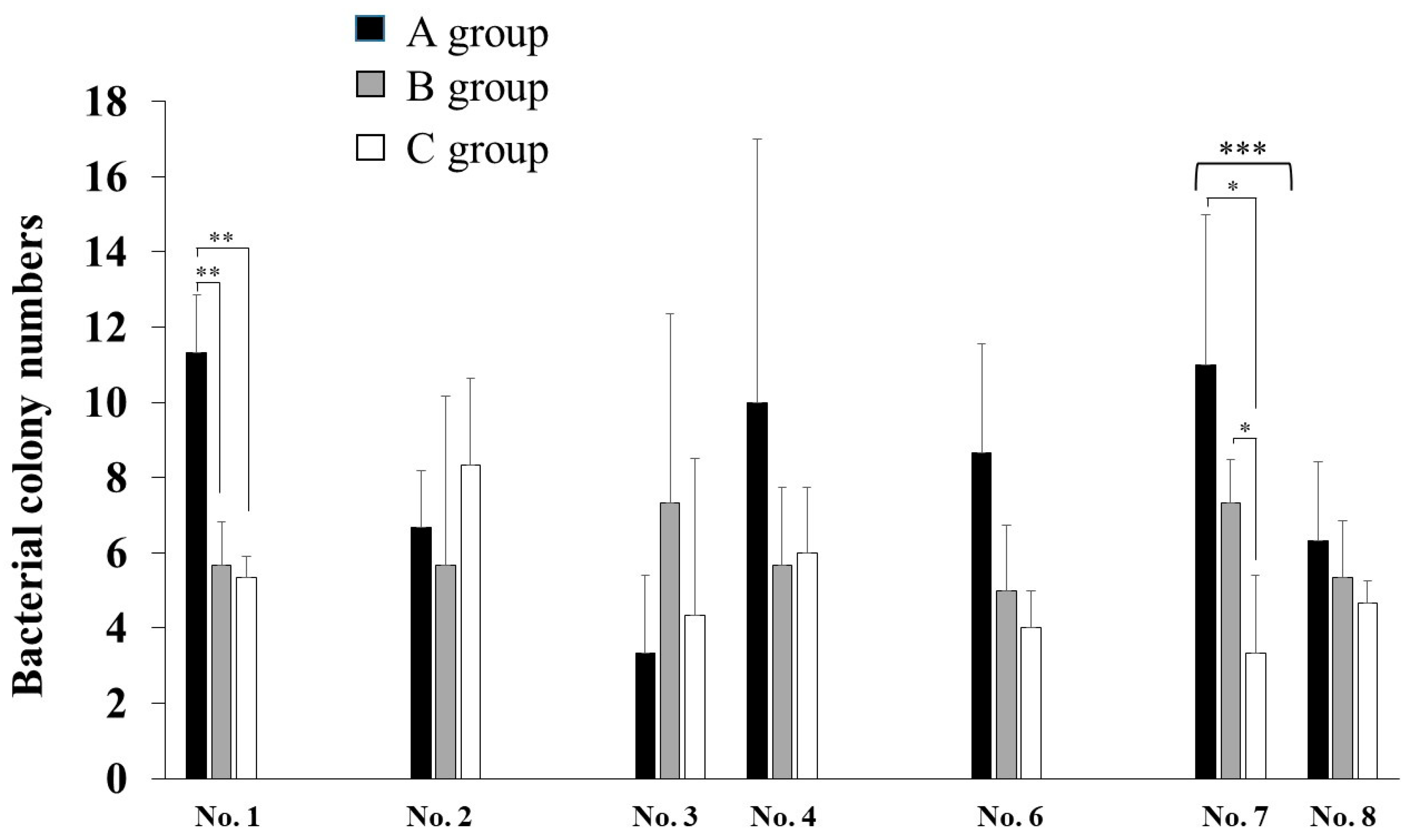
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ge, Z.Y.; Yang, L.M.; Xia, J.J.; Fu, X.H.; Zhang, X.Z. Possible aerosol transmission of COVID-19 and special precautions in dentistry. J. Zhejiang Univ. Sci. B 2020, 21, 361–368. [Google Scholar] [CrossRef] [Green Version]
- Peng, X.; Xu, X.; Li, Y.; Cheng, L.; Zhou, X.; Ren, B. Transmission routes of 2019-nCoV and controls in dental practice. Int. J. Oral Sci. 2020, 12, 9. [Google Scholar] [CrossRef]
- Xu, H.; Zhong, L.; Deng, J.; Peng, J.; Dan, H.; Zeng, X.; Li, T.; Chen, Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020, 12, 8. [Google Scholar] [CrossRef]
- Li, Z.Y.; Meng, L.Y. The prevention and control of a new coronavirus infection in department of stomatology. Zhonghua Kou Qiang Yi Xue Za Zhi 2020, 55, E001. [Google Scholar]
- Meng, L.; Hua, F.; Bian, Z. Coronavirus disease 2019 (COVID-19): Emerging and future challenges for dental and oral medicine. J. Dent. Res. 2020, 99, 481–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Wei, Q.; Alvarez, X.; Wang, H.; Du, Y.; Zhu, H.; Jiang, H.; Zhou, J.; Lam, P.; Zhang, L.; et al. Epithelial cells lining salivary gland ducts are early target cells of severe acute respiratory syndrome coronavirus infection in the upper respiratory tracts of rhesus macaques. J. Virol. 2011, 85, 4025–4030. [Google Scholar] [CrossRef] [Green Version]
- Chen, J. Pathogenicity and transmissibility of 2019-nCoV-A quick overview and comparison with other emerging viruses. Microbes Infect. 2020, 22, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Kampf, G.; Todt, D.; Pfaender, S.; Steinmann, E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020, 104, 246–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ing, E.B.; Xu, Q.A.; Salimi, A.; Torun, N. Physician deaths from corona virus (COVID-19) disease. Occup. Med. 2020, 70, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Reports about COVID-19 in Japan by Ministry of Health, Labour and Welfare. Available online: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000164708_00001.html (accessed on 26 March 2021). (In Japanese)
- Tada, A.; Watanabe, M.; Senpuku, H. Factors influencing compliance with infection control practice in Japanese dentists. Int. J. Occup. Environ. Med. 2014, 5, 24–31. [Google Scholar]
- Tada, A.; Watanabe, M.; Senpuku, H. Factors affecting changes in compliance with infection control practices by dentists in Japan. Am. J. Infect. Control. 2015, 43, 95–97. [Google Scholar] [CrossRef]
- Alawia, R.; Riad, A.; Kateeb, E. Risk perception and readiness of dental students to treat patients amid, COVID-19: Implication for dental education. Oral Dis. 2020. [Google Scholar] [CrossRef]
- Alawia, R.; Riad, A.; Kateeb, E. Knowledge and attitudes among dental students about COVID-19 and its precautionary measures: A cross-sectional study. J. Oral Med. Oral Surg. 2021, 27, 17. [Google Scholar] [CrossRef]
- Prati, C.; Pelliccioni, G.A.; Sambri, V.; Chersoni, S.; Gandolfi, M.G. COVID-19: Its impact on dental schools in Italy, clinical problems in endodontic therapy and general considerations. Int. Endod. J. 2020, 53, 723–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izzetti, R.; Nisi, M.; Gabriele, M.; Graziani, F. COVID-19 transmission in dental practice: Brief review of preventive measures in Italy. J. Dent. Res. 2020, 99, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Cochran, M.A.; Miller, C.H.; Sheldrake, M.A. The efficacy of the rubber dam as a barrier to the spread of microorganisms during dental treatment. J. Am. Dent. Assoc. 1989, 119, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Chavis, S.E.; Hines, S.E.; Dyalram, D.; Wilken, N.C.; Dalby, R.N. Can extraoral suction units minimize droplet spatter during a stimulated dental procedure? JADA 2021, 152, 157–165. [Google Scholar] [PubMed]
- Shahdad, S.; Patel, T.; Hindocha, A.; Cagney, N.; Mueller, J.D.; Seoudi, N.; Morgan, C.; Din, A. The efficacy of an extraoral scavenging device on reduction of splatter contamination during dental aerosol generating procedures: An exploratory study. Br. Dent. 2020, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Whiteley, G.S.; Glasbey, T.O.; Fahey, P.P. A suggested sampling algorithm for use with ATP testing in cleanliness measurement. Infect. Dis. Health 2016, 21, 169–175. [Google Scholar] [CrossRef] [Green Version]
- Budnyak, M.A.; Gurevich, K.G.; Fabrikant, K.G.; Miller, K.; Puttaiah, R. Dental infection control and occupational safety in the Russian Federation. J. Contemp. Dent. Pract. 2012, 13, 703–712. [Google Scholar]
- Puttaiah, R.; Miller, K.; Bedi, D.R.; Shetty, S.; Almas, K.; Tse, E.; Kim, B.O.; Youngblood, D.; Minquan, D. Comparison of knowledge, attitudes and practice of dental safety from eight countries at the turn of the century. J. Contemp. Dent. Pract. 2011, 12, 1–7. [Google Scholar] [CrossRef]
- Cheng, H.C.; Su, C.Y.; Huang, C.F.; Chuang, C.Y. Changes in compliance with recommended infection control practices and affecting factors among dentists in Taiwan. J. Dent. Educ. 2012, 76, 1684–1690. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, H.; Bayat, F.; Hooshmand, B.; Maleki, Z. Determinants of Iranian dentists’ behaviour regarding infection control. Int. Dent. J. 2011, 61, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.M.; Wang, W.; Song, Z.G.; Hu, Y.; Tao, Z.W.; Tian, J.H.; Pei, Y.Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senpuku, H.; Fukumoto, M.; Uchiyama, T.; Taguchi, C.; Suzuki, I.; Arikawa, K. Effects of Extraoral Suction on Droplets and Aerosols for Infection Control Practices. Dent. J. 2021, 9, 80. https://doi.org/10.3390/dj9070080
Senpuku H, Fukumoto M, Uchiyama T, Taguchi C, Suzuki I, Arikawa K. Effects of Extraoral Suction on Droplets and Aerosols for Infection Control Practices. Dentistry Journal. 2021; 9(7):80. https://doi.org/10.3390/dj9070080
Chicago/Turabian StyleSenpuku, Hidenobu, Masahiko Fukumoto, Toshikazu Uchiyama, Chieko Taguchi, Itaru Suzuki, and Kazumune Arikawa. 2021. "Effects of Extraoral Suction on Droplets and Aerosols for Infection Control Practices" Dentistry Journal 9, no. 7: 80. https://doi.org/10.3390/dj9070080
APA StyleSenpuku, H., Fukumoto, M., Uchiyama, T., Taguchi, C., Suzuki, I., & Arikawa, K. (2021). Effects of Extraoral Suction on Droplets and Aerosols for Infection Control Practices. Dentistry Journal, 9(7), 80. https://doi.org/10.3390/dj9070080





