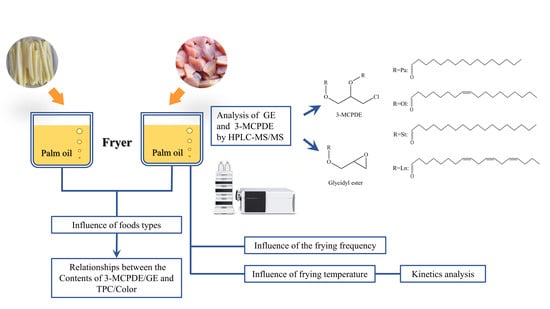
Effects of Food Types, Frying Frequency, and Frying Temperature on 3-Monochloropropane-1,2-diol Esters and Glycidyl Esters Content in Palm Oil during Frying
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Samples and Pre-Treatment
2.3. Deep-Fat Frying
2.4. Fatty Acid Analysis
2.5. Quantitation of TPC
2.6. Color Analysis
2.7. Determination of 3-MCPDE and GE
2.8. Kinetics and Statistical Analysis
3. Results
3.1. HPLC-MS/MS Analysis
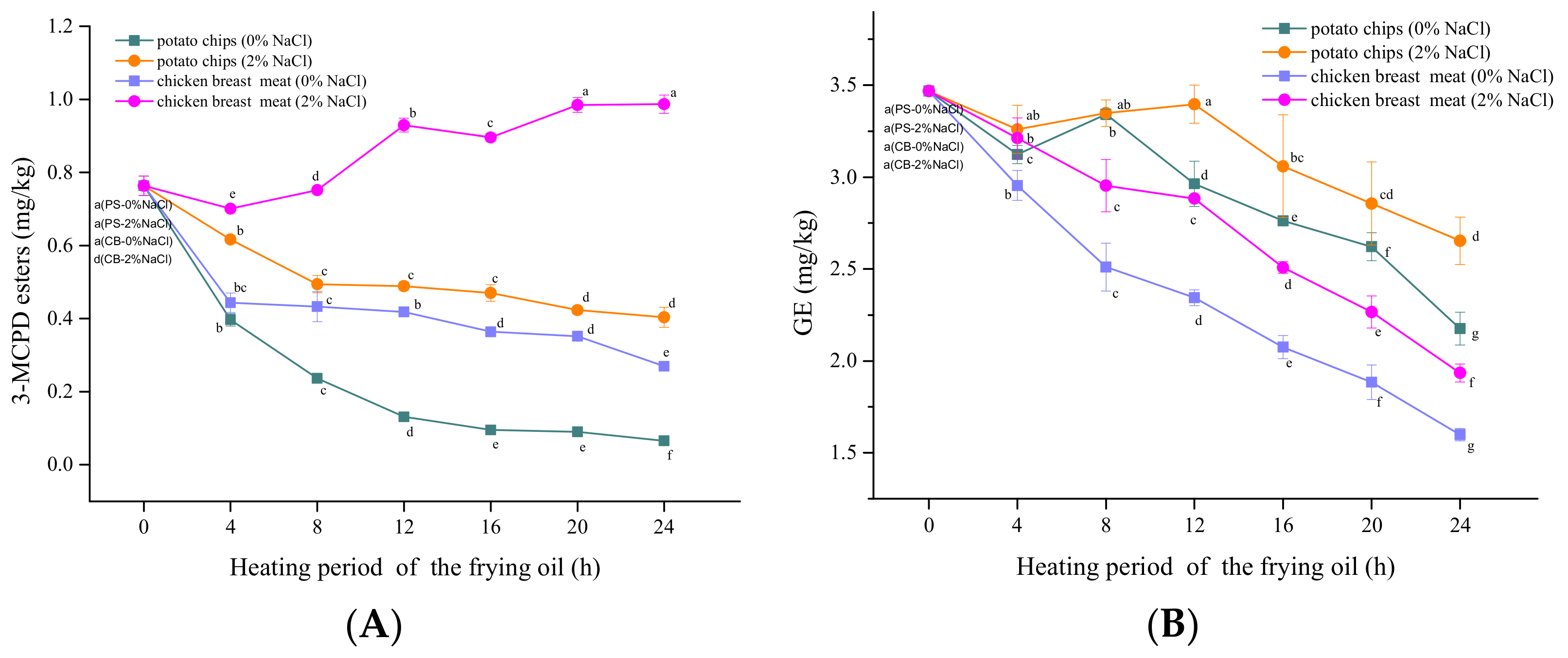
3.2. Influence of Foods Types
3.3. Relationships between the Contents of 3-MCPDE/GE and TPC/Color
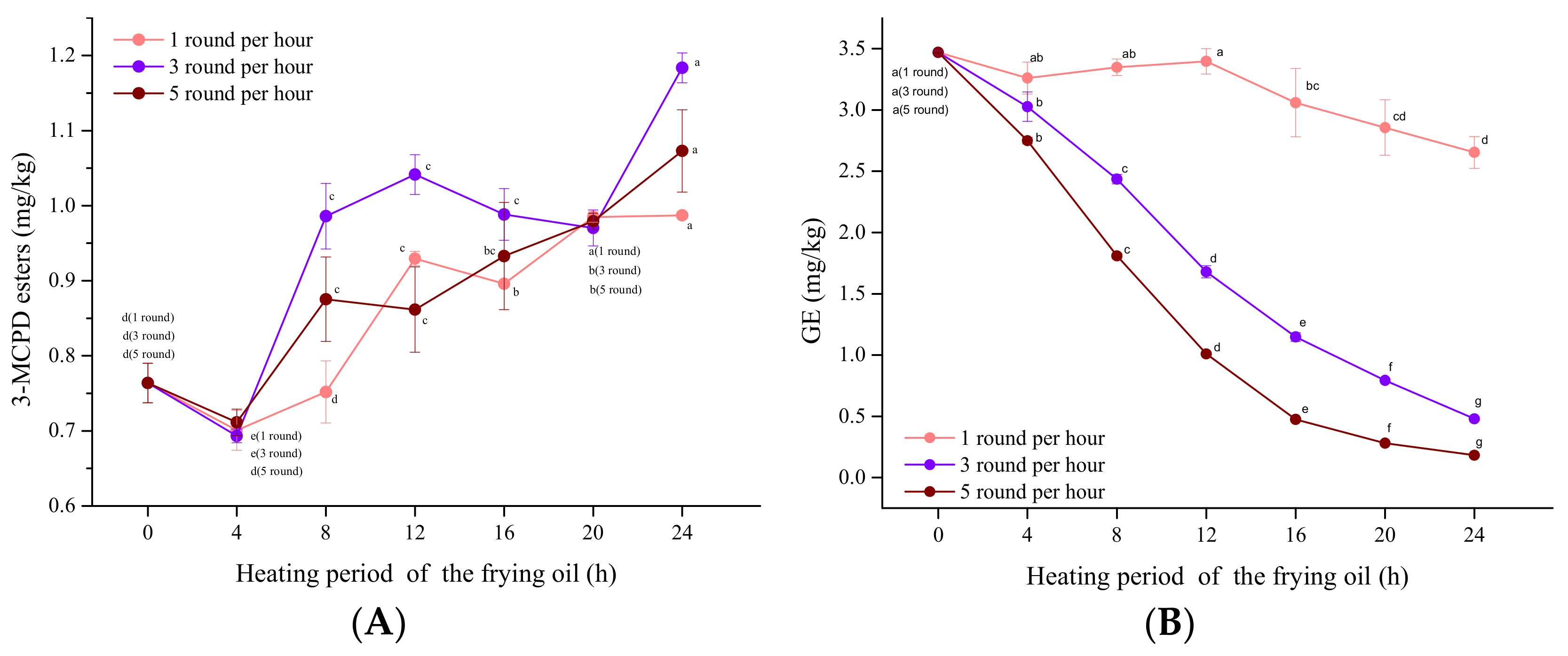
3.4. Influence of the Frying Frequency
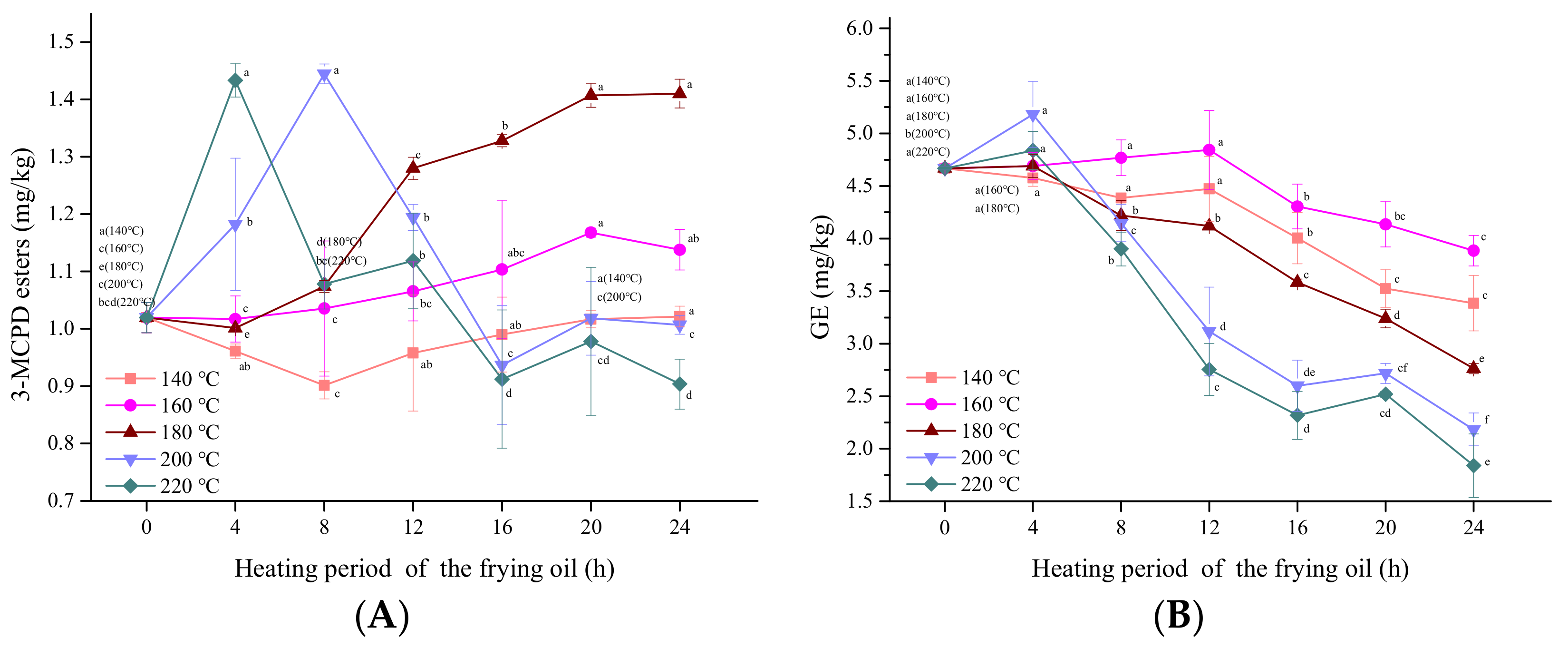
3.5. Influence of Frying Temperature
3.6. Kinetics Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC monographs on the evaluation of carcinogenic risk of chemicals to humans. In IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Humans; IARC: Lyon, France, 2000; pp. 469–486. [Google Scholar]
- European Food Safety Authority. Risks for Human Health Related to the Presence of 3- and 2-Monochloropropanediol (MCPD), and Their Fatty Acid Esters, and Glycidyl Fatty Acid Esters in Food. EFSA J. 2016, 4, 4426–4585. [Google Scholar]
- Kuhlmann, J. Determination of bound 2,3-epoxy-1-propanol (glycidol) and bound monochloropropanediol (MCPD) in refined oils. Eur. J. Lipid Sci. Technol. 2011, 113, 335–344. [Google Scholar] [CrossRef]
- Chung, H.; Chung, S.W.; Chan, B.; Ho, Y.Y.; Xiao, Y. Dietary exposure of Hong Kong adults to fatty acid esters of 3-monochloropropane-1,2-diol. Food Addit. Contam. Part A 2013, 30, 1508–1512. [Google Scholar] [CrossRef]
- Spungen, J.H.; MacMahon, S.; Leigh, J.; Flannery, B.; Kim, G.; Chirtel, S.; Smegal, D. Estimated US infant exposures to 3-MCPD esters and glycidyl esters from consumption of infant formula. Food Addit. Contam. Part A 2018, 35, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Haines, T.D.; Adlaf, K.J.; Pierceall, R.M.; Lee, I.; Venkitasubramanian, P.; Collison, M.W. Direct Determination of MCPD Fatty Acid Esters and Glycidyl Fatty Acid Esters in Vegetable Oils by LC–TOFMS. J. Am. Oil Chem. Soc. 2010, 88, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Zhou, Y.; Zhu, J.; Wang, S.; Nie, S.; Xie, M. Formation of 3-chloropropane-1,2-diol esters in model systems simulating thermal processing of edible oil. LWT 2016, 69, 586–592. [Google Scholar] [CrossRef]
- Franke, K.; Strijowski, U.; Fleck, G.; Pudel, F. Influence of chemical refining process and oil type on bound 3-chloro-1,2-propanediol contents in palm oil and rapeseed oil. LWT 2009, 42, 1751–1754. [Google Scholar] [CrossRef]
- Arisseto, A.P.; Marcolino, P.F.C.; Augusti, A.C.; Scaranelo, G.R.; Berbari, S.A.G.; Miguel, A.M.R.O.; Morgano, M.A.; Vicente, E. Contamination of Fried Foods by 3-Monochloropropane-1,2-diol Fatty Acid Esters During Frying. J. Am. Oil Chem. Soc. 2017, 94, 449–455. [Google Scholar] [CrossRef]
- Wang, S.; Liu, G.; Cheng, W. Chloride-mediated co-formation of 3-monochloropropanediol esters and glycidyl esters in both model vegetable oils and chemical model systems. Food Res. Int. 2021, 140, 109879. [Google Scholar] [CrossRef]
- Yao, Y.; Cao, R.; Liu, W.; Zhou, H.; Li, C.; Wang, S. Molecular Reaction Mechanism for the Formation of 3-Chloropropanediol Esters in Oils and Fats. J. Agric. Food Chem. 2019, 67, 2700–2708. [Google Scholar] [CrossRef]
- Merkle, S.; Ostermeyer, U.; Rohn, S.; Karl, H.; Fritsche, J. Mitigation strategies for ester bound 2-/3-MCPD and esterified glycidol in pre-fried breaded and frozen fish products. Food Chem. 2018, 245, 196–204. [Google Scholar] [CrossRef]
- Wong, Y.H.; Lai, O.M.; Abas, F.; Nyam, K.L.; Nehdi, I.; Muhamad, H.; Tan, C.P. Factors Impacting the Formation of 3-MCPD Esters and Glycidyl Esters During Deep Fat Frying of Chicken Breast Meat. J. Am. Oil Chem. Soc. 2017, 94, 759–765. [Google Scholar] [CrossRef]
- Wong, Y.H.; Muhamad, H.; Abas, F.; Lai, O.M.; Nyam, K.L.; Tan, C.P. Effects of temperature and NaCl on the formation of 3-MCPD esters and glycidyl esters in refined, bleached and deodorized palm olein during deep-fat frying of potato chips. Food Chem. 2017, 219, 126–130. [Google Scholar] [CrossRef]
- MacMahon, S.; Begley, T.H.; Diachenko, G.W. Occurrence of 3-MCPD and glycidyl esters in edible oils in the United States. Food Addit. Contam. Part A 2013, 30, 2081–2092. [Google Scholar] [CrossRef]
- Quan, W.; Li, Y.; Jiao, Y.; Xue, C.; Liu, G.; Wang, Z.; He, Z.; Qin, F.; Zeng, M.; Chen, J. Simultaneous generation of acrylamide, β-carboline heterocyclic amines and advanced glycation ends products in an aqueous Maillard reaction model system. Food Chem. 2020, 332, 127387. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Song, Y.; Hu, X.-S.; Liao, X.-J.; Ni, Y.-Y.; Li, Q.-H. Effects of sugars in batter formula and baking conditions on 5-hydroxymethylfurfural and furfural formation in sponge cake models. Food Res. Int. 2012, 49, 439–445. [Google Scholar] [CrossRef]
- Parker, J.K.; Balagiannis, D.P.; Higley, J.; Smith, G.; Wedzicha, B.L.; Mottram, D.S. Kinetic Model for the Formation of Acrylamide during the Finish-Frying of Commercial French Fries. J. Agric. Food Chem. 2012, 60, 9321–9331. [Google Scholar] [CrossRef]
- The State Food and Drug Administration. GB5009.168—2016 Determination of Fatty Acids in Foods; National Health and Family Planning Commission of China, and China Food and Drug Administration: Beijing, China, 2016. [Google Scholar]
- National Health and Family Planning Commission. GB 5009.202-2016 Determination of Polar Components in Edible Oil; National Health and Family Planning Commission of China: Beijing, China, 2016. [Google Scholar]
- Mogol, B.A.; Pye, C.; Anderson, W.; Crews, C.; Gökmen, V. Formation of Monochloropropane-1,2-diol and Its Esters in Biscuits during Baking. J. Agric. Food Chem. 2014, 62, 7297–7301. [Google Scholar] [CrossRef]
- Ross, T. Indices for Performance Evaluation of Predictive Models in Food Microbiology. J. Appl. Bacteriol. 1996, 81, 501–508. [Google Scholar]
- Crews, C.; Chiodini, A.; Granvogl, M.; Hamlet, C.; Hrncirik, K.; Kuhlmann, J.; Lampen, A.; Scholz, G.; Weisshaar, R.; Wenzl, T.; et al. Analytical approaches for MCPD esters and glycidyl esters in food and biological samples: A review and future perspectives. Food Addit. Contam. Part A 2013, 30, 11–45. [Google Scholar] [CrossRef]
- Chaijan, M.; Panpipat, W. Pre-neutralized crude palm oil as natural colorant and bioactive ingredient in fish sausage prepared from tilapia (Oreochromis niloticus). LWT 2021, 135, 110289. [Google Scholar] [CrossRef]
- European Food Safety Authority. Analysis of occurrence of 3-monochloropropane-1,2-diol (3-MCPD) in food in Europe in the years 2009-2011 and preliminary exposure assessment. EFSA J. 2013, 11, 3381. [Google Scholar] [CrossRef]
- Givens, D.; Gibbs, R.; Rymer, C.; Brown, R. Effect of intensive vs. free range production on the fat and fatty acid composition of whole birds and edible portions of retail chickens in the UK. Food Chem. 2011, 127, 1549–1554. [Google Scholar] [CrossRef]
- Li, C.; Li, L.; Jia, H.; Wang, Y.; Shen, M.; Nie, S.; Xie, M. Formation and reduction of 3-monochloropropane-1,2-diol esters in peanut oil during physical refining. Food Chem. 2015, 199, 605–611. [Google Scholar] [CrossRef]
- Food, M.; Worldwide, C.; Hellmann, H.; Goyer, A. Antioxidants in Potatoes: A Functional View on One of the Major Food Crops Worldwide. Molecules 2021, 26, 2446. [Google Scholar]
- Li, C.; Jia, H.; Shen, M.; Wang, Y.; Nie, S.; Chen, Y.; Zhou, Y.; Wang, Y.; Xie, M. Antioxidants Inhibit Formation of 3-Monochloropropane-1,2-diol Esters in Model Reactions. J. Agric. Food Chem. 2015, 63, 9850–9854. [Google Scholar] [CrossRef]
- Hammouda, I.B.; Zribi, A.; Mansour, A.B.; Matthäus, B.; Bouaziz, M. Effect of deep-frying on 3-MCPD esters and glycidyl esters contents and quality control of refined olive pomace oil blended with refined palm oil. Eur. Food Res. Technol. 2017, 243, 1219–1227. [Google Scholar] [CrossRef]
- Mba, O.I.; Dumont, M.-J.; Ngadi, M. Deterioration Kinetics of Crude Palm Oil, Canola Oil and Blend During Repeated Deep-Fat Frying. J. Am. Oil Chem. Soc. 2016, 93, 1243–1253. [Google Scholar] [CrossRef]
- Abdulkarim, S.; Long, K.; Lai, O.-M.; Muhammad, K.; Ghazali, H. Frying quality and stability of high-oleic Moringa oleifera seed oil in comparison with other vegetable oils. Food Chem. 2007, 105, 1382–1389. [Google Scholar] [CrossRef]
- Zhou, H.; Jin, Q.; Wang, X.; Xu, X. Effects of temperature and water content on the formation of 3-chloropropane-1,2-diol fatty acid esters in palm oil under conditions simulating deep fat frying. Eur. Food Res. Technol. 2013, 238, 495–501. [Google Scholar] [CrossRef]
- Aniołowska, M.; Kita, A. The Effect of Type of Oil and Degree of Degradation on Glycidyl Esters Content During the Frying of French Fries. J. Am. Oil Chem. Soc. 2015, 92, 1621–1631. [Google Scholar] [CrossRef] [Green Version]
- Cheng, W.; Liu, G.; Guo, Z.; Chen, F.; Cheng, K.-W. Kinetic Study and Degradation Mechanism of Glycidyl Esters in both Palm Oil and Chemical Models during High-Temperature Heating. J. Agric. Food Chem. 2020, 68, 15319–15326. [Google Scholar] [CrossRef] [PubMed]
- Ermacora, A.; Hrncirik, K. Study on the thermal degradation of 3-MCPD esters in model systems simulating deodorization of vegetable oils. Food Chem. 2014, 150, 158–163. [Google Scholar] [CrossRef]
- Cheng, W.-W.; Liu, G.-Q.; Wang, L.-Q.; Liu, Z.-S. Glycidyl Fatty Acid Esters in Refined Edible Oils: A Review on Formation, Occurrence, Analysis, and Elimination Methods. Compr. Rev. Food Sci. Food Saf. 2017, 16, 263–281. [Google Scholar] [CrossRef] [Green Version]
- Zelinkova, Z.; Svejkovska, B.; Velisek, J.; Dolezal, M. Fatty acid esters of 3-chloropropane-1,2-diol in edible oils. Food Addit. Contam. 2006, 23, 1290–1298. [Google Scholar] [CrossRef] [PubMed]
- Matthäus, B.; Pudel, F.; Fehling, P.; Vosmann, K.; Freudenstein, A. Strategies for the reduction of 3-MCPD esters and related compounds in vegetable oils. Eur. J. Lipid Sci. Technol. 2011, 113, 380–386. [Google Scholar] [CrossRef]
- Pudel, F.; Benecke, P.; Fehling, P.; Freudenstein, A.; Matthäus, B.; Schwaf, A. On the necessity of edible oil refining and possible sources of 3-MCPD and glycidyl esters. Eur. J. Lipid Sci. Technol. 2011, 113, 368–373. [Google Scholar] [CrossRef]
- Kimura, W.; Endo, Y. Decomposition products of glycidyl esters of fatty acids by heating. Biosci. Biotechnol. Biochem. 2017, 81, 581–586. [Google Scholar] [CrossRef] [Green Version]
| Compounds | Abbreviation | RT (min) | Precursor Ion [M + NH4] (m/z) | Production (m/z) | Collision Energy (eV) | Internal Standard |
|---|---|---|---|---|---|---|
| GE | ||||||
| Glycidyl linoleate | Li-GE | 3.88 | 354.3 | 81.1 * | 32 | Ol-GE-d5 |
| 95.1 | 28 | |||||
| Glycidyl palmitate | Pa-GE | 4.77 | 330.3 | 57.2 * | 32 | Ol-GE-d5 |
| 71.1 | 24 | |||||
| Glycidyl oleate | Ol-GE | 5.31 | 356.3 | 57.1 * | 32 | Ol-GE-d5 |
| 69.1 | 36 | |||||
| Glycidyl stearate | St-GE | 7.65 | 358.3 | 57.2 * | 36 | St-GE-d5 |
| 71.1 | 32 | |||||
| 3-MCPDE | ||||||
| 1,2-Bis-palmitoyl-3-chloropropanediol | Pa-Pa | 18.87 | 604.5 | 331.1 * | 14 | Pa-Pa-d5 |
| 239.2 | 20 | |||||
| Oleoyl-palmitoyl-3-chloropropanediol | Ol-Pa | 19.05 | 630.5 | 331.3 * | 20 | Pa-Pa-d5 |
| 357.3 | 20 | |||||
| 1,2-Bis-oleoyl-3-chloropropanediol | Ol-Ol | 19.21 | 656.5 | 357.3 * | 22 | Ol-Ol-d5 |
| 265.2 | 22 | |||||
| Oleoyl-stearoyl-3-chloropropanediol | Ol-St | 19.23 | 658.5 | 359.3 * | 20 | Ol-Ol-d5 |
| 357.3 | 24 | |||||
| Internal standard | ||||||
| Glycidyl oleate-d5 | Ol-GE-d5 | 5.23 | 361.0 | 69.3 * | 40 | |
| 83.2 | 28 | |||||
| Glycidyl stearate-d5 | St-GE-d5 | 7.56 | 363 | 57.2 * | 36 | |
| 85.1 | 28 | |||||
| 1,2-Bis-palmitoyl-3-chloropropanediol-d5 | Pa-Pa-d5 | 18.82 | 609.3 | 239.2 | 24 | |
| 336.3 * | 12 | |||||
| 1,2-Bis-oleoyl-3-chloropropanediol-d5 | Ol-Ol-d5 | 19.18 | 661.53 | 362.3 * | 28 | |
| 265.1 | 20 |
| Correlation (r) of MCPD Esters and GE in the Frying Oil (µg/kg) with ΔE Value | Correlation (r) of MCPD Esters and GE in the Frying Oil (µg/kg) with the TPC Value [%] | Correlation of ΔE in the Frying Oil with the Different Heating Hour | Correlation of TPC in the Frying Oil with the Different Heating Hour | |||
|---|---|---|---|---|---|---|
| 3-MCPDE | GE | 3-MCPDE | GE | |||
| Potato chips (0% NaCl) | −0.992 ** | −0.765 | −0.892 * | −0.916 * | 0.937 ** | 0.973 ** |
| Potato chips (2% NaCl) | −0.954 ** | −0.542 | −0.935 * | −0.811 | 0.866 * | 0.991 * |
| Chicken breast meat (0% NaCl) | −0.781 | −0.943 ** | −0.961 * | −0.912 * | 0.909 * | 0.956 * |
| Chicken breast meat (2% NaCl) | 0.949 * | −0.895 * | 0.857 | −0.990 ** | 0.941 ** | 0.979 * |
| T(℃) | Arrhenius Equation | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 140 | 160 | 180 | 200 | 220 | A (h−1) | Ea (kJ/mol) | R2 | ||
| Pa-Pa | K (×10−3, h−1) | - | 2.66 | 4.06 | 14.61 | 10.41 | 6.89 × 105 | 41.05 | 0.81 |
| R2 | - | 0.914 | 0.953 | 0.861 | 0.863 | ||||
| RRMSE% | - | 22.82 | 14.57 | 29.84 | 15.42 | ||||
| Ol-Pa | K (×10−3, h−1) | 4.22 | 4.40 | 9.36 | 46.14 | 56.88 | 7.46 × 104 | 53.58 | 0.89 |
| R2 | 0.937 | 0.85 | 0.958 | 0.794 | 0.863 | ||||
| RRMSE% | 17.49 | 20.24 | 7.51 | 12.28 | 23.16 | ||||
| Ol-Ol | K (×10−3, h−1) | - | 1.98 | 2.84 | 10.93 | 22.83 | 5.86 × 105 | 65.09 | 0.95 |
| R2 | - | 0.884 | 0.676 | 0.865 | 0.878 | ||||
| RRMSE% | - | 20.40 | 30.14 | 28.83 | 14.10 | ||||
| Ol-St | K (×10−3, h−1) | - | 2.03 | 3.18 | 14.03 | 23.13 | 8.39 × 105 | 66.00 | 0.93 |
| R2 | - | 0.835 | 0.832 | 0.843 | 0.884 | ||||
| RRMSE% | - | 26.83 | 22.27 | 29.66 | 13.23 | ||||
| Li-GE | K (×10−3, h−1) | 19.11 | 26.76 | 30.92 | 52.38 | 49.29 | 7.06 | 18.49 | 0.93 |
| R2 | 0.99 | 0.966 | 0.988 | 0.823 | 0.947 | ||||
| RRMSE% | 6.86 | 25.95 | 4.46 | 27.09 | 12.10 | ||||
| Pa-GE | K (×10−3, h−1) | 16.85 | 16.48 | 23.86 | 59.04 | 50.57 | 38.91 | 24.88 | 0.81 |
| R2 | 0.925 | 0.893 | 0.828 | 0.951 | 0.962 | ||||
| RRMSE% | 5.52 | 22.06 | 16.15 | 26.12 | 16.49 | ||||
| St-GE | K (×10−3, h−1) | 7.42 | 6.00 | 9.17 | 37.23 | 34.01 | 2.01 | 20.89 | 0.96 |
| R2 | 0.931 | 0.958 | 0.805 | 0.958 | 0.949 | ||||
| RRMSE% | 14.53 | 17.41 | 17.97 | 16.29 | 21.04 | ||||
| Ol-GE | K (×10−3, h−1) | 2.44 | 3.52 | 5.89 | 6.28 | 7.72 | 145.22 | 32.06 | 0.72 |
| R2 | 0.806 | 0.945 | 0.945 | 0.925 | 0.951 | ||||
| RRMSE% | 28.58 | 24.28 | 9.06 | 21.06 | 21.80 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Zhang, W.; Zhang, Y.; Huang, M.; Sun, B. Effects of Food Types, Frying Frequency, and Frying Temperature on 3-Monochloropropane-1,2-diol Esters and Glycidyl Esters Content in Palm Oil during Frying. Foods 2021, 10, 2266. https://doi.org/10.3390/foods10102266
Zhang J, Zhang W, Zhang Y, Huang M, Sun B. Effects of Food Types, Frying Frequency, and Frying Temperature on 3-Monochloropropane-1,2-diol Esters and Glycidyl Esters Content in Palm Oil during Frying. Foods. 2021; 10(10):2266. https://doi.org/10.3390/foods10102266
Chicago/Turabian StyleZhang, Jinglin, Wendi Zhang, Yuanzheng Zhang, Mingquan Huang, and Baoguo Sun. 2021. "Effects of Food Types, Frying Frequency, and Frying Temperature on 3-Monochloropropane-1,2-diol Esters and Glycidyl Esters Content in Palm Oil during Frying" Foods 10, no. 10: 2266. https://doi.org/10.3390/foods10102266