Effects of Pressure Level and Time Treatment of High Hydrostatic Pressure (HHP) on Inulin Gelation and Properties of Obtained Hydrogels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
Formation of Inulin Gels
2.2. Methods
2.2.1. Volumetric Gel Index (VGI)
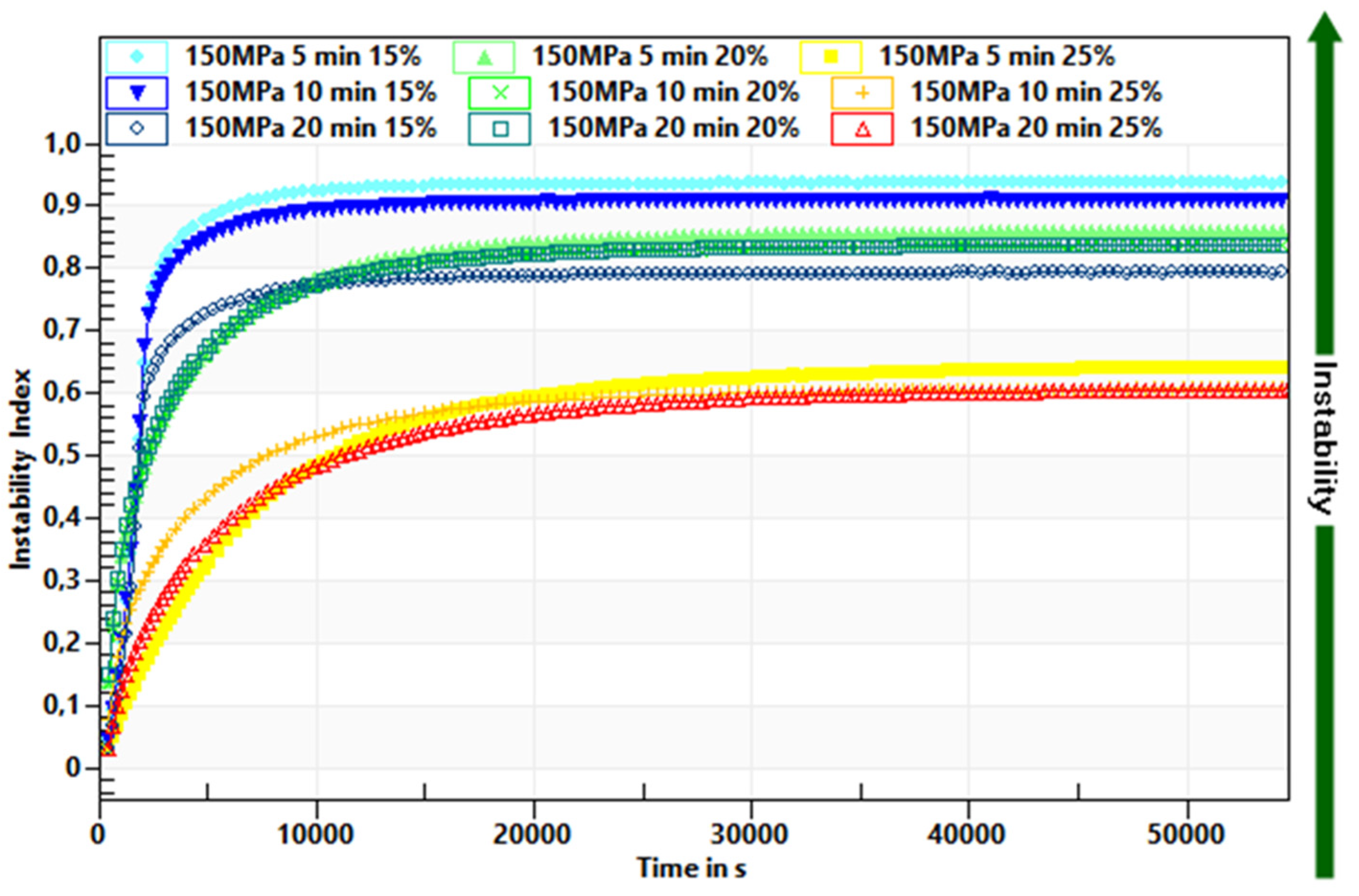
2.2.2. Determination of Physical Stability
2.2.3. Microstructure
2.2.4. Yield Stress
2.2.5. Textural Properties
2.2.6. Color Parameters
2.2.7. Statistical Analysis
3. Results and Discussion
3.1. The Influence of HHP Level and Time Treatment on Formation and Stability of Inulin Hydrogels
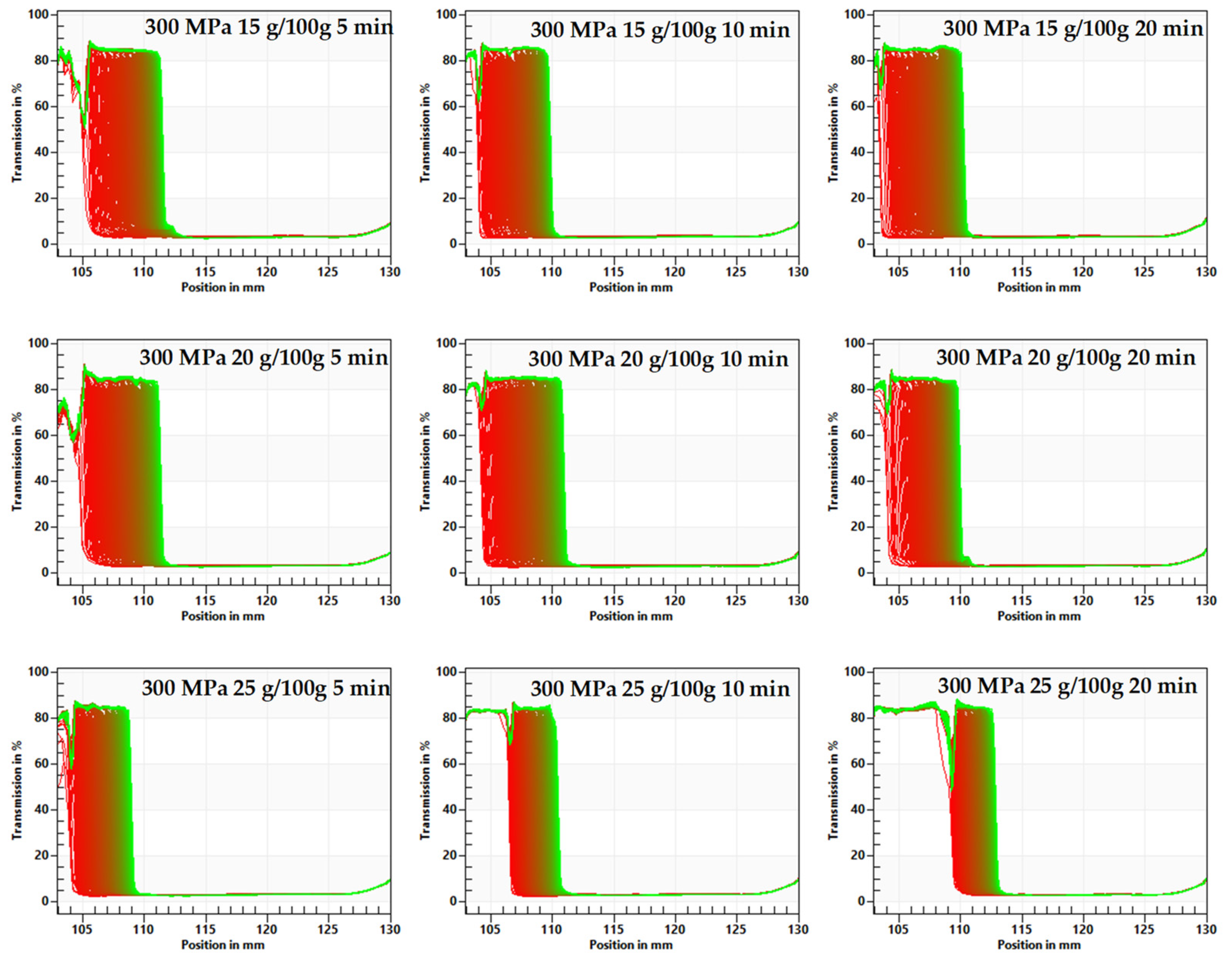
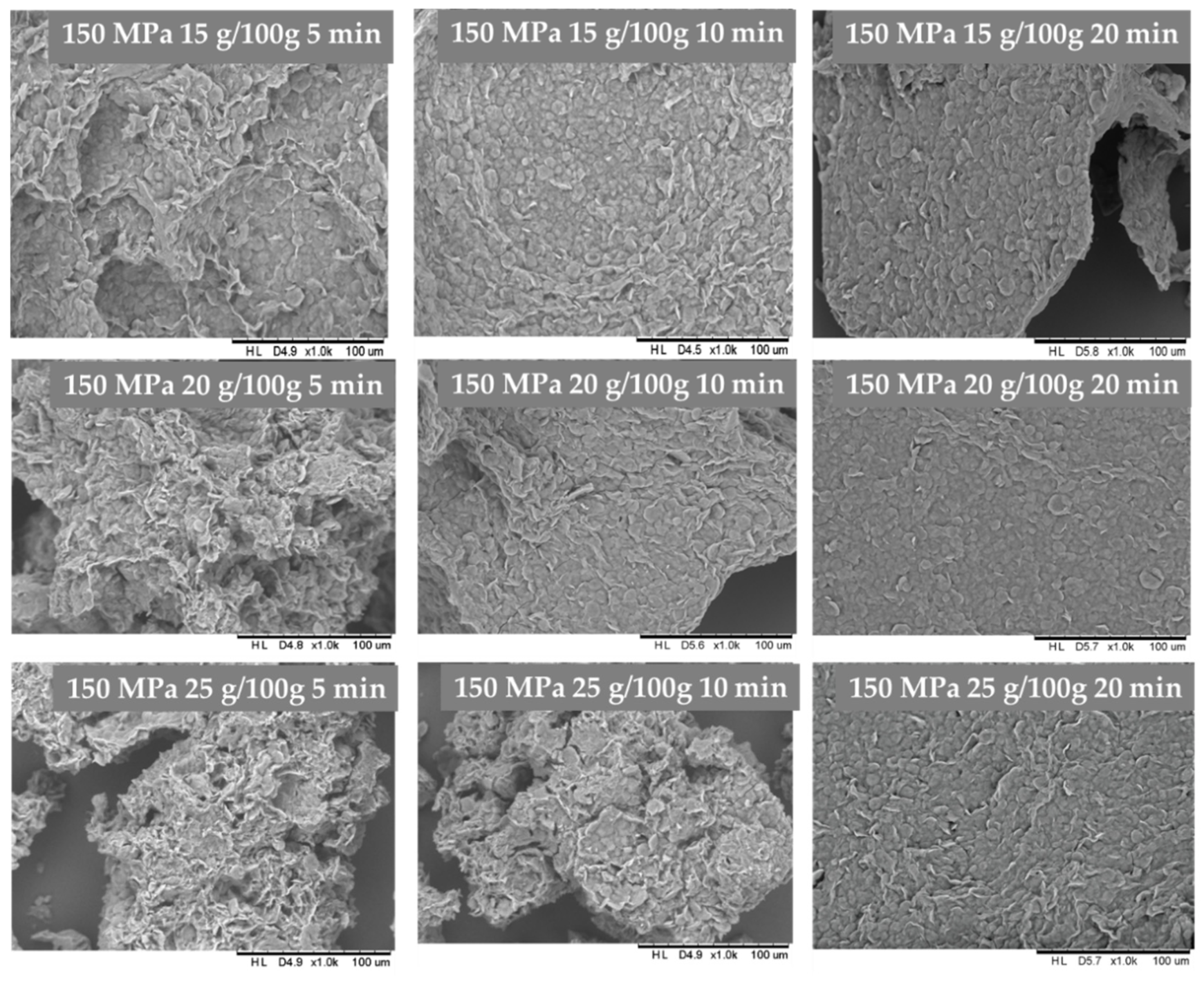
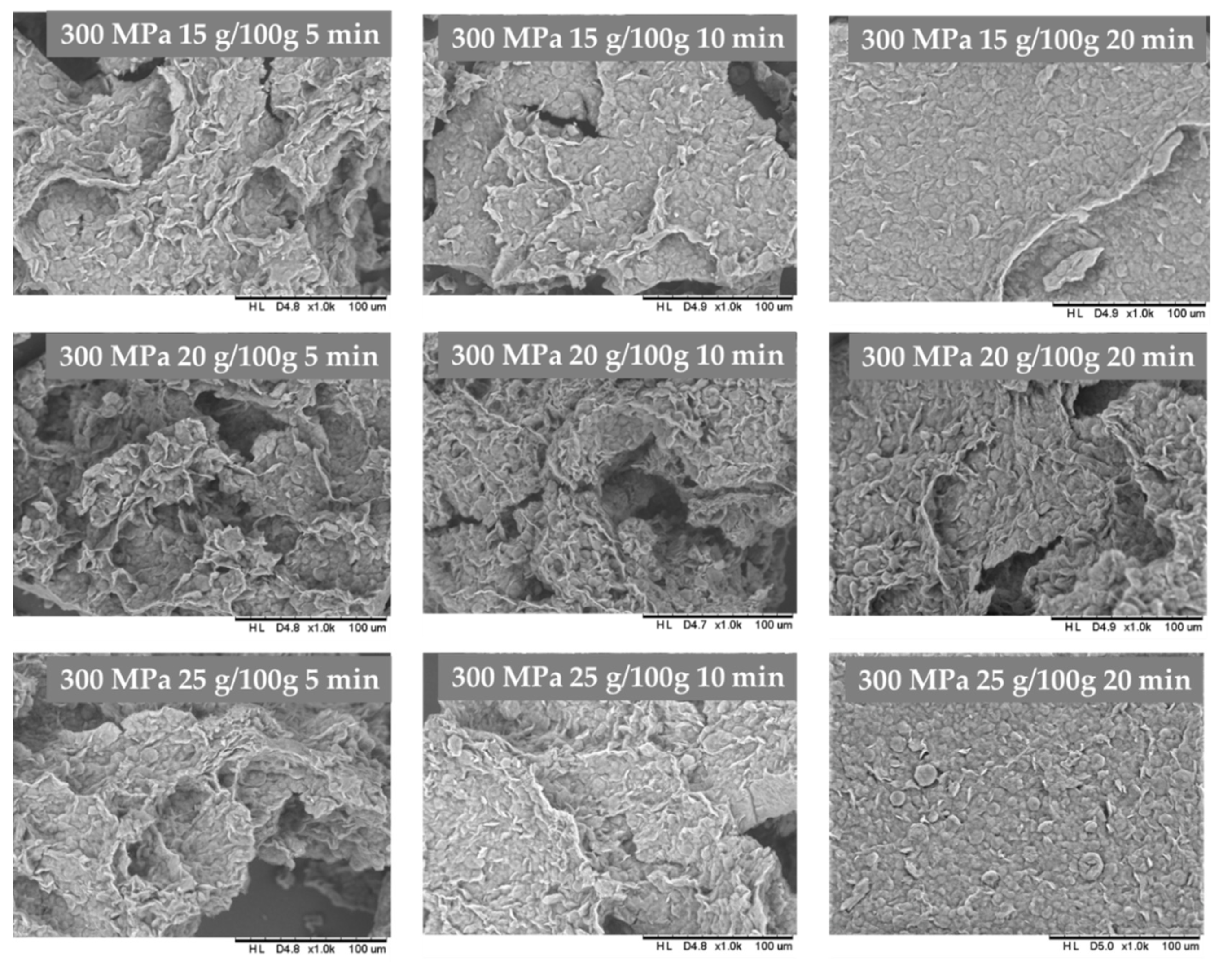
3.2. The Influence of HHP Level and Time Treatment on Microstructure of Inulin Hydrogels
3.3. The Influence of HHP Level and Time Treatment on Yield Stress and Textural Properties of Inulin Hydrogels
3.4. The Influence of HHP Level and Time Treatment on Colour Parameters of Inulin Hydrogels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Mahinroosta, M.; Farsangi, Z.J.; Allahverdi, A.; Shakoori, Z. Hydrogels as intelligent materials: A brief of synthesis, properties and applications. Mater. Today Chem. 2018, 8, 42–45. [Google Scholar] [CrossRef]
- Hennink, W.E.; Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 13–36. [Google Scholar] [CrossRef]
- Barbucci, R.; Leone, G.; Vecchiullo, A. Novel carboxymethyl cellulose based microporous hydrogels suitable for drug delivery. J. Biomater. Sci. Polym. Ed. 2004, 15, 607–619. [Google Scholar] [CrossRef]
- Said, H.M.; Alla, S.G.A.; El-Naggar, A.W.M. Synthesis and characterization of novel gels based on carboxymethyl cellulose/acrylic acid prepared by electron beam irradiation. React. Funct. Polym. 2004, 61, 397–404. [Google Scholar] [CrossRef]
- Fei, B.; Wach, R.A.; Mitomo, H.; Yoshii, F.; Kume, T. Hydrogel of biodegradable cellulose derivatives. I. Radiation induced crosslinking of CMC. J. Appl. Polym. Sci. 2000, 78, 278–283. [Google Scholar] [CrossRef]
- Funami, T.; Hiroe, M.; Noda, S.; Asai, I.; Ikeda, S.; Nishimari, K.; Ikeda, S.; Nishimari, K. Influence of molecular structure imaged with atomic force microscopy on the rheological behavior of carrageenan aqueous systems in the presence or absence of cations. Food Hydrocoll. 2007, 21, 617–629. [Google Scholar] [CrossRef]
- Zhao, Q.S.; Ji, Q.X.; Xing, K.; Li, X.Y.; Liu, C.S.; Chen, X.G. Preparation and characteristics of novel porous hydrogel films based on chitosan and glycerophosphate. Carbohydr. Polym. 2009, 76, 410–416. [Google Scholar] [CrossRef]
- Magnin, D.; Lefebvre, J.; Chornet, E.; Dumitriu, S. Physicochemical and structural characterization of a polyionic matrix of interest in biotechnology, in the pharmaceutical and biomedical fields. Carbohydr. Polym. 2004, 55, 437–453. [Google Scholar] [CrossRef]
- Ibrahim, S.M.; El Salmawi, K.M. Preparation and Properties of Carboxymethyl Cellulose (CMC)/Sodium alginate (SA) Blends Induced by Gamma Irradiation. J. Polym. Environ. 2013, 21, 520–527. [Google Scholar] [CrossRef]
- Williams, P.A.; Phillips, G.O. Physicochemical characterisation of gum Arabic arabinogalactan protein complex. Foods Food Ingred. J. Jpn. 2006, 211, 181–188. [Google Scholar]
- Giannouli, P.; Morris, E.R. Cryogelation of xanthan. Food Hydrocoll. 2003, 17, 495–501. [Google Scholar] [CrossRef]
- Balny, C. High pressure and protein oligomeric dissociation. High Press. Res. 2002, 22, 737–741. [Google Scholar] [CrossRef]
- Rahman, S.M. Food preservation methods. In Handbook of Food Preservation; Rahman, S.M., Ed.; CRC Press: London, UK; Taylor and Francis Group: Boca Raton, FL, USA, 2007; pp. 8–10. [Google Scholar]
- Kazutaka, Y. Food processing by high hydrostatic pressure. Biosci. Biotechnol. Biochem. 2017, 81, 672–679. [Google Scholar] [CrossRef] [Green Version]
- Considine, K.M.; Kelly, A.L.; Fitzgerald, G.F.; Hill, C.; Sleator, R.D. High-pressure processing effects on microbial food safety and food quality. FEMS Microbiol. Lett. 2008, 281, 1–9. [Google Scholar] [CrossRef]
- Bauer, B.A.; Knorr, D. The impact of pressure, temperature and treatment time on starches: Pressure-induced starch gelatinisation as pressure time temperature indicator for high hydrostatic pressure processing. J. Food Eng. 2005, 68, 329–334. [Google Scholar] [CrossRef]
- Colussi, R.; Kaur, L.; Zavareze, E.R.; Dias, A.R.G.; Stewart, R.B.; Singh, J. High pressure processing and retrogradation of potato starch: Influence on functional properties and gastro-small intestinal digestion in vitro. Food Hydrocoll. 2018, 75, 131–137. [Google Scholar] [CrossRef]
- Moreno, H.M.; Herranz, B.; Borderías, A.J.; Tovar, C.A. Effect of high pressure treatment on the structural, mechanical and rheological properties of glucomannan gels. Food Hydrocoll. 2016, 60, 437–444. [Google Scholar] [CrossRef]
- Steyer, B.; Bera, F.; Massaux, M.; Sindic, M.; Blecker, C.; Deroanne, C. Carrageenan Gelification Under High Hydrostatic Pressure: Preparation and Processing of Solutions. In Advances in High Pressure Bioscience and Biotechnology; Ludwig, H., Ed.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 353–356. [Google Scholar] [CrossRef]
- Ismail, H.; Irani, M.; Ahmad, Z. Starch-based hydrogels: Present status and applications. Int. J. Polym. Mater. Polym. Biomater. 2013, 62, 411–420. [Google Scholar] [CrossRef]
- Afinjuomo, F.; Barclay, T.G.; Song, Y.; Parikh, A.; Petrovsky, N. Synthesis and characterization of a novel inulin hydrogel crosslinked with pyromellitic dianhydride. React. Funct. Polym. 2019, 134, 104–111. [Google Scholar] [CrossRef]
- Florowska, A.; Krygier, K.; Florowski, T.; Dłuzewska, E. Prebiotics as functional food ingredients preventing diet-related diseases. Food Funct. 2016, 7, 2147–2155. [Google Scholar] [CrossRef] [PubMed]
- Florowska, A.; Florowski, T.; Sokołowska, B.; Janowicz, M.; Adamczak, L.; Pietrzak, D. Effect of high hydrostatic pressure on formation and rheological properties of inulin gels. LWT Food Sci. Technol. 2020, 121, 108995. [Google Scholar] [CrossRef]
- Kim, Y.; Faqih, M.N.; Wang, S.S. Factors affecting gel formation of inulin. Carbohydrate Polym. 2001, 46, 135–145. [Google Scholar] [CrossRef]
- Lerche, D.; Sobisch, T. Direct and accelerated characterization of formulation stability. J. Dispers. Sci. Technol. 2011, 32, 1799–1811. [Google Scholar] [CrossRef]
- Mokrzycki, W.; Tatol, M. Colour difference ∆E—A survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- Gehrich, K.; Wouters, R.; Zielbauer, B.; Beccard, S.; Mezger, M.; Bernard, J.; Vilgis, T.A. Alteration of the structural properties of inulin gels. Food Hydrocoll. 2018, 89, 302–310. [Google Scholar] [CrossRef]
- Glibowski, P.; Pikus, S.; Jurek, J.; Kotowoda, M. Factors affecting inulin crystallization after its complete dissolution. Carbohydr. Polym. 2014, 110, 107–112. [Google Scholar] [CrossRef]
- Glibowski, P. Effect of thermal and mechanical factors on rheological properties of high performance inulin gels and spreads. J. Food Eng. 2010, 99, 106–113. [Google Scholar] [CrossRef]
- Heinz, V.; Buckow, R. Food preservation by high pressure. J. Verbrauch. Lebensm. 2009, 5, 73–81. [Google Scholar] [CrossRef]
- Huang, H.W.; Lung, H.M.; Binghuei, B.Y.; Wang, C.Y. Responses of microorganisms to high hydrostatic pressure processing. Food Control 2014, 40, 250–259. [Google Scholar] [CrossRef]
- Huang, H.-W.; Hsu, C.P.; Wang, C.Y. Healthy expectations of high hydrostatic pressure treatment in food processing industry. J. Food Drug Anal. 2019, 28, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Ramaswamy, H.S.; Hiremath, N. The effect of high pressure treatment on rheological characteristics and colour of mango pulp. Int. J. Food Sci. Technol. 2005, 40, 885–895. [Google Scholar] [CrossRef]
- Li, G.; Zhu, F. Effect of high pressure on rheological and thermal properties of quinoa and maize starches. Food Chem. 2018, 241, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Jolie, R.P.; Christiaens, S.; De Roeck, A.; Fraeye, I.; Houben, K.; Van Buggenhout, S.; Van Loey, A.M.; Hendrickx, M.E. Pectin conversions under high pressure: Implications for the quality characteristics of plant-based foods. Trends Food Sci. Technol. 2012, 24, 103–118. [Google Scholar] [CrossRef]
- Opazo-Navarrete, M.; Tabilo-Munizaga, G.; Vega-Gálvez, A.; Miranda, M.; Pérez-Won, M. Effects of high hydrostatic pressure (HHP ) on the rheological properties of Aloe vera suspensions (Aloe barbadensis Miller ). Innov. Food Sci. Emerg. Technol. 2012, 16, 243–250. [Google Scholar] [CrossRef]
- Lopez-Sánchez, P.; Svelander, C.; Bialek, L.; Schumm, S.; Langton, M. Rheology and microstructure of carrot and tomato emulsions as a result of high-pressure homogenization conditions. J. Food Sci. 2011, 76, 130–140. [Google Scholar] [CrossRef]
- Li, W.; Bai, Y.; Mousaa, S.A.S.; Zhang, Q.; Shen, Q. Effect of High Hydrostatic Pressure on Physicochemical and Structural Properties of Rice Starch. Food Bioprocess Technol. 2012, 5, 2233–2241. [Google Scholar] [CrossRef]
- Gonçalves, S.M.; Chavez, D.W.H.; Oliveira LM de Sarantopoulos CIG de, L.; de Carvalho, C.W.P.; de Melo, N.R.; Rosenthal, A. Effects of high hydrostatic pressure processing on structure and functional properties of biodegradable film. Heliyon 2020, 6, e05213. [Google Scholar] [CrossRef]
- Daubert, C.R.; Tkachunk, J.A.; Truong, V.D. Quantitative measurement of food spreadability using the vane method. J. Texture Stud. 1998, 29, 427–435. [Google Scholar] [CrossRef]
- Ghaderi, S.; Hesarinejad, A.M.; Shekarforoush, E.; Mirzababaee, M.S.; Karimpour, F. Effects of high hydrostatic pressure on the rheological properties and foams / emulsions stability of Alyssum homolocarpum seed gum. Food Sci. Nutr. 2020, 6, 5571–5579. [Google Scholar] [CrossRef]
- Bourne, M. Food Texture and Viscosity: Concept and Measurement, 2nd ed.; Academic Press: San Diego, CA, USA, 2002; pp. 427–456. ISBN 0-12-119062-5. [Google Scholar]
- Larrea-Wachtendorff, D.; Sousa, I.; Ferrari, G. Starch-Based Hydrogels Produced by High-Pressure Processing (HPP): Effect of the Starch Source and Processing Time. Food Eng. Rev. 2021, 13, 622–633. [Google Scholar] [CrossRef]
- Knorr, D.; Heinz, V.; Buckow, R. High pressure application for food biopolymers. Biochim. Biophys. Acta Proteins Proteom. 2006, 1764, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Pei-Ling, L.; Xiao-Song, H.; Qun, S. Effect of high hydrostatic pressure on starches: A review. Starch-Stärke 2010, 62, 615–628. [Google Scholar] [CrossRef]
- Vega-Gálvez, A.; Giovagnoli, C.; Pérez-Won, M.; Reyes, J.E.; Vergara, J.; Miranda, M.; Di Scala, K. Application of high hydrostatic pressure to aloe vera (Aloe barbadensis Miller) gel: Microbial inactivation and evaluation of quality parameters. Innov. Food Sci. Emerg. Technol. 2012, 13, 57–63. [Google Scholar] [CrossRef]
- Tabilo-Munizaga, G.; Barbosa-Cánovas, G.V. Color and textural parameters of pressurized and heat-treated surimi gels as affected by potato starch and egg white. Food Res. Int. 2004, 37, 767–775. [Google Scholar] [CrossRef]
- Kim, D.; Fan, J.P.; Chung, H.C.; Han, G.D. Changes in Extractability and Antioxidant Activity of Jerusalem Artichoke (Helianthus tuberosus L.) Tubers by Various High Hydrostatic Pressure Treatments. Food Sci. Biotechnol. 2010, 19, 1365–1371. [Google Scholar] [CrossRef]



| Inulin Concentration | HHP Parameters | VGI [%] | Yield Stress [Pa] | Spreadability [N*s] | Firmness [N] | Adhesiveness [N*s] |
|---|---|---|---|---|---|---|
| 15 g/100 g | 150 MPa/5 min | 100 aA | 0 aA ± 0 | 0.1 aA ± 0.0 | 0.0 aA ± 0.0 | 0.0 bC ± 0.0 |
| 150 MPa/10 min | 100 aA | 0 aA ± 0 | 0.1 aA ± 0.0 | 0.1 aA ± 0.0 | 0.0 bC ± 0.0 | |
| 150 MPa/20 min | 100 aA | 0 aA ± 0 | 0.2 aA ± 0.0 | 0.1 aA ± 0.0 | 0.0 bC ± 0.0 | |
| 300 MPa/5 min | 100 aA | 367 bA ± 24 | 2.4 bA ± 0.0 | 0.8 bA ± 0.1 | −0.3 aC ± 0.1 | |
| 300 MPa/10 min | 100 aA | 434 cA ± 12 | 2.4 bA ± 0.1 | 0.9 bA ± 0.3 | −0.3 aC ± 0.1 | |
| 300 MPa/20 min | 100 aA | 598 dA ± 10 | 2.7 cA ± 0.1 | 1.0 bA ± 0.1 | −0.3 aC ± 0.0 | |
| 20 g/100 g | 150 MPa/5 min | 100 aA | 0 aA ± 0 | 0.6 aB ± 0.0 | 0.1 aA ± 0.0 | −0.1 cB ± 0.0 |
| 150 MPa/10 min | 100 aA | 38 bB ± 1 | 0.6 aB ± 0.0 | 0.1 aAB ± 0.0 | −0.1 cB ± 0.0 | |
| 150 MPa/20 min | 100 aA | 28 bB ± 1 | 0.6 aB ± 0.0 | 0.1 aA ± 0.0 | −0.1 cB ± 0.0 | |
| 300 MPa/5 min | 100 aA | 759 dB ± 4 | 4.2 bB ± 0.1 | 1.2 bB ± 0.0 | −0.6 bB ± 0.0 | |
| 300 MPa/10 min | 100 aA | 712 cB ± 7 | 4.6 cB ± 0.0 | 1.5 cB ± 0.0 | −0.6 aB ± 0.0 | |
| 300 MPa/20 min | 100 aA | 875 eB ± 5 | 4.8 dB ± 0.0 | 1.5 cA ± 0.0 | −0.7 aB ± 0.0 | |
| 25 g/100 g | 150 MPa/5 min | 100 aA | 41 aB ± 4 | 1.2 aC ± 0.1 | 0.3 aB ± 0.1 | −0.1 dA ± 0.0 |
| 150 MPa/10 min | 100 aA | 68 aC ± 3 | 1.5 bC ± 0.1 | 0.2 aB ± 0.1 | −0.1 dA ± 0.0 | |
| 150 MPa/20 min | 100 aA | 65 aC ± 2 | 1.6 cC ± 0.0 | 0.2 aB ± 0.0 | −0.1 dA ± 0.0 | |
| 300 MPa/5 min | 100 aA | 775 bB ± 7 | 6.3 dC ± 0.1 | 1.9 bC ± 0.1 | −0.9 cA ± 0.0 | |
| 300 MPa/10 min | 100 aA | 932 cC ± 4 | 8.0 eC ± 0.0 | 2.1 bC ± 0.1 | −1.0 bA ± 0.0 | |
| 300 MPa/20 min | 100 aA | 1274 dC ± 50 | 8.4 fC ± 0.0 | 3.0 cB ± 0.6 | −1.3 aA ± 0.0 |
| Inulin Concentration | HHP Parameters | L* | a* | b* |
|---|---|---|---|---|
| 15 g/100 g | 150 MPa/5 min | 85.8 bA ± 1.5 | −1.5 bA ± 0.2 | 0.9 cdA ± 0.0 |
| 150 MPa/10 min | 84.0 abA ± 0.1 | −1.6 bA ± 0.1 | 1.1 dA ± 0.0 | |
| 150 MPa/20 min | 83.3 aA ± 1.0 | −1.7 abA ± 0.1 | 0.7 cA ± 0.1 | |
| 300 MPa/5 min | 85.3 abA ± 1.1 | −1.8 abA ± 0.1 | 0.4 bA ± 0.0 | |
| 300 MPa/10 min | 85.8 bA ± 0.2 | −1.9 aA ± 0.0 | 0.0 aA ± 0.0 | |
| 300 MPa/20 min | 85.1 abA ± 0.4 | −1.8 abA ± 0.1 | 0.0 aA ± 0.2 | |
| 20 g/100 g | 150 MPa/5 min | 87.5 abA ± 1.5 | −1.4 dA ± 0.0 | 0.9 bA ± 0.0 |
| 150 MPa/10 min | 87.1 abB ± 0.2 | −1.5 bcA ± 0.0 | 1.6 dB ± 0.1 | |
| 150 MPa/20 min | 85.5 aA ± 1.5 | −1.6 bB ± 0.0 | 1.2 cB ± 0.1 | |
| 300 MPa/5 min | 86.3 abAB ± 0.1 | −1.7 aAB ± 0.0 | 0.3 aA ± 0.0 | |
| 300 MPa/10 min | 88.13 bC ± 0.1 | −1.4 cdC ± 0.0 | 0.9 bB ± 0.0 | |
| 300 MPa/20 min | 86.3 abB ± 0.1 | −1.7 aB ± 0.0 | 0.3 aB ± 0.0 | |
| 25 g/100 g | 150 MPa/5 min | 87.8 aA ± 0.3 | −1.4 cdA ± 0.0 | 2.7 bcB ± 0.1 |
| 150 MPa/10 min | 87.8 aB ± 1.5 | −1.3 eB ± 0.0 | 3.1 dC ± 0.2 | |
| 150 MPa/20 min | 88.4 aB ± 0.6 | −1.4 cA ± 0.0 | 2.7 cC ± 0.1 | |
| 300 MPa/5 min | 87.0 aB ± 0.0 | −1.6 bB ± 0.0 | 2.3 abB ± 0.1 | |
| 300 MPa/10 min | 86.8 aB ± 0.1 | −1.8 aB ± 0.0 | 2.1 aC ± 0.1 | |
| 300 MPa/20 min | 87.2 aC ± 0.1 | −1.3 deC ± 0.0 | 2.3 aC ± 0.0 |
| Inulin Concentration and HHP Parameters | Inulin 25 g/100 g | Inulin 20 g/100 g | Inulin 15 g/100 g | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 300 MPa | 150 MPa | 300 MPa | 150 MPa | 300 MPa | 150 MPa | |||||||||||||||
| 20 min | 10 min | 5 min | 20 min | 10 min | 5 min | 20 min | 10 min | 5 min | 20 min | 10 min | 5 min | 20 min | 10 min | 5 min | 20 min | 10 min | 5 min | |||
| inulin 15 g/100 g | 150 MPa | 5 min | 2.11 | 1.81 | 2.08 | 3.19 | 3.41 | 2.75 | 1.36 | 2.29 | 1.32 | 0.96 | 1.54 | 1.64 | 2.00 | 1.68 | 1.73 | 2.61 | 1.91 | - |
| 10 min | 3.49 | 2.96 | 3.27 | 4.75 | 4.37 | 4.18 | 2.50 | 4.18 | 2.45 | 1.60 | 3.17 | 3.56 | 1.71 | 2.18 | 1.69 | 1.00 | - | |||
| 20 min | 4.32 | 3.77 | 4.11 | 5.58 | 5.15 | 5.02 | 3.07 | 4.88 | 3.03 | 2.31 | 3.95 | 4.25 | 2.05 | 2.61 | 2.09 | - | ||||
| 300 MPa | 5 min | 2.80 | 2.32 | 2.69 | 3.98 | 3.73 | 3.52 | 1.04 | 2.89 | 0.97 | 1.41 | 2.25 | 2.40 | 0.96 | 1.07 | - | ||||
| 10 min | 2.83 | 2.35 | 2.70 | 3.90 | 3.97 | 3.45 | 0.71 | 2.59 | 0.67 | 1.90 | 2.14 | 2.21 | 0.65 | - | ||||||
| 20 min | 3.22 | 2.69 | 3.06 | 4.37 | 4.26 | 3.88 | 1.26 | 3.16 | 1.23 | 1.95 | 2.59 | 2.76 | - | |||||||
| inulin 20 g/100 g | 150 MPa | 5 min | 1.80 | 1.86 | 1.92 | 2.16 | 3.19 | 2.13 | 1.64 | 1.31 | 1.63 | 2.07 | 1.37 | - | ||||||
| 10 min | 0.78 | 0.68 | 0.80 | 1.79 | 2.11 | 1.33 | 1.51 | 1.25 | 1.53 | 1.81 | - | |||||||||
| 20 min | 2.20 | 1.82 | 2.12 | 3.35 | 3.02 | 2.90 | 1.61 | 2.62 | 1.55 | - | ||||||||||
| 300 MPa | 5 min | 2.24 | 1.82 | 2.16 | 3.28 | 3.36 | 2.86 | 0.10 | 1.97 | - | ||||||||||
| 10 min | 1.68 | 1.85 | 1.85 | 1.95 | 2.57 | 1.81 | 1.93 | - | ||||||||||||
| 20 min | 2.22 | 1.82 | 2.15 | 3.26 | 3.34 | 2.84 | - | |||||||||||||
| Inulin 25 g/100 g | 150 MPa | 5 min | 0.74 | 1.30 | 0.94 | 0.59 | 1.40 | - | ||||||||||||
| 10 min | 1.57 | 1.86 | 1.65 | 1.54 | - | |||||||||||||||
| 20 min | 1.28 | 1.84 | 1.50 | - | ||||||||||||||||
| 300 MPa | 5 min | 0.39 | 0.41 | - | ||||||||||||||||
| 10 min | 0.71 | - | ||||||||||||||||||
| 20 min | - | |||||||||||||||||||
| Values are mean (n = 3). | ||||||||||||||||||||
| 0 < ΔE < 1 | 1 < ΔE < 2 | 2< ΔE < 3.5 | 3.5 < ΔE < 5 | 5 < ΔE | ||||||||||||||||
| observer does not notice the difference | only experienced observer can notice the difference | unexperienced observer also notices the difference | clear difference in color is noticed | observer notices two different colors | ||||||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Florowska, A.; Florowski, T.; Sokołowska, B.; Adamczak, L.; Szymańska, I. Effects of Pressure Level and Time Treatment of High Hydrostatic Pressure (HHP) on Inulin Gelation and Properties of Obtained Hydrogels. Foods 2021, 10, 2514. https://doi.org/10.3390/foods10112514
Florowska A, Florowski T, Sokołowska B, Adamczak L, Szymańska I. Effects of Pressure Level and Time Treatment of High Hydrostatic Pressure (HHP) on Inulin Gelation and Properties of Obtained Hydrogels. Foods. 2021; 10(11):2514. https://doi.org/10.3390/foods10112514
Chicago/Turabian StyleFlorowska, Anna, Tomasz Florowski, Barbara Sokołowska, Lech Adamczak, and Iwona Szymańska. 2021. "Effects of Pressure Level and Time Treatment of High Hydrostatic Pressure (HHP) on Inulin Gelation and Properties of Obtained Hydrogels" Foods 10, no. 11: 2514. https://doi.org/10.3390/foods10112514







