Developing Soybean Protein Gel-Based Foods from Okara Using the Wet-Type Grinder Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of WG-Treated Okara
2.3. Viscosity of WG-Treated Okara
2.4. Particle Size Distribution (PSD) of WG-Treated Okara
2.5. Preparation of SPI Gel
2.6. Compression Measurements of SPI Gels Containing WG-Treated Okara
2.7. Water Holding Capacity (WHC) of SPI Gels Containing WG-Treated Okara
2.8. Statistical Analyses
3. Results
3.1. PSD, Viscosity, and Dispersion Ability of WG-Treated Okara
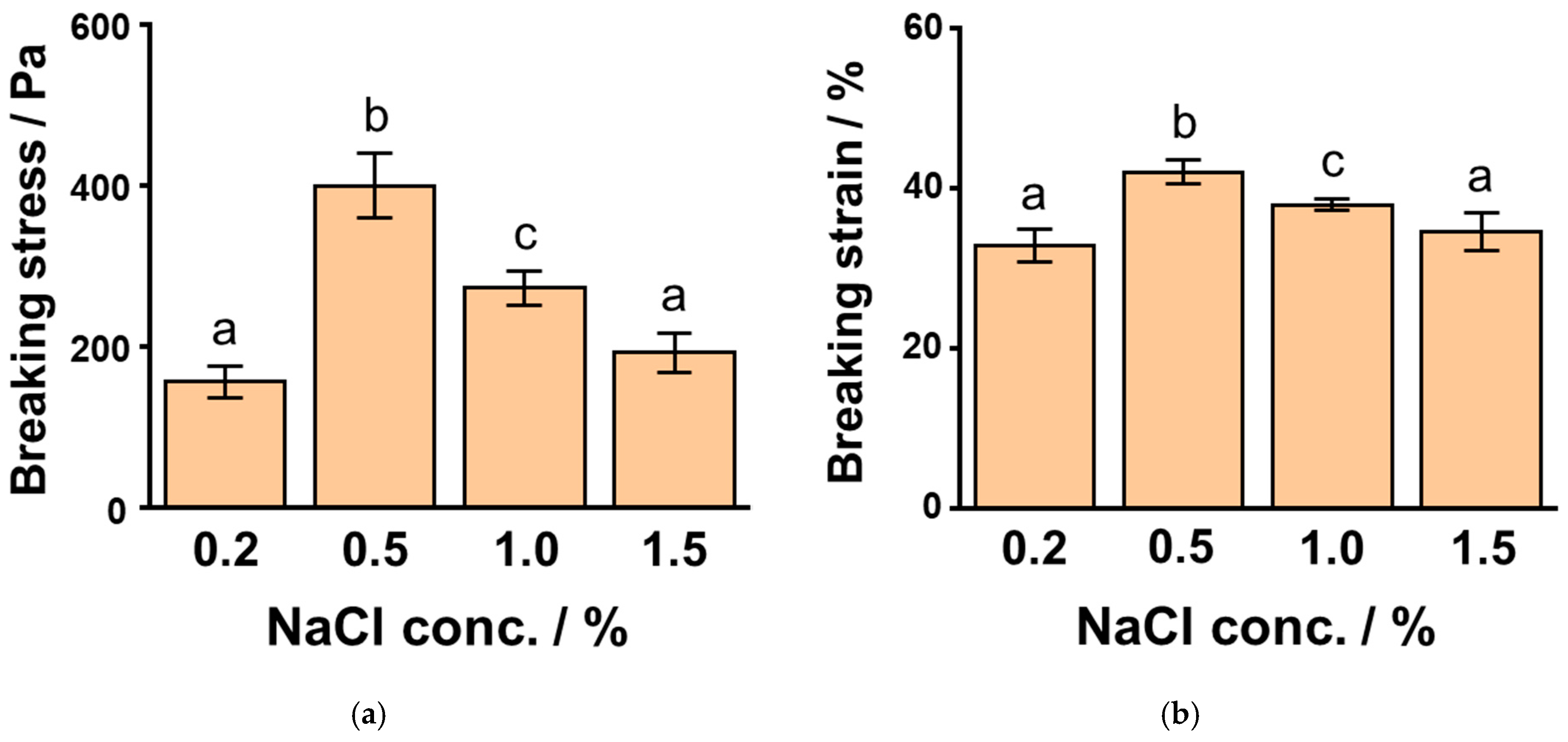
3.2. Effects of NaCl Concentrations on SPI Gels
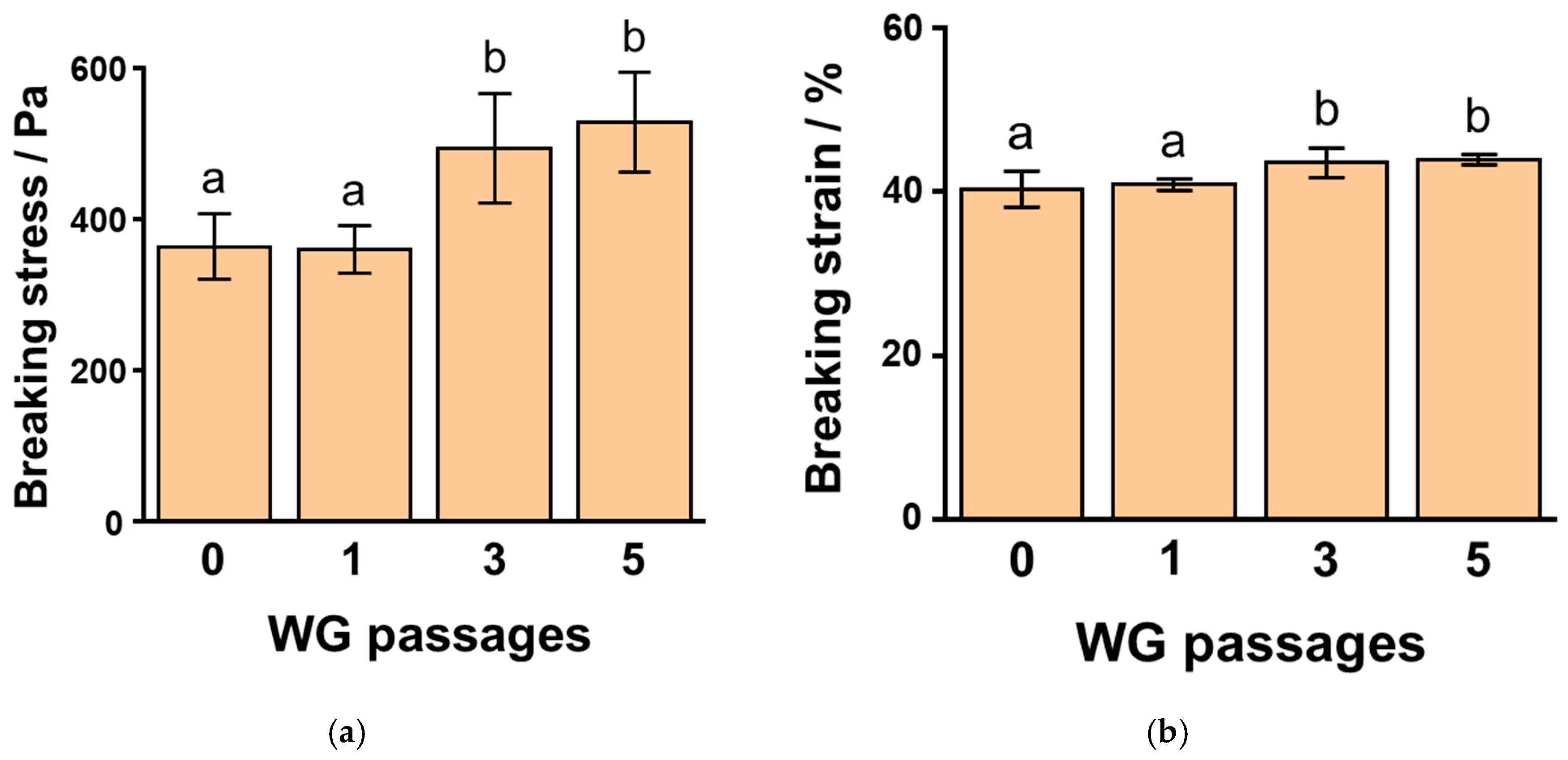
3.3. Effects of WG-Treated Okara, with Varying Passages, on SPI Gels
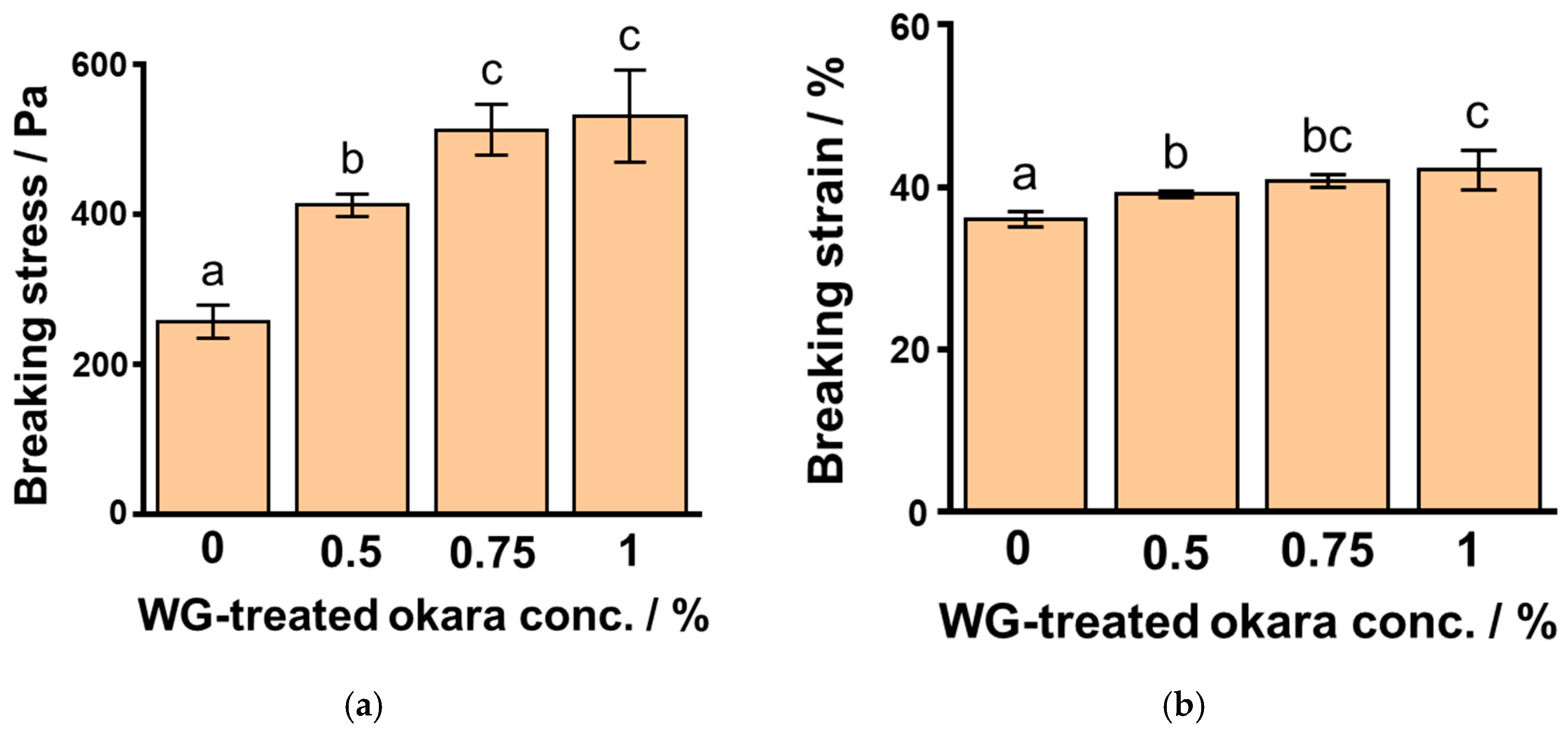
3.4. Effects of WG-Treated Okara Concentration on SPI Gels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nagano, T.; Arai, Y.; Yano, H.; Aoki, T.; Kurihara, S.; Hirano, R.; Nishinari, K. Improved physicochemical and functional properties of okara, a soybean residue, by nanocellulose technologies for food development—A review. Food Hydrocoll. 2020, 109, 105964. [Google Scholar] [CrossRef]
- O’Toole, D.K. Characteristics and use of okara, the soybean residue from soy milk production—A review. J. Agric. Food Chem. 1999, 47, 363–371. [Google Scholar] [CrossRef]
- Li, S.; Zhu, D.; Li, K.; Yang, Y.; Lei, Z.; Zhang, Z. Soybean curd residue: Composition, utilization, and related limiting factors. ISRN Ind. Eng. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Huang, S.; He, Y.; Zou, Y.; Liu, Z. Modification of insoluble dietary fibres in soya bean okara and their physicochemical properties. Int. J. Food Sci. Technol. 2015, 50, 2606–2613. [Google Scholar] [CrossRef]
- Pérez-López, E.; Mateos-Aparicio, I.; Rupérez, P. High hydrostatic pressure aided by food-grade enzymes as a novel approach for Okara valorization. Innov. Food Sci. Emerg. Technol. 2017, 42, 197–203. [Google Scholar] [CrossRef]
- Ullah, I.; Yin, T.; Xiong, S.; Zhang, J.; Din, Z.-U.; Zhang, M. Structural characteristics and physicochemical properties of okara (soybean residue) insoluble dietary fiber modified by high-energy wet media milling. LWT 2017, 82, 15–22. [Google Scholar] [CrossRef]
- Nagano, T.; Hirano, R.; Kurihara, S.; Nishinari, K. Improved effects of okara atomized by a water jet system on alpha-amylase inhibition and butyrate production by Roseburia intestinalis. Biosci. Biotechnol. Biochem. 2020, 84, 1467–1474. [Google Scholar] [CrossRef] [PubMed]
- Fayaz, G.; Plazzotta, S.; Calligaris, S.; Manzocco, L.; Nicoli, M.C. Impact of high pressure homogenization on physical properties, extraction yield and biopolymer structure of soybean okara. LWT 2019, 113, 108324. [Google Scholar] [CrossRef]
- Dai, B.; Huang, S.; Deng, Y. Modified insoluble dietary fibers in okara affect body composition, serum metabolic properties, and fatty acid profiles in mice fed high-fat diets: An NMR investigation. Food Res. Int. 2019, 116, 1239–1246. [Google Scholar] [CrossRef]
- Pérez-López, E.; Veses, A.M.; Redondo, N.; Tenorio-Sanz, M.D.; Villanueva, M.J.; Redondo-Cuenca, A.; Marcos, A.; Nova, E.; Mateos-Aparicio, I.; Rupérez, P. Soybean okara modulates gut microbiota in rats fed a high-fat diet. Bioact. Carbohydr. Diet. Fibre 2018, 16, 100–107. [Google Scholar] [CrossRef]
- Ullah, I.; Hu, Y.; You, J.; Yin, T.; Xiong, S.; Din, Z.-U.; Huang, Q.; Liu, R. Influence of okara dietary fiber with varying particle sizes on gelling properties, water state and microstructure of tofu gel. Food Hydrocoll. 2019, 89, 512–522. [Google Scholar] [CrossRef]
- Yin, T.; Yao, R.; Ullah, I.; Xiong, S.; Huang, Q.; You, J.; Hu, Y.; Shi, L. Effects of nanosized okara dietary fiber on gelation properties of silver carp surimi. LWT 2019, 111, 111–116. [Google Scholar] [CrossRef]
- Lu, H.; Gui, Y.; Zheng, L.; Liu, X. Morphological, crystalline, thermal and physicochemical properties of cellulose nanocrystals obtained from sweet potato residue. Food Res. Int. 2013, 50, 121–128. [Google Scholar] [CrossRef]
- García, A.; Gandini, A.; Labidi, J.; Belgacem, N.; Bras, J. Industrial and crop wastes: A new source for nanocellulose biorefinery. Ind. Crops Prod. 2016, 93, 26–38. [Google Scholar] [CrossRef]
- Costa, A.L.R.; Gomes, A.; Tibolla, H.; Menegalli, F.C.; Cunha, R.L. Cellulose nanofibers from banana peels as a Pickering emulsifier: High-energy emulsification processes. Carbohydr. Polym. 2018, 194, 122–131. [Google Scholar] [CrossRef]
- Watanabe, Y.; Kitamura, S.; Kawasaki, K.; Kato, T.; Uegaki, K.; Ogura, K.; Ishikawa, K. Application of a water jet system to the pretreatment of cellulose. Biopolymers 2011, 95, 833–839. [Google Scholar] [CrossRef]
- Nagano, T.; Yano, H. Dietary cellulose nanofiber modulates obesity and gut microbiota in high-fat-fed mice. Bioact. Carbohydr. Diet. Fibre 2020, 22, 100214. [Google Scholar] [CrossRef]
- Nagano, T.; Yano, H. Effect of dietary cellulose nanofiber and exercise on obesity and gut microbiota in mice fed a high-fat-diet. Biosci. Biotechnol. Biochem. 2020, 84, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Ifuku, S.; Yamada, K.; Morimoto, M.; Saimoto, H. Nanofibrillation of dry chitin powder by star burst system. J. Nanomater. 2012, 2012, 645624. [Google Scholar] [CrossRef] [Green Version]
- Dutta, A.K.; Kawamoto, N.; Sugino, G.; Izawa, H.; Morimoto, M.; Saimoto, H.; Ifuku, S. Simple preparation of chitosan nanofibers from dry chitosan powder by the Star Burst system. Carbohydr. Polym. 2013, 97, 363–367. [Google Scholar] [CrossRef]
- Azuma, K.; Ifuku, S. Nanofibers based on chitin: A new functional food. Pure Appl. Chem. 2016, 88, 605–619. [Google Scholar] [CrossRef]
- Iwamoto, S.; Nakagaito, A.N.; Yano, H.; Nogi, M. Optically transparent composites reinforced with plant fiber-based nanofibers. Appl. Phys. A 2005, 81, 1109–1112. [Google Scholar] [CrossRef]
- Iwamoto, S.; Nakagaito, A.N.; Yano, H. Nano-fibrillation of pulp fibers for the processing of transparent nanocomposites. Appl. Phys. A 2007, 89, 461–466. [Google Scholar] [CrossRef]
- Nechyporchuk, O.; Belgacem, M.N.; Bras, J. Production of cellulose nanofibrils: A review of recent advances. Ind. Crops Prod. 2016, 93, 2–25. [Google Scholar] [CrossRef]
- Liu, H.H.; Chien, J.T.; Kuo, M.I. Ultra high pressure homogenized soy flour for tofu making. Food Hydrocoll. 2013, 32, 278–285. [Google Scholar] [CrossRef]
- Pojić, M.; Mišan, A.; Tiwari, B. Eco-innovative technologies for extraction of proteins for human consumption from renewable protein sources of plant origin. Trends Food Sci. Technol. 2018, 75, 93–104. [Google Scholar] [CrossRef]
- Jones, O.G. Recent advances in the functionality of non-animal-sourced proteins contributing to their use in meat analogs. Curr. Opin. Food Sci. 2016, 7, 7–13. [Google Scholar] [CrossRef]
- Geerts, M.E.J.; Dekkers, B.L.; van der Padt, A.; van der Goot, A.J. Aqueous fractionation processes of soy protein for fibrous structure formation. Innov. Food Sci. Emerg. Technol. 2018, 45, 313–319. [Google Scholar] [CrossRef]
- Nishinari, K.; Fang, Y.; Nagano, T.; Guo, S.; Wang, R. 6—Soy as a food ingredient. In Proteins in Food Processing, 2nd ed.; Yada, R.Y., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 149–186. [Google Scholar]
- Nagano, T.; Fukuda, Y.; Akasaka, T. Dynamic viscoelastic study on the gelation properties of β-conglycinin-rich and glycinin-rich soybean protein isolates. J. Agric. Food Chem. 1996, 44, 3484–3488. [Google Scholar] [CrossRef]
- Nagano, T. Contribution of disulfide bonding to viscoelastic properties and microstructures of 11S globulin gels from soybeans: Magnesium chloride-induced gels. Food Sci. Technol. Res. 2013, 19, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Peters, J.P.C.M.; Vergeldt, F.J.; Boom, R.M.; van der Goot, A.J. Water-binding capacity of protein-rich particles and their pellets. Food Hydrocoll. 2017, 65, 144–156. [Google Scholar] [CrossRef]
- Nagano, T.; Tokita, M. Viscoelastic properties and microstructures of 11S globulin and soybean protein isolate gels: Magnesium chloride-induced gels. Food Hydrocoll. 2011, 25, 1647–1654. [Google Scholar] [CrossRef]
- Desmond, E. Reducing salt: A challenge for the meat industry. Meat Sci. 2006, 74, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Cando, D.; Herranz, B.; Borderias, A.J.; Moreno, H.M. Different additives to enhance the gelation of surimi gel with reduced sodium content. Food Chem. 2016, 196, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Alakhrash, F.; Anyanwu, U.; Tahergorabi, R. Physicochemical properties of Alaska pollock (Theragra chalcograma) surimi gels with oat bran. LWT 2016, 66, 41–47. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, W.; Wang, H.; Ye, Q. Effects of a highly resistant rice starch and pre-incubation temperatures on the physicochemical properties of surimi gel from grass carp (Ctenopharyn Odon Idellus). Food Chem. 2014, 145, 212–219. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, G.; Zhang, W. Effects of regenerated cellulose fiber on the characteristics of myofibrillar protein gels. Carbohydr. Polym. 2019, 209, 276–281. [Google Scholar] [CrossRef]



| Untreated | One Passage | Three Passages | Five Passages | |
|---|---|---|---|---|
| Median size (μm) | 68.5 | 13.5 | 9.9 | 8.9 |
| Viscosity (mPas) | 10 ± 4 a | 40 ± 8 b | 70 ± 10 c | 120 ± 16 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arai, Y.; Nishinari, K.; Nagano, T. Developing Soybean Protein Gel-Based Foods from Okara Using the Wet-Type Grinder Method. Foods 2021, 10, 348. https://doi.org/10.3390/foods10020348
Arai Y, Nishinari K, Nagano T. Developing Soybean Protein Gel-Based Foods from Okara Using the Wet-Type Grinder Method. Foods. 2021; 10(2):348. https://doi.org/10.3390/foods10020348
Chicago/Turabian StyleArai, Yuya, Katsuyoshi Nishinari, and Takao Nagano. 2021. "Developing Soybean Protein Gel-Based Foods from Okara Using the Wet-Type Grinder Method" Foods 10, no. 2: 348. https://doi.org/10.3390/foods10020348






