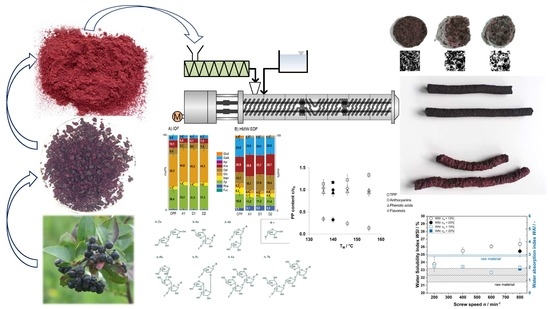
Figure 1.
Effect of screw speed (n) and water content (cw) on (A) specific mechanical energy (SME) and (B) the chokeberry pomace powder (CPP) material temperature (TM) at 100 °C barrel temperature (TB).
Figure 1.
Effect of screw speed (n) and water content (cw) on (A) specific mechanical energy (SME) and (B) the chokeberry pomace powder (CPP) material temperature (TM) at 100 °C barrel temperature (TB).
Figure 2.
Monosaccharide composition of (A) insoluble dietary fiber (IDF) and (B) high molecular weight soluble dietary fiber (HMW-SDF) from chokeberry pomace powder (CPP) and samples after extrusion processing at different water contents (cw) and specific mechnical energies (SME) applied resulting in different material temperatures (TM) (mol%, range/2, n = 2). (A1) SME 145 ± 6 Whkg−1, TM 136 °C; (D1) SME 222 ± 10 Whkg−1, TM 155 °C; (D2) SME 190 ± 9 Whkg−1, TM 140 °C. GlcA, d-glucuronic acid; GalA, d-galacturonic acid; Api, d-apiose; Ara, l-arabinose; Gal, d-galactose; Glc, d-glucose; Man, d-mannose; Rha, l-rhamnose; Xyl, d-xylose; Fuc, l-fucose.
Figure 2.
Monosaccharide composition of (A) insoluble dietary fiber (IDF) and (B) high molecular weight soluble dietary fiber (HMW-SDF) from chokeberry pomace powder (CPP) and samples after extrusion processing at different water contents (cw) and specific mechnical energies (SME) applied resulting in different material temperatures (TM) (mol%, range/2, n = 2). (A1) SME 145 ± 6 Whkg−1, TM 136 °C; (D1) SME 222 ± 10 Whkg−1, TM 155 °C; (D2) SME 190 ± 9 Whkg−1, TM 140 °C. GlcA, d-glucuronic acid; GalA, d-galacturonic acid; Api, d-apiose; Ara, l-arabinose; Gal, d-galactose; Glc, d-glucose; Man, d-mannose; Rha, l-rhamnose; Xyl, d-xylose; Fuc, l-fucose.
Figure 3.
Pectic arabinan oligosaccharides liberated by endo-arabinanase from dietary fiber fractions isolated from chokeberry pomace powder (CPP) before and after extrusion processing. T, terminal end; R, reducing end [
20].
Figure 3.
Pectic arabinan oligosaccharides liberated by endo-arabinanase from dietary fiber fractions isolated from chokeberry pomace powder (CPP) before and after extrusion processing. T, terminal end; R, reducing end [
20].
Figure 4.
Thermomechanical impact on polyphenol (PP) stability in chokeberry pomace powder (CPP) after extrusion processing (mean ± SD, n = 2). c0, based on CPP; TM, material temperature. Open symbols, water content (cw) 13% (A1, B1, D1). Filled symbols, cw 23% (D2). TPP, total PP.
Figure 4.
Thermomechanical impact on polyphenol (PP) stability in chokeberry pomace powder (CPP) after extrusion processing (mean ± SD, n = 2). c0, based on CPP; TM, material temperature. Open symbols, water content (cw) 13% (A1, B1, D1). Filled symbols, cw 23% (D2). TPP, total PP.
Figure 5.
Cross section and porosity of chokeberry pomace powder (CPP) extrudates after extrusion processing at different specific mechanical energies (SME) applied at different water contents (cw). (A1) SME 145 ± 6 Whkg−1, cw 13%; (D1) SME 222 ± 10 Whkg−1, cw 13%; (D2) SME 190 ± 9 Whkg−1, cw 23%.
Figure 5.
Cross section and porosity of chokeberry pomace powder (CPP) extrudates after extrusion processing at different specific mechanical energies (SME) applied at different water contents (cw). (A1) SME 145 ± 6 Whkg−1, cw 13%; (D1) SME 222 ± 10 Whkg−1, cw 13%; (D2) SME 190 ± 9 Whkg−1, cw 23%.
Table 1.
Experiments performed. Extrusion parameters (n, screw speed (min−1); TB, barrel temperature (°C); cw, water content (%); SME, specific mechanical energy (Whkg−1); TM, material temperature (°C); total mass flow for all trials was 10 kgh−1).
Table 1.
Experiments performed. Extrusion parameters (n, screw speed (min−1); TB, barrel temperature (°C); cw, water content (%); SME, specific mechanical energy (Whkg−1); TM, material temperature (°C); total mass flow for all trials was 10 kgh−1).
| Sample Name | n | TB | cw | SME | TM |
|---|
| A1 | 200 | 100 | 13 | 145 ± 6 | 136 |
| A2 | 200 | 100 | 23 | 122 ± 5 | 123 |
| B1 | 400 | 100 | 13 | 174 ± 4 | 146 |
| B2 | 400 | 100 | 23 | 155 ± 3 | 134 |
| C1 | 600 | 100 | 13 | 195 ± 10 | 152 |
| C2 | 600 | 100 | 23 | 180 ± 7 | 139 |
| D1 | 800 | 100 | 13 | 222 ± 10 | 155 |
| D2 | 800 | 100 | 23 | 190 ± 9 | 140 |
Table 2.
Dietary fiber (DF) contents of chokeberry pomace powder (CPP) before and after extrusion processing (g/100 g dm, mean ± SD, n = 2) at water contents of 13% (A1, B1, and D1) and 23% (D2), respectively. SME, specific mechanical energy (Whkg−1); TM, material temperature (°C); IDF, insoluble DF; HMW-SDF, high molecular weight soluble DF; LMW-SDF, low molecular weight soluble DF; TDF, total DF.
Table 2.
Dietary fiber (DF) contents of chokeberry pomace powder (CPP) before and after extrusion processing (g/100 g dm, mean ± SD, n = 2) at water contents of 13% (A1, B1, and D1) and 23% (D2), respectively. SME, specific mechanical energy (Whkg−1); TM, material temperature (°C); IDF, insoluble DF; HMW-SDF, high molecular weight soluble DF; LMW-SDF, low molecular weight soluble DF; TDF, total DF.
| Sample | SME | TM | IDF | HMW-SDF | LMW-SDF | TDF |
|---|
| CPP | --- | --- | 43.8 ± 1.4 A | 11.5 ± 1.2 A | 2.4 ± 2.2 A | 57.8 ± 2.0 A |
| A1 | 145 ± 6 | 136 | 44.3 ± 1.1 A | 14.5 ± 0.9 AB | 0.8 ± 0.4 A | 59.6 ± 0.7 A |
| B1 | 174 ± 4 | 146 | 42.9 ± 1.8 A | 13.9 ± 0.7 AB | 1.1 ± 0.3 A | 58.0 ± 2.3 A |
| D1 | 222 ± 10 | 155 | 41.5 ± 2.8 A | 16.1 ± 0.8 B | 1.5 ± 0.1 A | 59.0 ± 1.8 A |
| D2 | 190 ± 9 | 140 | 43.3 ± 2.2 A | 14.3 ± 1.2 AB | 1.1 ± 0.4 A | 58.7 ± 3.8 A |
Table 3.
Partially methylated alditol acetates (PMAA) from insoluble dietary fiber (IDF) and high molecular weight soluble dietary fiber (HMW-SDF) fractions isolated from chokeberry pomace powder (CPP) before and after extrusion processing 1 (mol%, range/2, n = 2).
Table 3.
Partially methylated alditol acetates (PMAA) from insoluble dietary fiber (IDF) and high molecular weight soluble dietary fiber (HMW-SDF) fractions isolated from chokeberry pomace powder (CPP) before and after extrusion processing 1 (mol%, range/2, n = 2).
| | IDF | HMW-SDF |
|---|
| Glycosidic Linkage 2 | CPP | A1 | D1 | D2 | CPP | A1 | D1 | D2 |
|---|
| t-Araf | 7.0 ± 0.6 | 5.2 ± 0.7 | 3.8 ± 0.5 | 4.9 ± 1.0 | 15.7 ± 0.9 | 18.5 ± 0.6 | 16.9 ± 2.4 | 18.6 ± 0.2 |
| t-Arap | 0.9 ± 0.1 | 0.8 ± 0.1 | 0.9 ± 0.1 | 1.1 ± 0.2 | 1.9 ± 0.1 | 1.2 ± 0.4 | 1.5 ± 0.1 | 1.5 ± 0.4 |
| 1,2-Araf | 0.3 ± 0.1 | 0.5 ± 0.2 | 0.5 ± 0.1 | 0.8 ± 0.2 | 0.9 ± 0.3 | 0.6 ± 0.3 | 0.5 ± 0.1 | 0.6 ± 0.2 |
| 1,3-Araf | 1.8 ± 0.3 | 1.5 ± 0.1 | 1.2 ± 0.1 | 2.0 ± 0.4 | 3.3 ± 0.4 | 4.4 ± 0.5 | 4.1 ± 0.1 | 4.2 ± 0.3 |
| 1,5-Araf | 10.4 ± 1.3 | 6.9 ± 0.2 | 5.9 ± 1.9 | 9.7 ± 4.2 | 17.0 ± 1.0 | 27.6 ± 0.3 | 24.9 ± 4.2 | 28.2 ± 0.8 |
| 1,2,5-Araf | 0.8 ± 0.4 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.7 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 |
| 1,3,5-Araf | 3.9 ± 0.2 | 2.2 ± 0.1 | 1.4 ± 0.1 | 2.0 ± 0.2 | 3.1 ± 0.2 | 7.8 ± 0.3 | 7.1 ± 1.5 | 8.2 ± 0.1 |
| 1,2,3,5-Araf | 0.8 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.1 | 0.8 ± 0.1 | 1.2 ± 0.3 | 1.3 ± 0.1 | 1.4 ± 0.2 | 1.4 ± 0.2 |
| Σ Ara | 25.8 ± 1.7 | 17.8 ± 0.8 | 14.2 ± 2.7 | 21.6 ± 6.0 | 43.8 ± 2.3 | 62.3 ± 0.1 | 57.3 ± 8.4 | 63.5 ± 1.3 |
| t-Galp | 3.1 ± 0.3 | 3.3 ± 0.1 | 3.1 ± 0.1 | 3.5 ± 0.4 | 4.7 ± 0.5 | 4.6 ± 0.2 | 4.7 ± 0.6 | 4.5 ± 0.2 |
| 1,2-Galp | ND | ND | ND | ND | 1.2 ± 0.3 | 0.1 ± 0.1 | 0.5 ± 0.2 | 0.1 ± 0.1 |
| 1,3-Galp | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.1 ± 0.1 | 0.2 ± 0.1 | 1.8 ± 0.1 | 0.9 ± 0.3 | 1.0 ± 0.2 | 0.9 ± 0.3 |
| 1,4-Galp | 2.9 ± 0.4 | 3.3 ± 0.2 | 2.7 ± 0.2 | 3.3 ± 0.8 | 1.4 ± 0.6 | 4.3 ± 1.0 | 6.9 ± 1.6 | 4.2 ± 0.7 |
| 1,6-Galp | 0.7 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 2.2 ± 0.2 | 1.2 ± 0.2 | 1.3 ± 0.2 | 1.1 ± 0.1 |
| 1,3,4-Galp | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.4 ± 0.1 | ND | ND | ND | ND |
| 1,3,6-Galp | 0.7 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.5 ± 0.2 | 6.5 ± 0.8 | 3.2 ± 0.7 | 3.0 ± 0.4 | 2.6 ± 0.1 |
| Σ Gal | 7.8 ± 0.7 | 8.2 ± 0.2 | 7.4 ± 0.1 | 8.9 ± 0.7 | 17.8 ± 1.0 | 14.4 ± 2.3 | 17.4 ± 3.1 | 13.6 ± 0.9 |
| t-Glcp | 1.1 ± 0.3 | 1.2 ± 0.1 | 1.4 ± 0.3 | 1.1 ± 0.2 | 1.7 ± 0.6 | 0.9 ± 0.3 | 0.6 ± 0.1 | 0.9 ± 0.2 |
| 1,4-Glcp | 40.4 ± 0.1 | 47.6 ± 0.7 | 48.6 ± 3.9 | 42.6 ± 4.5 | 8.8 ± 1.0 | 3.6 ± 0.9 | 5.7 ± 3.4 | 3.4 ± 0.9 |
| 1,4,6-Glcp | 3.7 ± 0.4 | 4.0 ± 0.1 | 4.0 ± 0.1 | 3.8 ± 0.5 | 2.8 ± 0.6 | 1.9 ± 0.1 | 2.6 ± 0.5 | 1.9 ± 0.7 |
| Σ Glc | 45.2 ± 0.2 | 52.8 ± 0.8 | 54.1 ± 4.2 | 47.4 ± 4.2 | 13.3 ± 2.1 | 6.4 ± 1.2 | 9.0 ± 4.0 | 6.2 ± 1.8 |
| t-Manp | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.2 ± 0.1 | 2.4 ± 1.3 | 0.7 ± 0.2 | 0.4 ± 0.1 | 0.6 ± 0.2 |
| 1,4-Manp | 2.6 ± 0.4 | 3.3 ± 0.3 | 3.4 ± 0.1 | 3.2 ± 0.2 | 2.2 ± 0.4 | 1.6 ± 0.2 | 1.7 ± 0.1 | 2.0 ± 0.6 |
| 1,4,6-Manp | 0.5 ± 0.1 | 0.6 ± 0.1 | 0.7 ± 0.1 | 0.6 ± 0.1 | 0.8 ± 0.2 | ND | ND | ND |
| Σ Man | 3.3 ± 0.4 | 4.2 ± 0.3 | 4.3 ± 0.1 | 4.1 ± 0.3 | 5.4 ± 1.8 | 2.2 ± 0.3 | 2.2 ± 0.2 | 2.7 ± 0.8 |
| 1,2-Rhap | 0.6 ± 0.3 | 0.5 ± 0.1 | 0.3 ± 0.1 | 0.4 ± 0.2 | 0.8 ± 0.1 | 1.7 ± 0.1 | 1.6 ± 0.3 | 1.6 ± 0.1 |
| 1,2,4-Rhap | 0.3 ± 0.1 | 0.6 ± 0.1 | 0.5 ± 0.1 | 0.6 ± 0.2 | 0.4 ± 0.1 | 0.9 ± 0.1 | 1.1 ± 0.1 | 0.8 ± 0.1 |
| Σ Rha | 0.9 ± 0.4 | 1.1 ± 0.1 | 0.8 ± 0.2 | 1.0 ± 0.1 | 1.2 ± 0.1 | 2.7 ± 0.1 | 2.6 ± 0.4 | 2.4 ± 0.1 |
| t-Xylp | 5.0 ± 0.1 | 5.2 ± 0.4 | 4.7 ± 0.1 | 5.4 ± 0.3 | 4.3 ± 0.5 | 4.7 ± 0.4 | 4.9 ± 0.2 | 5.1 ± 0.2 |
| 1,2-Xylp3 | 2.1 ± 0.1 | 2.3 ± 0.1 | 2.5 ± 0.1 | 2.2 ± 0.1 | 1.8 ± 0.3 | 1.5 ± 0.1 | 1.7 ± 0.3 | 1.7 ± 0.1 |
| 1,4-Xylp3 | 9.8 ± 3.6 | 8.4 ± 1.4 | 11.9 ± 1.6 | 9.5 ± 3.0 | 12.3 ± 0.7 | 5.8 ± 0.2 | 4.8 ± 0.2 | 4.8 ± 0.6 |
| Σ Xyl | 17.0 ± 3.5 | 15.9 ± 1.0 | 19.2 ± 1.7 | 17.1 ± 2.8 | 18.5 ± 0.5 | 12.1 ± 0.7 | 11.5 ± 0.7 | 11.6 ± 0.4 |
Table 4.
Relative composition of pectic arabinan oligosaccharides liberated from chokeberry pomace powder (CPP) and extruded dietary fiber (DF) fractions 1 after enzymatic cleavage using endo-arabinanase (mol%, range/2, n = 2). IDF, insoluble dietary fiber; HMW-SDF, high molecular weight dietary fiber.
Table 4.
Relative composition of pectic arabinan oligosaccharides liberated from chokeberry pomace powder (CPP) and extruded dietary fiber (DF) fractions 1 after enzymatic cleavage using endo-arabinanase (mol%, range/2, n = 2). IDF, insoluble dietary fiber; HMW-SDF, high molecular weight dietary fiber.
| | IDF | HMW-SDF |
|---|
| Compound | CPP | A1 | D1 | D2 | CPP | A1 | D1 | D2 |
|---|
| A-2a | 81.2 ± 0.1 | 80.4 ± 0.1 | 79.8 ± 0.5 | 80.3 ± 0.1 | 84.9 ± 0.9 | 84.9 ± 0.2 | 84.4 ± 0.1 | 84.2 ± 0.3 |
| A-4a | 5.1 ± 0.1 | 7.2 ± 0.2 | 8.6 ± 0.7 | 8.4 ± 0.2 | 5.4 ± 0.4 | 5.2 ± 0.1 | 6.0 ± 0.1 | 5.6 ± 0.1 |
| A-4b | 0.9 ± 0.1 | 1.0 ± 0.1 | <LOQ | <LOQ | 1.1 ± 0.1 | 0.9 ± 0.2 | 1.1 ± 0.2 | 1.0 ± 0.1 |
| A-5b | 9.1 ± 0.1 | 8.8 ± 0.1 | 8.3 ± 0.2 | 8.4 ± 0.3 | 7.6 ± 0.1 | 7.9 ± 0.2 | 7.0 ± 0.3 | 7.9 ± 0.1 |
| A-5c | 2.4 ± 0.1 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| A-6a | 0.6 ± 0.1 | 1.1 ± 0.1 | 1.6 ± 0.2 | 1.4 ± 0.1 | 0.4 ± 0.4 | 0.6 ± 0.1 | 0.7 ± 0.1 | 0.6 ± 0.1 |
| A-7b | 0.7 ± 0.1 | 1.4 ± 0.1 | 1.8 ± 0.2 | 1.6 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.8 ± 0.1 | 0.6 ± 0.1 |
Table 5.
Glucose, fructose, and sucrose contents of chokeberry pomace powder (CPP) before and after extrusion processing (g/100 g dm, mean ± SD, n = 2) at water contents of 13% (A1, B1, and D1) and 23% (D2), respectively. SME, specific mechanical energy (Whkg−1); TM, material temperature (°C).
Table 5.
Glucose, fructose, and sucrose contents of chokeberry pomace powder (CPP) before and after extrusion processing (g/100 g dm, mean ± SD, n = 2) at water contents of 13% (A1, B1, and D1) and 23% (D2), respectively. SME, specific mechanical energy (Whkg−1); TM, material temperature (°C).
| Sample | SME | TM | Glucose | Fructose | Sucrose |
|---|
| CPP | --- | --- | 5.2 ± 0.2 A | 4.5 ± 0.2 A | 0.4 ± 0.2 A |
| A1 | 145 ± 6 | 136 | 4.3 ± 0.0 B | 3.9 ± 0.1 B | 0.5 ± 0.4 A |
| B1 | 174 ± 4 | 146 | 4.2 ± 0.2 B | 3.3 ± 0.2 C | 0.4 ± 0.2 A |
| D1 | 222 ± 10 | 155 | 3.9 ± 0.0 B | 3.7 ± 0.1 BC | 0.3 ± 0.3 A |
| D2 | 190 ± 9 | 140 | 4.4 ± 0.2 B | 3.9 ± 0.1 B | 0.5 ± 0.5 A |
Table 6.
Anthocyanins contents of chokeberry pomace powder (CPP) and samples after extrusion processing (g/100 g dm, mean ± SD, n = 2) at water contents of 13% (A1, B1, and D1) and 23% (D2), respectively. SME, specific mechanical energy (Whkg−1); TM, material temperature (°C); Cy-3-gal, cyanidin-3O-galactoside; Cy-3-glu, cyanidin-3O-glucoside; Cy-3-ara, cyanidin-3O-arabinoside; Cy-3-xyl, cyanidin-3O-xyloside; Cy, cyanidin aglycon; M, monomers.
Table 6.
Anthocyanins contents of chokeberry pomace powder (CPP) and samples after extrusion processing (g/100 g dm, mean ± SD, n = 2) at water contents of 13% (A1, B1, and D1) and 23% (D2), respectively. SME, specific mechanical energy (Whkg−1); TM, material temperature (°C); Cy-3-gal, cyanidin-3O-galactoside; Cy-3-glu, cyanidin-3O-glucoside; Cy-3-ara, cyanidin-3O-arabinoside; Cy-3-xyl, cyanidin-3O-xyloside; Cy, cyanidin aglycon; M, monomers.
| Sample | SME | TM | Cy-3-gal | Cy-3-glu | Cy-3-ara | Cy-3-xyl | Cy | Anthocyanins | M |
|---|
| CPP | --- | --- | 1.15 ± 0.06 A | 0.063 ± 0.010 A | 0.51 ± 0.03 A | 0.079 ± 0.013 A | <LOQ | 1.80 ± 0.09 A | 85 ± 3 A |
| A1 | 145 ± 6 | 136 | 0.38 ± 0.13 B | 0.021 ± 0.005 B | 0.17 ± 0.04 B | 0.025 ± 0.006 B | 0.010 ± 0.002 A | 0.61 ± 0.17 B | 73 ± 6 AB |
| B1 | 174 ± 4 | 146 | 0.27 ± 0.02 B | 0.015 ± 0.002 B | 0.11 ± 0.01 BC | 0.016 ± 0.002 B | 0.014 ± 0.003 A | 0.43 ± 0.04 B | 68 ± 5 AB |
| D1 | 222 ± 10 | 155 | 0.15 ± 0.04 B | 0.009 ± 0.003 B | 0.06 ± 0.02 C | 0.008 ± 0.003 B | 0.013 ± 0.002 A | 0.24 ± 0.06 B | 61 ± 9 B |
| D2 | 190 ± 9 | 140 | 0.37 ± 0.07 B | 0.020 ± 0.004 B | 0.15 ± 0.03 BC | 0.022 ± 0.004 B | 0.013 ± 0.003 A | 0.58 ± 0.11 B | 73 ± 5 AB |
Table 7.
Phenolic acids contents of chokeberry pomace powder (CPP) and samples after extrusion processing (g/100 g dm, mean ± SD, n = 2) at water contents of 13% (A1, B1, and D1) and 23% (D2), respectively. SME, specific mechanical energy (Whkg−1); TM, material temperature (°C); 5 CQA, 5-caffeoylquinic acids acid; 4-CQA, 4-caffeoylquinic acids acid; 3-CQA, 3-caffeoylquinic acids acid.
Table 7.
Phenolic acids contents of chokeberry pomace powder (CPP) and samples after extrusion processing (g/100 g dm, mean ± SD, n = 2) at water contents of 13% (A1, B1, and D1) and 23% (D2), respectively. SME, specific mechanical energy (Whkg−1); TM, material temperature (°C); 5 CQA, 5-caffeoylquinic acids acid; 4-CQA, 4-caffeoylquinic acids acid; 3-CQA, 3-caffeoylquinic acids acid.
| Sample | SME | TM | 5-CQA | 4-CQA | 3-CQA | Phenolic Acids |
|---|
| CPP | --- | --- | 0.165 ± 0.019 A | 0.009 ± 0.001 A | 0.136 ± 0.013 A | 0.306 ± 0.027 A |
| A1 | 145 ± 6 | 136 | 0.156 ± 0.000 A | 0.010 ± 0.000 AC | 0.126 ± 0.003 A | 0.292 ± 0.003 A |
| B1 | 174 ± 4 | 146 | 0.159 ± 0.009 A | 0.012 ± 0.001 BC | 0.126 ± 0.005 A | 0.297 ± 0.014 A |
| D1 | 222 ± 10 | 155 | 0.156 ± 0.006 A | 0.016 ± 0.001 D | 0.119 ± 0.002 A | 0.290 ± 0.008 A |
| D2 | 190 ± 9 | 140 | 0.154 ± 0.004 A | 0.010 ± 0.001 AB | 0.125 ± 0.001 A | 0.288 ± 0.004 A |
Table 8.
Polyphenols contents of chokeberry pomace powder (CPP) and samples after extrusion processing (g/100 g dm, mean ± SD, n = 2) at water contents of 13% (A1, B1, and D1) and 23% (D2), respectively. SME, specific mechanical energy (Whkg−1); TM, material temperature (°C); TPP, total polyphenols contents analyzed by the Folin–Ciocalteu test; TPP-HPLC, total polyphenols contents calculated as the sum of monomeric anthocyanins, phenolic acids, and flavonols.
Table 8.
Polyphenols contents of chokeberry pomace powder (CPP) and samples after extrusion processing (g/100 g dm, mean ± SD, n = 2) at water contents of 13% (A1, B1, and D1) and 23% (D2), respectively. SME, specific mechanical energy (Whkg−1); TM, material temperature (°C); TPP, total polyphenols contents analyzed by the Folin–Ciocalteu test; TPP-HPLC, total polyphenols contents calculated as the sum of monomeric anthocyanins, phenolic acids, and flavonols.
| Sample | SME | TM | TPP | TPP-HPLC |
|---|
| CPP | --- | --- | 5.5 ± 0.2 A | 2.29 ± 0.12 A |
| A1 | 145 ± 6 | 136 | 6.2 ± 0.5 AB | 1.10 ± 0.18 B |
| B1 | 174 ± 4 | 146 | 6.7 ± 0.6 AB | 0.92 ± 0.02 B |
| D1 | 222 ± 10 | 155 | 7.3 ± 0.1 B | 0.71 ± 0.05 B |
| D2 | 190 ± 9 | 140 | 6.4 ± 0.1 AB | 1.05 ± 0.10 B |
Table 9.
Techno-functional and sensory properties of chokeberry pomace powder (CPP) and samples after extrusion processing at water contents of 13% (A1, B1, and D1) and 23% (D2), respectively. SME, specific mechanical energy (Whkg−1); TM, material temperature (°C); WSI, water solubility index; WAI, water absorption index; SEI, sectional expansion index; LEI, longitudinal expansion index; H, hardness (Nm−2); C, color (L* lightness, a* red/green value, and b* blue/yellow value).
Table 9.
Techno-functional and sensory properties of chokeberry pomace powder (CPP) and samples after extrusion processing at water contents of 13% (A1, B1, and D1) and 23% (D2), respectively. SME, specific mechanical energy (Whkg−1); TM, material temperature (°C); WSI, water solubility index; WAI, water absorption index; SEI, sectional expansion index; LEI, longitudinal expansion index; H, hardness (Nm−2); C, color (L* lightness, a* red/green value, and b* blue/yellow value).
| Sample | SME | TM | WSI | WAI | SEI | LEI | H | C |
|---|
| | | | | | | | | L* | a* | b* |
|---|
| CPP | --- | --- | 22.3 ± 0.9 A | 2.9 ± 0.1 A | -- | -- | -- | 29.8 ± 0.6 A | 21.1 ± 0.3 A | 5.1 ± 0.1 A |
| A1 | 145 ± 6 | 136 | 23.8 ± 1.0 AB | 2.1 ± 0.0 B | 1.95 ± 0.13 A | 1.13 ± 0.06 A | 17 ± 6 AB | 21.7 ± 0.2 B | 16.9 ± 0.1 BC | 1.9 ± 0.0 BC |
| A2 | 122 ± 5 | 123 | 24.4 ± 0.3 BC | 2.0 ± 0.0 BC | 1.52 ± 0.11 B | 1.15 ± 0.06 A | 23 ± 8 A | 21.1 ± 0.0 B | 15.3 ± 0.3 D | 1.8 ± 0.0 BCD |
| B1 | 174 ± 4 | 146 | 25.5 ± 0.4 CD | 2.1 ± 0.0 B | 1.05 ± 0.05 CD | 1.57 ± 0.21 B | 9 ± 3 B | 22.1 ± 0.2 B | 17.2 ± 0.3 B | 1.9 ± 0.0 BCD |
| B2 | 122 ± 5 | 134 | 25.2 ± 0.1 BCD | 1.6 ± 0.0 D | 1.49 ± 0.11 B | 1.47 ± 0.08 B | 10 ± 2 B | 22.0 ± 0.4 B | 16.6 ± 0.3 BCE | 2.1 ± 0.1 B |
| C1 | 195 ± 10 | 152 | 26.1 ± 0.2 D | 1.6 ± 0.0 D | 0.85 ± 0.07 E | 2.00 ± 0.19 C | 8 ± 7 B | 21.6 ± 0.8 B | 16.7 ± 0.4 BCE | 1.7 ± 0.1 DE |
| C2 | 180 ± 7 | 139 | 25.5 ± 0.2 CD | 2.0 ± 0.1 BC | 1.20 ± 0.05 C | 1.53 ± 0.17 B | 9 ± 5 B | 21.7 ± 0.3 B | 16.2 ± 0.3 CE | 1.7 ± 0.1 CDE |
| D1 | 222 ± 10 | 155 | 26.4 ± 0.7 D | 2.0 ± 0.1 BC | 0.85 ± 0.05 E | NA | 8 ± 2 B | 23.5 ± 0.5 C | 17.4 ± 0.2 B | 1.7 ± 0.0 CDE |
| D2 | 190 ± 9 | 140 | 25.4 ± 0.7 CD | 1.9 ± 0.0 C | 1.00 ± 0.12 DE | 1.83 ± 0.09 C | 13 ± 4 AB | 22.3 ± 0.2 BC | 16.0 ± 0.3 DE | 1.5 ± 0.1 E |