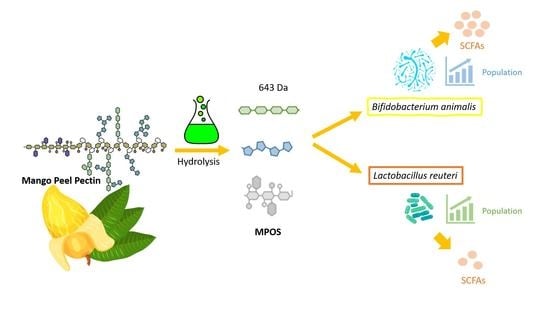
Crude Pectic Oligosaccharide Recovery from Thai Chok Anan Mango Peel Using Pectinolytic Enzyme Hydrolysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Microorganisms
2.3. Optimisation Condition of Pectic Oligosaccharide Preparation from Mango Peel
2.4. MPOS (Mango Peel Pectic Oligosaccharide) Quality Assessments
2.4.1. Determination of Molecular Weight
2.4.2. Determination of Monosaccharide Compositions
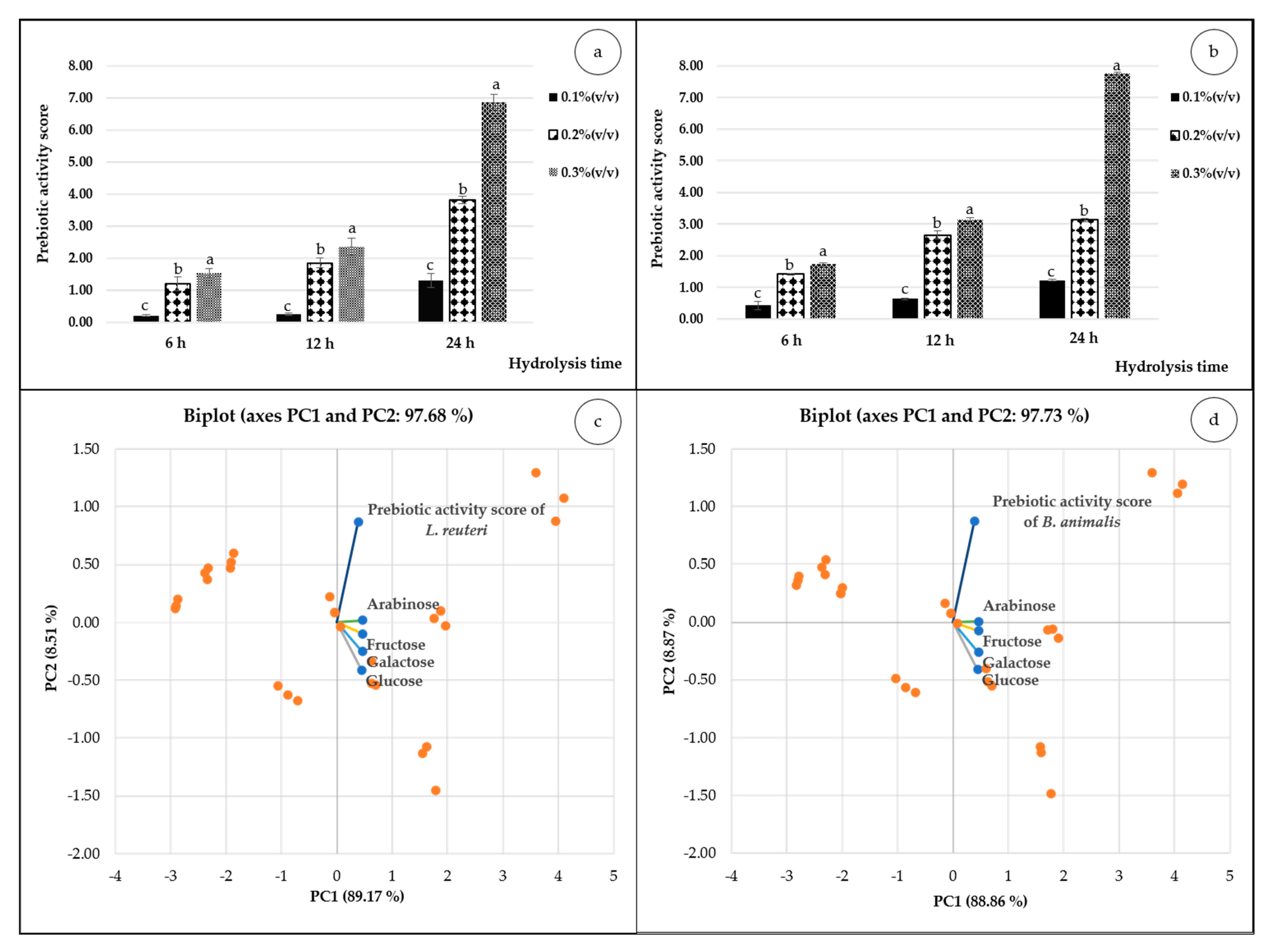
2.4.3. Selection of MPOS Condition Using Prebiotic Activity
2.5. Fermentation of MPOS on Probiotic Growth and Products
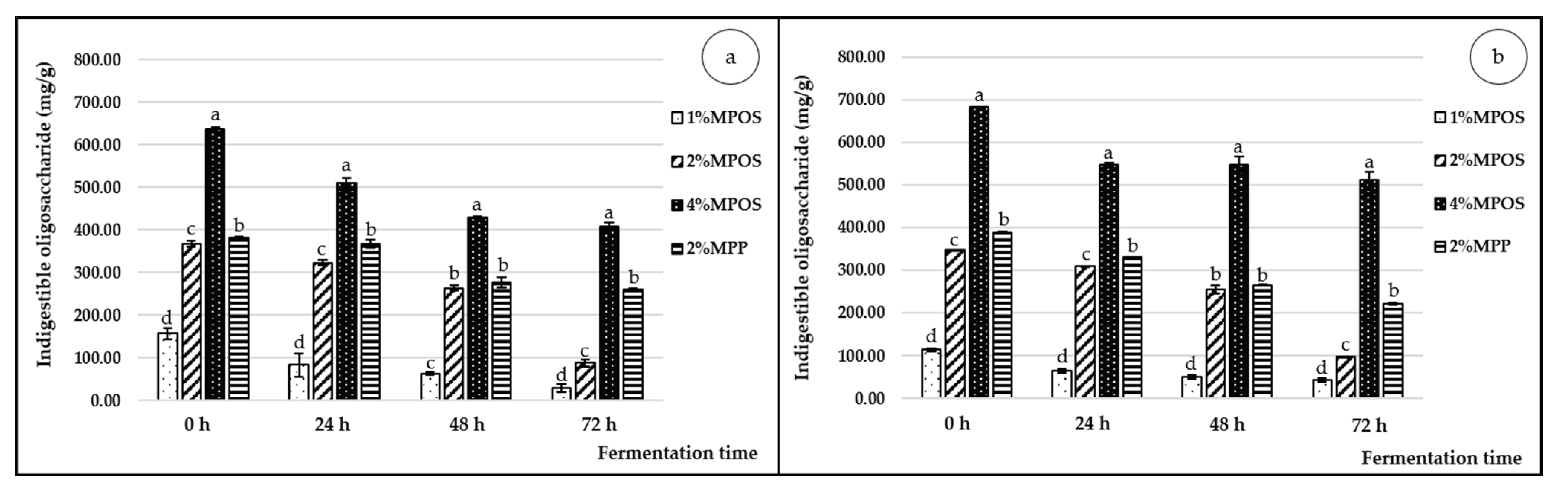
2.5.1. Indigestible Oligosaccharide
2.5.2. Simulation of the Fermentation
Probiotics Population
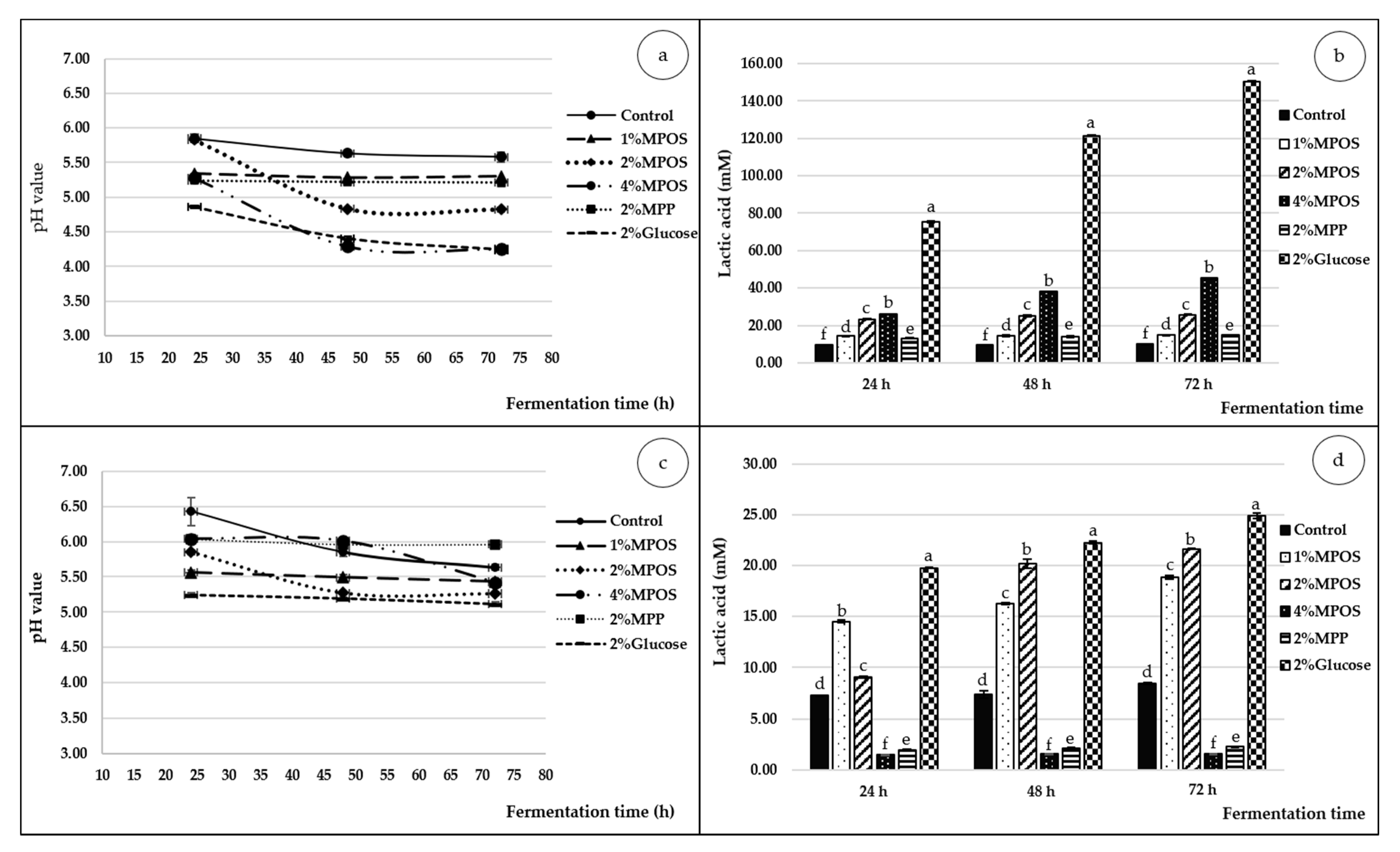
pH Value
2.5.3. By-Products of Probiotics
Lactic Acid
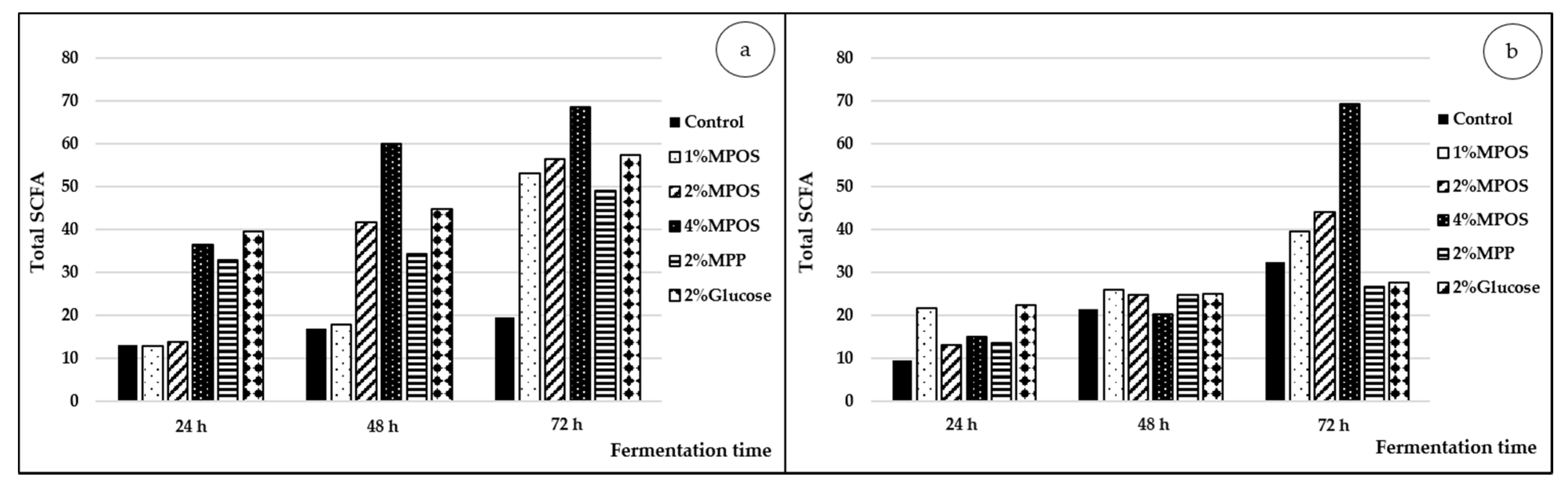
Short Chain Fatty Acid Production
2.6. Statistical Analysis
3. Results and Discussion
3.1. Optimisation Condition of MPOS on Probiotic Growth
3.1.1. Monosaccharide Contents and Molecular Weight of MPOS s
3.1.2. Prebiotic Assessment
3.2. Fermentation of MPOS on Probiotic Growth and Products
3.2.1. Indigestible Oligosaccharide
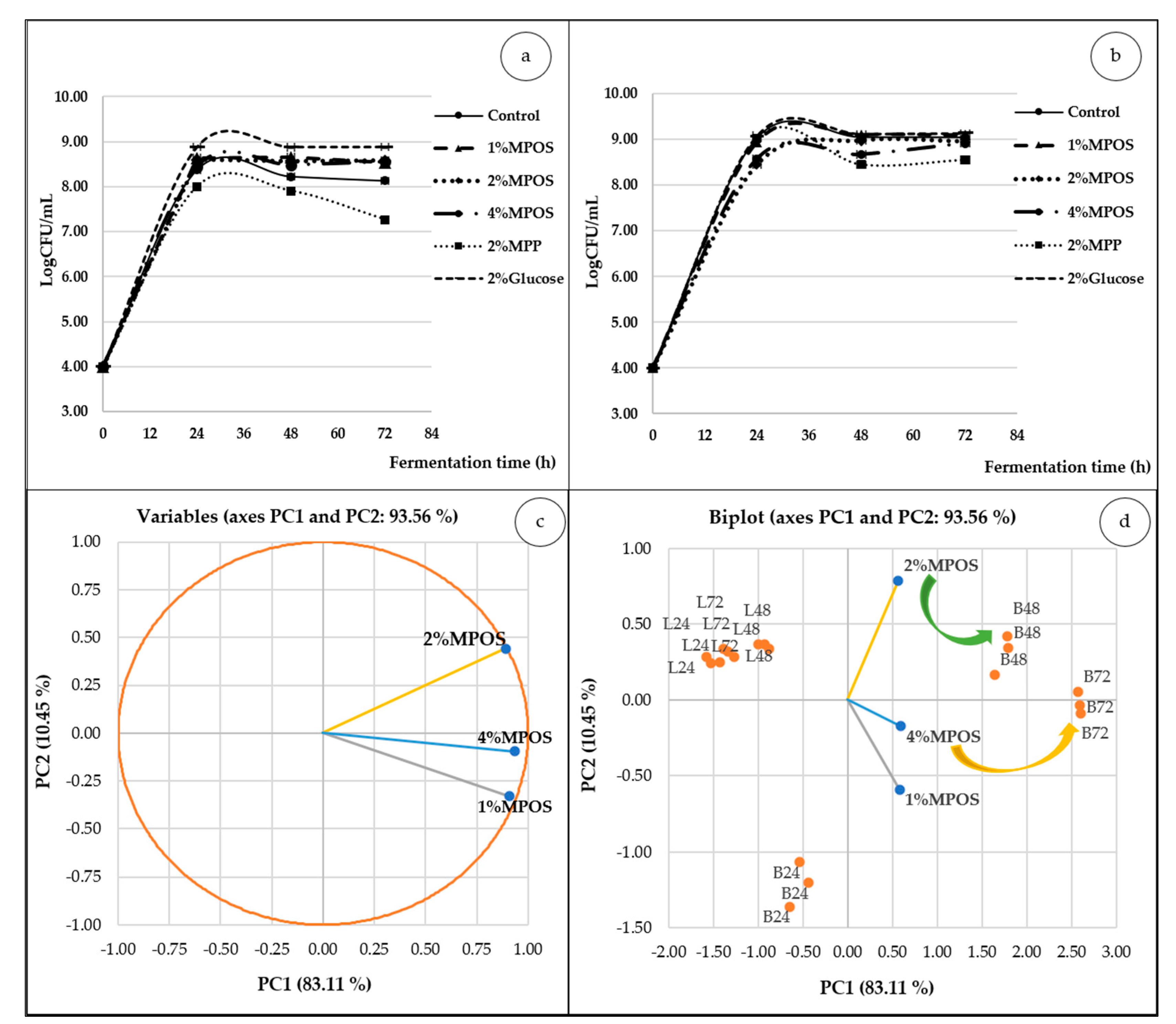
3.2.2. Probiotics Population
3.2.3. pH and Lactic Acid
3.2.4. Short Chain Fatty Acid Production (SCFA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Cindio, B.; Gabriele, D.; Lupi, F.R. Pectin: Properties Determination and Uses. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 294–300. [Google Scholar]
- Moslemi, M. Reviewing the recent advances in application of pectin for technical and health promotion purposes: From laboratory to market. Carbohydr. Polym. 2021, 254, 117324. [Google Scholar] [CrossRef]
- Wicker, L.; Kim, Y. Pectin and Health. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 289–293. [Google Scholar]
- Fissore, E.N.; Rojas, A.M.; Gerschenson, L.N. Rheological performance of pectin-enriched products isolated from red beet (Beta vulgaris L. var. conditiva) through alkaline and enzymatic treatments. Food Hydrocoll. 2012, 26, 249–260. [Google Scholar] [CrossRef]
- Vincken, J.P.; Schols, H.A.; Oomen, R.J.F.J.; McCann, M.C.; Ulvskov, P.; Voragen, A.G.J.; Visser, R.G.F. If homogalac-turonan were a side chain of rhamnogalacturonan I. implications for cell wall architecture. Plant Physiol. 2003, 132, 1781–1789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gullón, B.; Gómez, B.; Martínez-Sabajanes, M.; Yáñez, R.; Parajó, J.; Alonso, J. Pectic oligosaccharides: Manufacture and functional properties. Trends Food Sci. Technol. 2013, 30, 153–161. [Google Scholar] [CrossRef]
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef]
- Voragen, A.G.J.; Coenen, G.-J.; Verhoef, R.P.; Schols, H.A. Pectin, a versatile polysaccharide present in plant cell walls. Structural Chem. 2009, 20, 263–275. [Google Scholar] [CrossRef] [Green Version]
- Ho, Y.-Y.; Lin, C.-M.; Wu, M.-C. Evaluation of the prebiotic effects of citrus pectin hydrolysate. J. Food Drug Anal. 2017, 25, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Babbar, N.; Dejonghe, W.; Gatti, M.; Sforza, S.; Elst, K. Pectic oligosaccharides from agricultural by-products: Production, characterization and health benefits. Crit. Rev. Biotechnol. 2015, 36, 594–606. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Hu, H.; Wang, L.; Liu, F.; Pan, S. Preparation and prebiotic potential of pectin oligosaccharides obtained from citrus peel pectin. Food Chem. 2018, 244, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Probert, H.M.; Van Loo, J.; Rastall, R.A.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr. Res. Rev. 2004, 17, 259–275. [Google Scholar] [CrossRef] [Green Version]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Mu, Q.; Tavella, V.J.; Luo, X.M. Role of Lactobacillus reuteri in human health and diseases. Front. Microbiol. 2018, 9, 757. [Google Scholar] [CrossRef]
- Jungersen, M.; Anette, W.; Eric Johansen, E.; Jeffrey, E.; Christensen, J.E.; Stuer-Lauridsen, B.; Eskesen, D. The science behind the probiotic strain Bifidobacterium animalis subsp. lactis BB-12®. Microorganisms 2014, 2, 92–110. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.M.; Eisen, J.A.; Zivkovic, A.M. The microbes we eat: Abundance and taxonomy of microbes consumed in a day’s worth of meals for three diet types. PeerJ 2014, 2, e659. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Aziz, Q.; Doré, J.; Emmanuel, A.; Guarner, F.; Quigley, E.M.M. Gut microbiota and gastrointestinal health: Current concepts and future directions. Neurogastroenterol. Motil. 2013, 25, 4–15. [Google Scholar] [CrossRef]
- Binns, N. Probiotics, Prebiotics and the Gut Microbiota; ILSI Europe International Life Sciences Institute: Washington, DC, USA, 2013; p. 33. [Google Scholar]
- Olano-Martin, E.; Gibson, G.R.; Rastell, R.A. Comparison of the in vitro bifidogenic properties of pectins and pectic oligosaccharides. J. Appl. Microbiol. 2002, 93, 505–511. [Google Scholar] [CrossRef] [Green Version]
- Manderson, K.; Pinart, M.; Tuohy, K.M.; Grace, W.E.; Hotchkiss, A.T.; Widmer, W.; Yadhav, M.P.; Gibson, G.R.; Rastall, R.A. In vitro determination of prebiotic properties of oligosaccharides derived from an orange juice manufacturing by-product stream. Appl. Environ. Microbiol. 2005, 71, 8383–8389. [Google Scholar] [CrossRef] [Green Version]
- Mandalari, G.; Palop, C.N.; Tuohy, K.; Gibson, G.R.; Bennett, R.N.; Waldron, K.W.; Bisignano, G.; Narbad, A.; Faulds, C.B. In vitro evaluation of the prebiotic activity of a pectic oligosaccharide-rich extract enzymatically derived from bergamot peel. Appl. Microbiol. Biotechnol. 2006, 73, 1173–1179. [Google Scholar] [CrossRef]
- Kang, H.J.; Jo, C.; Kwon, J.H.; Son, J.H.; An, B.J.; Byun, M.W. Antioxidant and cancer cell proliferation inhibition effect of citrus pectin-oligosaccharide prepared by irradiation. J. Med. Food 2006, 9, 313–320. [Google Scholar] [CrossRef]
- Li, P.-J.; Xia, J.-L.; Nie, Z.-Y.; Shan, Y. Pectic oligosaccharides hydrolyzed from orange peel by fungal multi-enzyme complexes and their prebiotic and antibacterial potentials. LWT-Food Sci. Technol. 2016, 69, 203–210. [Google Scholar] [CrossRef]
- Li, T.; Li, S.; Dong, Y.; Zhu, R.; Liu, Y. Antioxidant activity of penta-oligogalacturonide, isolated from haw pectin, suppresses triglyceride synthesis in mice fed with a high-fat diet. Food Chem. 2014, 145, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Olano-Martin, E.; Williams, M.; Gibson, G.; Rastall, R. Pectins and pectic-oligosaccharides inhibit Escherichia coli O157:H7 Shiga toxin as directed towards the human colonic cell line HT29. FEMS Microbiol. Lett. 2003, 218, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Concha, O.J.; Zúñiga, H.M.E. Enzymatic depolymerization of sugar beet pulp: Production and characterization of pectin and pectic-oligosaccharides as a potential source for functional carbohydrates. Chem. Eng. J. 2012, 192, 29–36. [Google Scholar] [CrossRef]
- Sabajanes, M.M.; Yáñez, R.; Alonso, J.L.; Parajó, J.C. Pectic oligosaccharides production from orange peel waste by enzymatic hydrolysis. Int. J. Food Sci. Technol. 2012, 47, 747–754. [Google Scholar] [CrossRef]
- Huang, P.-H.; Fu, L.-C.; Huang, C.-S.; Wang, Y.-T.; Wu, M.-C. The uptake of oligogalacturonide and its effect on growth inhibition, lactate dehydrogenase activity and galactin-3 release of human cancer cells. Food Chem. 2012, 132, 1987–1995. [Google Scholar] [CrossRef]
- Babbar, N.; Dejonghe, W.; Sforza, S.; Elst, K. Enzymatic pectic oligosaccharides (POS) production from sugar beet pulp using response surface methodology. J. Food Sci. Technol. 2017, 54, 3707–3715. [Google Scholar] [CrossRef]
- Moura, F.; Macagnan, F.; Silva, L. Oligosaccharide production by hydrolysis of polysaccharides: A review. Int. J. Food Sci. Technol. 2014, 50, 275–281. [Google Scholar] [CrossRef]
- Gómez, B.; Gullón, B.; Yáñez, R.; Parajó, J.C.; Alonso, J.L. Pectic oligosacharides from lemon peel wastes: Production, purification, and chemical characterization. J. Agric. Food Chem. 2013, 61, 10043–10053. [Google Scholar] [CrossRef]
- Zhu, R.; Mengling, H.; Chunyun, Z.; Lijiao, Z.; Congya, W.; Liu, J.; Zhenhua, D.; Shang, F.; Hu, F.; Tiejing, L.; et al. Pectin oligosaccharides from hawthorn (Crataegus pinnatifida Bunge. var. major) inhibit the formation of advanced glycation end products in infant formula milk powder. Food Funct. 2019, 10, 8081–8093. [Google Scholar] [CrossRef]
- Gómez, B.; Gullón, B.; Yáñez, R.A.; Schols, H.; Alonso, J.L. Prebiotic potential of pectins and pectic oligosaccharides derived from lemon peel wastes and sugar beet pulp: A comparative evaluation. J. Funct. Foods 2016, 20, 108–121. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Transformation of durian biomass into a highly valuable end commodity: Trends and opportunities. Biomass Bioenergy 2011, 35, 2470–2478. [Google Scholar] [CrossRef]
- Casabar, J.T.; Ramaraj, R.; Tipnee, S.; Unpaprom, Y. Enhancement of hydrolysis with Trichoderma harzianum for bioethanol production of sonicated pineapple fruit peel. Fuel 2020, 279, 118437. [Google Scholar] [CrossRef]
- Wilaipon, P. The effects of briquetting pressure on banana-peel briquette and the banana waste in Northern Thailand. American. J. Appl. Sci. 2009, 6, 167–171. [Google Scholar]
- Maneenpun, S.; Yunchalad, M. Developing processed mango products for international markets. ACTA Hortic. 2004, 645, 93–105. [Google Scholar] [CrossRef]
- Vieira, W.A.S.; Michereff, S.J.; de Morais, M.A.; Hyde, K.D.; Câmara, M.P.S. Endophytic species of Colletotrichum associated with mango in northeastern Brazil. Fungal Divers. 2014, 67, 181–202. [Google Scholar] [CrossRef]
- Larrauri, J.A.; Rupérez, P.; Borroto, B.; Saura-Calixto, F. Mango peels as a new tropical fibre: Preparation and characterization. LWT-Food Sci. Technol 1996, 29, 729–733. [Google Scholar] [CrossRef]
- Topuz, O.K.; Yerlikaya, P.; Uçak, İ.; Gümüş, B.; Büyükbenli, H.A.; Gökoğlu, N. Influence of pomegranate peel (Punica granatum) extract on lipid oxidation in anchovy fish oil under heat accelerated conditions. J. Food Sci. Technol. 2015, 52, 625–632. [Google Scholar] [CrossRef]
- Geerkens, C.; Nagel, A.; Just, K.; Miller-Rostek, P.; Kammerer, D.; Schweiggert, R.; Carle, R. Mango pectin quality as influenced by cultivar, ripeness, peel particle size, blanching, drying, and irradiation. Food Hydrocoll. 2015, 51, 241–251. [Google Scholar] [CrossRef]
- Sayago-Ayerdi, S.G.; Zamora-Gasga, V.M.; Venema, K. Prebiotic effect of predigested mango peel on gut microbiota assessed in a dynamic in vitro model of the human colon (TIM-2). Food Res. Int. 2019, 118, 89–95. [Google Scholar] [CrossRef]
- Ajila, C.M.; Prasada, R.U.J.S. Mango peel dietary fibre: Composition and associated bound phenolics. J. Funct. Foods 2013, 5, 444–450. [Google Scholar] [CrossRef]
- Sommano, S.; Ounamornmas, P.; Nisoa, M.; Sriwattana, S.; Page, P.; Colelli, G. Characterisation and physiochemical properties of mango peel pectin extracted by conventional and phase control microwave-assisted extractions. Int. Food Res. J. 2018, 25, 2657–2665. [Google Scholar]
- Garcia-Magaña, L.; Garcia, H.; Bello-Pérez, L.; Sayago-Ayerdi, S.; Oca, M. Functional properties and dietary fiber characterization of mango processing by-products (Mangifera indica L., cv Ataulfo and Tommy Atkins). Plant Foods Hum. Nutr. 2013, 68, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Sommano, S.; Ounamornmas, P.; Nisoa, M.; Sriwattana, S. Bioactive functionality of pectin from peels of seven Thai mango cultivars. ACTA Hortic. 2018, 423–428. [Google Scholar] [CrossRef]
- Holbrook, J.J. Protein fluorescence of lactate dehydrogenase. Biochem. J. 1972, 128, 921–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Man, J.C.; Rogosa, M.; Sharpe, M.E. A medium for the cultivation of lactobacilli. J. Appl. Bacteriol. 1960, 23, 130–135. [Google Scholar] [CrossRef]
- Nebra, Y.; Blanch, A.R. A New Selective Medium for Bifidobacterium spp. Appl. Environ. Microbiol. 1999, 65, 5173–5176. [Google Scholar] [CrossRef] [Green Version]
- Sezonov, G.; Joseleau-Petit, D.; D’Ari, R. Escherichia coli physiology in Luria-Bertani broth. J. Bacteriol. 2007, 189, 8746–8749. [Google Scholar] [CrossRef] [Green Version]
- Wongkaew, M.; Sommano, S.; Tangpao, T.; Rachtanapun, P.; Jantanasakulwong, K. Mango peel pectin by micro-wave-assisted extraction and its use as fat replacement in dried Chinese sausage. Foods 2020, 9, 450. [Google Scholar] [CrossRef] [Green Version]
- Guandalini, B.B.V.; Rodrigues, N.P.; Marczak, L.D.F. Sequential extraction of phenolics and pectin from mango peel assisted by ultrasound. Food Res. Int. 2019, 119, 455–461. [Google Scholar] [CrossRef]
- Huang, P.-H.; Lu, H.-T.; Wang, Y.-T.; Wu, M.-C. Antioxidant activity and emulsion-stabilizing effect of pectic enzyme treated pectin in soy protein isolate-stabilized oil/water emulsion. J. Agric. Food Chem. 2011, 59, 9623–9628. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, Y.; Cai, M.; Guan, R.; Neng, J.; Pi, X.; Sun, P. In vitro prebiotic activities of oligosaccharides from the by-products in Ganoderma lucidum spore polysaccharide extraction. RSC Adv. 2020, 10, 14794–14802. [Google Scholar] [CrossRef] [Green Version]
- Tieking, M.; Kühnl, W. Evidence for formation of heterooligosaccharides by Lactobacillus sanfranciscensis during growth in wheat sourdough. J. Agric. Food Chem. 2005, 53, 2456–2461. [Google Scholar] [CrossRef]
- Schwab, C.; Gänzle, M.G. Effect of membrane lateral pressure on the expression of fructosyltransferases in Lactobacillus reuteri. Syst. Appl. Microbiol. 2006, 29, 89–99. [Google Scholar] [CrossRef]
- Huebner, J.; Wehling, R.; Hutkins, R. Functional activity of commercial prebiotics. Int. Dairy J. 2007, 17, 770–775. [Google Scholar] [CrossRef]
- Wichienchot, S.P.T.; Jongjareonrak, A.; Chansuwan, W.; Preeya Hmadhlu, P.; Itharat, A.; Ooraikul, B. Extraction and analysis of prebiotics from selected plants from southern Thailand, Songklanakarin. J. Sci. Technol. 2011, 33, 517–523. [Google Scholar]
- DuBois, M.A.E.; Hamilton, J.K.; Rebers, P.; Smith, F. Calorimetric dubois method for determination of sugar and related substances. Anal. Chem. 2002, 28, 350–356. [Google Scholar] [CrossRef]
- Robertson, J.A.; Ryden, P.; Louise., B.R.; Reading., S.; Gibson., G.; Ring., S.G. Structural properties of diet-derived polysaccharides and their influence on butyrate production during fermentation. LWT-Food Sci. Technol. 2001, 34, 567–573. [Google Scholar] [CrossRef]
- Campbell, J. High-Throughput assessment of bacterial growth inhibition by optical density measurements. Curr. Protoc. Chem. Biol. 2010, 2, 195–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ginjupalli, K.; Karthik, A.; Shavi, G.; Averineni, R.K.; Bhat, M.; Udupa, N. Development of RP-HPLC method for simultaneous estimation of lactic acid and glycolic acid. Der Pharma. Chem. 2013, 5, 335–340. [Google Scholar]
- Filípek, J.; Dvořák, R. Determination of the volatile fatty acid content in the rumen liquid: Comparison of gas chromatography and capillary isotachophoresis. ACTA Vet. Brno 2009, 78, 627–633. [Google Scholar] [CrossRef]
- Ivanova, T.; Iliev, I.; Kirilov, N.; Vasileva, T.; Dalgalarrondo, M.; Haertlé, T.; Chobert, J.-M.; Ivanova, I. Effect of oligosaccharides on the growth of Lactobacillus delbrueckii subsp bulgaricus strains isolated from dairy products. J. Agric. Food Chem. 2009, 57, 9496–9502. [Google Scholar]
- Grahame, D.; Bryksa, B.; Yada, R. Factors affecting enzyme activity. In Improving and Tailoring Enzymes for Food Quality and Functionality; Elsevier BV, Woodhead Publishing: Cambridgeshire, UK, 2015; pp. 11–55. [Google Scholar]
- Cano, M.E.; García-Martín, A.; Morales, P.; Wojtusik, M.; Santos, V.; Kovensky, J.; Ladero, M. Production of oligosaccharides from agrofood wastes. Fermentation 2020, 6, 31. [Google Scholar] [CrossRef] [Green Version]
- Dasaesamoh, R.; Youravong, W.; Wichienchot, S. Optimization on pectinase extraction and purification by yeast fermentation of oligosaccharides from dragon fruit (Hyloceus undatus). Int. Food Res. J. 2016, 23, 2601–2607. [Google Scholar]
- Thitiratsakul, B.; Anprung, P. Prebiotic activity score and bioactive compounds in longan (Dimocarpus longan Lour.): Influence of pectinase in enzyme-assisted extraction. J. Food Sci. Technol. 2014, 51, 1947–1955. [Google Scholar] [CrossRef] [Green Version]
- Fasawang, N.; Anprung, P. Antioxidant and prebiotic activity of enzymatically hydrolyzed lychee fruit pulp. Food Technol. Biotechnol. 2014, 52, 300–306. [Google Scholar]
- Gopal, P.K.A.; Sullivan, P.; Smart, J.B. Utilisation of galactooligosaccharides as selective substrates for growth by lactic acid bacteria including Bifidobacterium lactis DR10 and Lactobacillus rhamnosus DR20. Int. Dairy J. 2001, 11, 19–25. [Google Scholar] [CrossRef]
- Mueller, M.; Čavarkapa, A.; Unger, F.M.; Viernstein, H.; Praznik, W. Prebiotic potential of neutral oligo- and polysaccharides from seed mucilage of Hyptis suaveolens. Food Chem. 2017, 221, 508–514. [Google Scholar] [CrossRef]
- Møller, P.L.; Jørgensen, F.; Hansen, O.C.; Madsen, S.M.; Stougaard, P. Intra- and extracellular beta-galactosidases from Bifidobacterium bifidum and B. infantis: Molecular cloning, heterologous expression, and comparative characterization. Appl. Environ. Microbiol. 2001, 67, 2276–2283. [Google Scholar] [CrossRef] [Green Version]
- Figueroa-González, I.; Rodríguez-Serrano, G.; Gómez-Ruiz, L.; García-Garibay, M.; Cruz-Guerrero, A. Prebiotic effect of commercial saccharides on probiotic bacteria isolated from commercial products. Food Sci. Technol. 2019, 39, 747–753. [Google Scholar] [CrossRef] [Green Version]
- Chengming, S.; Lu, J.; Li, B.; Lin, W.; Zhang, Z.; Weiwei, C.; Sun, C.; Chi, M.; Bingjun, Y.; Yang, B.; et al. Effect of Functional Oligosaccharides and Ordinary Dietary Fiber on Intestinal Microbiota Diversity. Front. Microbiol. 2017, 8, 1750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dongowski, G.; Lorenz, A. Unsaturated oligogalacturonic acids are generated by in vitro treatment of pectin with human faecal flora. Carbohydr. Res. 1998, 314, 237–244. [Google Scholar] [CrossRef]
- Kneifel, W.; Rajal, A.; Kulbe, K.D. In vitro growth behaviour of probiotic bacteria in culture media with carbohydrates of prebiotic importance. Microb. Ecol. Health Dis. 2000, 12, 27–34. [Google Scholar] [CrossRef]
- Paulton, R.J.L. The bacterial growth curve. J. Biol. Educ. 1991, 25, 92–94. [Google Scholar] [CrossRef]
- Soto, C. Effect of isomaltooligosaccharide and gentiooligosaccharide on the growth and fatty acid profile of Lactobacillus plantarum. Electron. J. Biotechnol. 2013, 16, 1–10. [Google Scholar] [CrossRef]
- Goderska, K.; Nowak, J.; Czarnecki, Z. Comparison of the growth of Lactobacillus acidophilus and Bifidobacterium bifidum species in media supplemented with selected saccharides including prebiotics. ACTA Sci. Pol. Technol. Aliment. 2008, 7, 5–20. [Google Scholar]
- Mora-Villalobos, J.A.; Montero-Zamora, J.; Barboza, N.; Rojas-Garbanzo, C.; Usaga, J.; Redondo-Solano, M.; Schroedter, L.; Olszewska-Widdrat, A.; López-Gómez, J.P. Multi-product lactic acid bacteria fermentations: A Review. Fermentation 2020, 6, 23. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Zhang, Y.; Li, H.; Zhang, C.; Zhang, J.; Uddin, J.; Liu, X. Effect of soybean oligopeptide on the growth and metabolism of Lactobacillus acidophilus JCM 1132. RSC Adv. 2020, 10, 16737–16748. [Google Scholar] [CrossRef]
- Narendranath, N.V.; Power, R. Relationship between pH and medium dissolved solids in terms of growth and metabolism of Lactobacilli and Saccharomyces cerevisiae during ethanol production. Appl. Environ. Microbiol. 2005, 71, 2239–2243. [Google Scholar] [CrossRef] [Green Version]
- Śliżewska, K.; Chlebicz-Wójcik, A. Growth kinetics of probiotic Lactobacillus strains in the alternative, cost-efficient semi-solid fermentation medium. Biology 2020, 9, 423. [Google Scholar] [CrossRef]
- Naifeh, J.; Dimri, M.; Varacallo, M. Biochemistry, Aerobic Glycolysis; StatPearls Publishing: Treasure Island, FL, USA, 2020; p. 37. [Google Scholar]
- Usta-Gorgun, B.; Yilmaz-Ersan, L. Short-chain fatty acids production by Bifidobacterium species in the presence of salep. Electron. J. Biotechnol. 2020, 47, 29–35. [Google Scholar] [CrossRef]
- Okpokwasili, G.; Nweke, C.O. Microbial growth and substrate utilization kinetics. Afr. J. Biotechnol. 2006, 5, 305–317. [Google Scholar]
- Chaia, A.P.A.; Oliver, G.J. Intestinal microflora and metabolic activity gut flora. Nutr. Immun. Health 2008, 77–98. [Google Scholar] [CrossRef]
- Markowiak-Kopeć, P.; Śliżewska, K. The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef] [PubMed]
- Jun, H.-I.; Lee, C.-H.; Song, G.-S.; Kim, Y.-S. Characterization of the pectic polysaccharides from pumpkin peel. LWT 2006, 39, 554–561. [Google Scholar] [CrossRef]
- Topping, D.L.; Clifton, P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef]
- Montoya, C.A.; Rutherfurd, S.M.; Moughan, P.J. Kiwifruit fibre level influences the predicted production and absorption of SCFA in the hindgut of growing pigs using a combined in vivo–in vitro digestion methodology. Br. J. Nutr. 2016, 115, 1317–1324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkowska, A.; Nowak, A.; Antczak-Chrobot, A.; Motyl, I.; Czyżowska, A.; Paliwoda, A. Structurally different pectic oligosaccharides produced from apple pomace and their biological activity in vitro. Foods 2019, 8, 365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Liang, R.-H.; Liu, W.; Li, T.; Liu, C.-M.; Wu, S.-S.; Wang, Z.-J. Pectic-oligosaccharides prepared by dynamic high-pressure microfluidization and their in vitro fermentation properties. Carbohydr. Polym. 2013, 91, 175–182. [Google Scholar] [CrossRef]
- Wang, M.; Wichienchot, S.; He, X.; Fu, X.; Huang, Q.; Zhang, B. In vitro colonic fermentation of dietary fibers: Fermentation rate, short-chain fatty acid production and changes in microbiota. Trends Food Sci. Technol. 2019, 88, 1–9. [Google Scholar] [CrossRef]
- Slavin, J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013, 5, 1417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebet, V.; Arrigoni, E.; Amadò, R. Measurement of fermentation products and substrate disappearance during incubation of dietary fibre sources with human faecal flora. LWT 1998, 31, 473–479. [Google Scholar] [CrossRef]
- Gulfi, M.; Arrigoni, E.; Amado, R. Influence of structure on in vitro fermentability of commercial pectins and partially hydrolysed pectin preparations. Carbohydr. Polym. 2005, 59, 247–255. [Google Scholar] [CrossRef]
- Hijova, E.; Chmelarova, A. Short chain fatty acids and colonic health. Bratisl Lek List. 2007, 108, 354–358. [Google Scholar]
- Hoque, R.; Farooq, A.; Ghani, A.; Gorelick, F.; Mehal, W.Z. Lactate reduces liver and pancreatic injury in toll-like receptor– and inflammasome-mediated inflammation via GPR81-mediated suppression of innate immunity. Gastroenterology 2014, 146, 1763–1774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Chemical Characteristic of “Chok Anan” Mango Peel Pectin (Initial MPP) | Pectin Yield (%) | Degree of Esterification (%) | Equivalent Weight (mg/mol) | Methoxyl Content (%) | |||
|---|---|---|---|---|---|---|---|
| 15.06 ± 0.29 | 56.88 ± 0.78 | 1037.30 ± 4.96 | 4.00 ± 0.03 | ||||
| Hydrolysis Time (h) | Enzyme Concentration (%v/v) | Molecular Weight (Da) | Monosaccharide Content (%w/w) | ||||
| Glucose | Fructose | Galactose | Arabinose | Xylose | |||
| Initial MPP | - | - | 6.28 ± 0.03 | 5.43 ± 0.08 | 0.75 ± 0.03 | 0.25 ± 0.02 | Tr |
| 6 | 0.1 | <1000 (790) | 14.08 ± 0.23 e | 18.79 ± 0.15 e | 2.07 ± 0.00 i | 1.07 ± 0.04 i | Tr |
| 0.2 | <1000 (759) | 14.35 ± 0.18 e | 18.72 ± 0.14 e | 2.18 ± 0.00 h | 1.33 ± 0.04 h | Tr | |
| 0.3 | <1000 (737) | 14.37 ± 0.17 e | 18.99 ± 0.13 e | 2.34 ± 0.02 g | 1.47 ± 0.00 g | Tr | |
| 12 | 0.1 | <1000 (697) | 15.61 ± 0.30 d | 20.47 ± 0.23 d | 2.76 ± 0.00 f | 1.68 ± 0.07 f | Tr |
| 0.2 | <1000 (693) | 16.52 ± 0.00 c | 20.56 ± 0.40 d | 2.81 ± 0.03 e | 1.91 ± 0.00 e | Tr | |
| 0.3 | <1000 (681) | 17.93 ± 0.30 b | 21.43 ± 0.19 c | 2.88 ± 0.02 d | 2.13 ± 0.03 d | Tr | |
| 24 | 0.1 | <1000 (666) | 19.01 ± 0.19 a | 22.49 ± 0.16 b | 3.05 ± 0.02 c | 2.31 ± 0.02 c | Tr |
| 0.2 | <1000 (660) | 19.21 ± 0.48 a | 22.70 ± 0.23 b | 3.10 ± 0.01 b | 2.40 ± 0.01 b | Tr | |
| 0.3 | <1000 (643) | 19.52 ± 0.55 a | 24.41 ± 1.02 a | 3.35 ± 0.01 a | 3.02 ± 0.03 a | Tr | |
| Time (T) | - | * | * | * | * | n/a | |
| Enzyme concentration (E) | - | * | * | * | * | n/a | |
| T*E | - | * | * | * | * | n/a | |
| Carbon Sources | L. reuteri DSM 17938 | B. animalis TISTR 2195 |
|---|---|---|
| Control | 0.08 ± 0.00 Da | 0.05 ± 0.00 Da |
| 1% MPOS | 0.01 ± 0.00 Fb | 0.24 ± 0.02 Ca |
| 2% MPOS | 0.06 ± 0.00 Eb | 0.41 ± 0.00 Ba |
| 4% MPOS | 0.62 ± 0.00 Ba | 0.002 ± 0.00 Eb |
| 2%MPP | 0.13 ± 0.01 Ca | 0.03 ± 0.01 DEb |
| 2%Glucose | 4.18 ± 0.04 Aa | 0.96 ± 0.05 Ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wongkaew, M.; Tinpovong, B.; Sringarm, K.; Leksawasdi, N.; Jantanasakulwong, K.; Rachtanapun, P.; Hanmoungjai, P.; Sommano, S.R. Crude Pectic Oligosaccharide Recovery from Thai Chok Anan Mango Peel Using Pectinolytic Enzyme Hydrolysis. Foods 2021, 10, 627. https://doi.org/10.3390/foods10030627
Wongkaew M, Tinpovong B, Sringarm K, Leksawasdi N, Jantanasakulwong K, Rachtanapun P, Hanmoungjai P, Sommano SR. Crude Pectic Oligosaccharide Recovery from Thai Chok Anan Mango Peel Using Pectinolytic Enzyme Hydrolysis. Foods. 2021; 10(3):627. https://doi.org/10.3390/foods10030627
Chicago/Turabian StyleWongkaew, Malaiporn, Bow Tinpovong, Korawan Sringarm, Noppol Leksawasdi, Kittisak Jantanasakulwong, Pornchai Rachtanapun, Prasert Hanmoungjai, and Sarana Rose Sommano. 2021. "Crude Pectic Oligosaccharide Recovery from Thai Chok Anan Mango Peel Using Pectinolytic Enzyme Hydrolysis" Foods 10, no. 3: 627. https://doi.org/10.3390/foods10030627