Encapsulation of Olive Pomace Extract in Rocket Seed Gum and Chia Seed Gum Nanoparticles: Characterization, Antioxidant Activity and Oxidative Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extractions of Rocket Seed and Chia Seed Gum
2.2. Proximate Analysis of Olive Pomace Powder, Rocket and Chia Seed Gum
2.3. Preparations of Olive Pomace Extract
2.4. Phenolic Compounds of OPE by HPLC Analysis
2.5. Preparation of RSG and CSG Solutions
2.6. Fabrication of RSG and CSG Nanoparticles
2.7. Characterization of Nanoparticles
2.7.1. Nanoparticle Size and Zeta Potential
2.7.2. Nanoparticle Morphology
2.7.3. Encapsulation Efficiency
2.7.4. Thermal Properties
2.7.5. Fourier-Transform Infrared Spectroscopy (FTIR)
2.7.6. In Vitro Release Study
2.7.7. Determination of TPC and Antioxidant Activity of Nanoparticles
2.8. Pickering Emulsion
2.8.1. Preparation Procedure
2.8.2. Oxidative Stability Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Analysis of OPP, RSG, and CSG
3.2. Characterization of NPs
3.2.1. Particle Size and Surface Charge
3.2.2. Encapsulation Efficiency
3.2.3. Nanoparticle Morphology
3.2.4. Thermal Properties
3.2.5. FTIR Spectroscopy
3.2.6. In Vitro Release
3.2.7. Antioxidant Activity of the Nanoparticles
3.2.8. Oxidative Stability of the Pickering Emulsions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aliakbarian, B.; Paini, M.; Adami, R.; Perego, P.; Reverchon, E. Use of Supercritical Assisted Atomization to produce nanoparticles from olive pomace extract. Innov. Food Sci. Emerg. Technol. 2017, 40, 2–9. [Google Scholar] [CrossRef]
- Alagna, F.; Mariotti, R.; Panara, F.; Caporali, S.; Urbani, S.; Veneziani, G.; Esposto, S.; Taticchi, A.; Rosati, A.; Rao, R.; et al. Olive phenolic compounds: Metabolic and transcriptional profiling during fruit development. BMC Plant Biol. 2012, 12, 162. [Google Scholar] [CrossRef] [Green Version]
- Rigane, G.; Bouaziz, M.; Baccar, N.; Abidi, S.; Sayadi, S.; Ben Salem, R. Recovery of Hydroxytyrosol Rich Extract from Two-Phase Chemlali Olive Pomace by Chemical Treatment. J. Food Sci. 2012, 77, C1077–C1083. [Google Scholar] [CrossRef] [PubMed]
- Speroni, C.S.; Guerra, D.R.; Bender, A.B.B.; Stiebe, J.; Ballus, C.A.; da Silva, L.P.; Lozano-Sánchez, J.; Emanuelli, T. Micronization increases the bioaccessibility of polyphenols from granulometrically separated olive pomace fractions. Food Chem. 2020, 344, 128689. [Google Scholar] [CrossRef] [PubMed]
- Chanioti, S.; Tzia, C. Extraction of phenolic compounds from olive pomace by using natural deep eutectic solvents and innovative extraction techniques. Innov. Food Sci. Emerg. Technol. 2018, 48, 228–239. [Google Scholar] [CrossRef]
- Chanioti, S.; Katsouli, M.; Tzia, C. Novel Processes for the Extraction of Phenolic Compounds from Olive Pomace and Their Protection by Encapsulation. Molecules 2021, 26, 1781. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Zandi, M.; Rezaei, M.; Farahmandghavi, F. Two-step method for encapsulation of oregano essential oil in chitosan nanoparticles: Preparation, characterization and in vitro release study. Carbohydr. Polym. 2013, 95, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Esfanjani, A.F.; Assadpour, E.; Jafari, S.M. Improving the bioavailability of phenolic compounds by loading them within lipid-based nanocarriers. Trends Food Sci. Technol. 2018, 76, 56–66. [Google Scholar] [CrossRef]
- Soppimath, K.S.; Aminabhavi, T.M.; Kulkarni, A.R.; Rudzinski, E.W. Biodegradable polymeric nanoparticles as drug delivery devices. J. Control. Release 2001, 70, 1–20. [Google Scholar] [CrossRef]
- Reis, C.; Neufeld, R.J.; Ribeiro, A.; Veiga, F. Nanoencapsulation I. Methods for preparation of drug-loaded polymeric nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2006, 2, 8–21. [Google Scholar] [CrossRef] [Green Version]
- Esfanjani, A.F.; Jafari, S.M. Biopolymer nano-particles and natural nano-carriers for nano-encapsulation of phenolic compounds. Colloids Surf. B Biointerfaces 2016, 146, 532–543. [Google Scholar] [CrossRef] [PubMed]
- de Campo, C.; Dos Santos, P.P.; Costa TM, H.; Paese, K.; Guterres, S.S.; de Oliveira Rios, A.; Flôres, S.H. Nanoencapsulation of chia seed oil with chia mucilage (Salvia hispanica L.) as wall material: Characterization and stability evaluation. Food Chem. 2017, 234, 1–9. [Google Scholar] [CrossRef]
- Mozafari, M.R.; Flanagan, J.; Matia-Merino, L.; Awati, A.; Omri, A.; Suntres, E.Z.; Singh, H. Recent trends in the lipid-based nanoencapsulation of antioxidants and their role in foods. J. Sci. Food Agric. 2006, 86, 2038–2045. [Google Scholar] [CrossRef]
- Taheri, A.; Razavi, S.M.A. Fabrication of Cress Seed Gum Nanoparticles, an Anionic Polysaccharide, Using Desolvation Technique: An Optimization Study. BioNanoScience 2015, 5, 104–116. [Google Scholar] [CrossRef]
- Rezaei, A.; Fathi, M.; Jafari, S.M. Nanoencapsulation of hydrophobic and low-soluble food bioactive compounds within different nanocarriers. Food Hydrocoll. 2019, 88, 146–162. [Google Scholar] [CrossRef]
- Tabasi, S.N.; Razavi, S.M.A.; Mehditabar, H. Fabrication of basil seed gum nanoparticles as a novel oral delivery system of glutathione. Carbohydr. Polym. 2017, 157, 1703–1713. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ballesta, M.; Gil-Izquierdo, Ángel; García-Viguera, C.; Domínguez-Perles, R. Nanoparticles and Controlled Delivery for Bioactive Compounds: Outlining Challenges for New “Smart-Foods” for Health. Foods 2018, 7, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doost, A.S.; Kassozi, V.; Grootaert, C.; Claeys, M.; Dewettinck, K.; Van Camp, J.; Van Der Meeren, P. Self-assembly, functionality, and in-vitro properties of quercetin loaded nanoparticles based on shellac-almond gum biological macromolecules. Int. J. Biol. Macromol. 2019, 129, 1024–1033. [Google Scholar] [CrossRef]
- Mishra, D.; Hubenak, J.R.; Mathur, A.B. 2013. Nanoparticle systems as tools to improve drug delivery and therapeutic efficacy. J. Biomed. Mater. Res. Part A 2013, 101, 3646–3660. [Google Scholar] [CrossRef]
- Prajapati, V.D.; Jani, G.K.; Moradiya, N.G.; Randeria, N.P. Pharmaceutical applications of various natural gums, mucilages and their modified forms. Carbohydr. Polym. 2013, 92, 1685–1699. [Google Scholar] [CrossRef]
- Koocheki, A.; Razavi, S.M.A.; Hesarinejad, M.A. Effect of Extraction Procedures on Functional Properties of Eruca sativa Seed Mucilage. Food Biophys. 2012, 7, 84–92. [Google Scholar] [CrossRef]
- da Silva Marineli, R.; Moraes, É.A.; Lenquiste, S.A.; Godoy, A.T.; Eberlin, M.N.; Maróstica, M.R., Jr. Chemical characterization and antioxidant potential of Chilean chia seeds and oil (Salvia hispanica L.). LWT Food Sci. Technol. 2014, 59, 1304–1310. [Google Scholar] [CrossRef]
- Timilsena, Y.; Adhikari, R.; Barrow, C.J.; Adhikari, B. Microencapsulation of chia seed oil using chia seed protein isolate⿿chia seed gum complex coacervates. Int. J. Biol. Macromol. 2016, 91, 347–357. [Google Scholar] [CrossRef]
- Soleimanifar, M.; Jafari, S.M.; Assadpour, E. Encapsulation of olive leaf phenolics within electrosprayed whey protein nanoparticles; production and characterization. Food Hydrocoll. 2020, 101, 105572. [Google Scholar] [CrossRef]
- Kesente, M.; Kavetsou, E.; Roussaki, M.; Blidi, S.; Loupassaki, S.; Chanioti, S.; Siamandoura, P.; Stamatogianni, C.; Philippou, E.; Papaspyrides, C.; et al. Encapsulation of Olive Leaves Extracts in Biodegradable PLA Nanoparticles for Use in Cosmetic Formulation. Bioengineering 2017, 4, 75. [Google Scholar] [CrossRef] [Green Version]
- Chanioti, S.; Siamandoura, P.; Tzia, C. Evaluation of Extracts Prepared from Olive Oil By-Products Using Microwave-Assisted Enzymatic Extraction: Effect of Encapsulation on the Stability of Final Products. Waste Biomass Valorization 2016, 7, 831–842. [Google Scholar] [CrossRef]
- Muzzalupo, I.; Badolati, G.; Chiappetta, A.; Picci, N.; Muzzalupo, R. In vitro Antifungal Activity of Olive (Olea europaea) Leaf Extracts Loaded in Chitosan Nanoparticles. Front. Bioeng. Biotechnol. 2020, 8, 151. [Google Scholar] [CrossRef] [Green Version]
- Razavi, S.M.A.; Mortazavi, S.A.; Matia-Merino, L.; Hosseini-Parvar, S.H.; Motamedzadegan, A.; Khanipour, E. Optimisation study of gum extraction from Basil seeds (Ocimum basilicum L.). Int. J. Food Sci. Technol. 2009, 44, 1755–1762. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis Association of Official Analytical Chemists; AOAC: Washington, DC, USA, 2000. [Google Scholar]
- Bak, J.H.; Yoo, B. Intrinsic viscosity of binary gum mixtures with xanthan gum and guar gum: Effect of NaCl, sucrose, and pH. Int. J. Biol. Macromol. 2018, 111, 77–81. [Google Scholar]
- Timilsena, Y.; Adhikari, R.; Kasapis, S.; Adhikari, B. Rheological and microstructural properties of the chia seed polysaccharide. Int. J. Biol. Macromol. 2015, 81, 991–999. [Google Scholar] [CrossRef]
- Kutlu, G.; Bozkurt, F.; Tornuk, F. Extraction of a novel water-soluble gum from nettle (Urtica dioica) seeds: Optimization and characterization. Int. J. Biol. Macromol. 2020, 162, 480–489. [Google Scholar]
- Kutlu, G.; Akcicek, A.; Bozkurt, F.; Karasu, S.; Tekin-Cakmak, Z.H. Rocket seed (Eruca sativa Mill) gum: Physicochemical and comprehensive rheological characterization. Food Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144. [Google Scholar]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E. Novel Total Antioxidant Capacity Index for Dietary Polyphenols and Vitamins C and E, Using Their Cupric Ion Reducing Capability in the Presence of Neocuproine: CUPRAC Method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef]
- Karazhiyan, H.; Razavi, S.M.; Phillips, G.O. Extraction optimization of a hydrocolloid extract from cress seed (Lepidium sativum) using response surface methodology. Food Hydrocoll. 2011, 25, 915–920. [Google Scholar] [CrossRef]
- Bustamante, M.; Oomah, B.D.; Rubilar, M.; Shene, C. Effective Lactobacillus plantarum and Bifidobacterium infantis encapsulation with chia seed (Salvia hispanica L.) and flaxseed (Linum usitatissimum L.) mucilage and soluble protein by spray drying. Food Chem. 2017, 216, 97–105. [Google Scholar] [CrossRef]
- Timilsena, Y.; Adhikari, R.; Kasapis, S.; Adhikari, B. Molecular and functional characteristics of purified gum from Australian chia seeds. Carbohydr. Polym. 2016, 136, 128–136. [Google Scholar] [CrossRef]
- Behrouzian, F.; Hojjat, K.; Seyed, M.A.R. Intrinsic viscosity of cress (Lepidium sativum) seed gum: Effect of salts and sugars. Food Hydrocoll. 2014, 35, 100–105. [Google Scholar] [CrossRef]
- Skaltsounis, A.L.; Argyropoulou, A.; Aligiannis, N.; Xynos, N. 11—Recovery of High Added Value Compounds from Olive Tree Products and Olive Processing Byproducts. In Olive and Olive Oil Bioactive Constituents; Boskou, D., Ed.; AOCS Press: Champaign, IL, USA, 2015; pp. 333–356. [Google Scholar]
- Nunes, M.A.; da Costa, A.G.; Bessada, S.; Santos, J.; Puga, H.; Alves, R.C.; Freitas, V.; Oliveira, B. Olive pomace as a valuable source of bioactive compounds: A study regarding its lipid- and water-soluble components. Sci. Total. Environ. 2018, 644, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Malapert, A.; Reboul, E.; Loonis, M.; Dangles, O.; Tomao, V. Direct and Rapid Profiling of Biophenols in Olive Pomace by UHPLC-DAD-MS. Food Anal. Methods 2017, 11, 1001–1010. [Google Scholar] [CrossRef]
- Pereira, M.C.; Oliveira, D.A.; Hill, L.E.; Zambiazi, R.C.; Borges, C.D.; Vizzotto, M.; Mertens-Talcott, S.; Talcott, S.; Gomes, C.L. Effect of nanoencapsulation using PLGA on antioxidant and antimicrobial activities of guabiroba fruit phenolic extract. Food Chem. 2018, 240, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Hashemi, M.; Hosseini, S.M. Effect of chitosan molecular weight as micro and nanoparticles on antibacterial activity against some soft rot pathogenic bacteria. LWT 2016, 71, 347–355. [Google Scholar] [CrossRef]
- Perduca, M.J.; Spotti, M.J.; Santiago, L.G.; Judis, M.A.; Rubiolo, A.C.; Carrara, C.R. Rheological characterization of the hydrocolloid from Gleditsia amorphoides seeds. LWT 2013, 51, 143–147. [Google Scholar] [CrossRef]
- Mainardes, R.M.; Evangelista, R.C. Praziquantel-loaded PLGA nanoparticles: Preparation and characterization. J. Microencapsul. 2005, 22, 13–24. [Google Scholar] [CrossRef]
- Joye, I.J.; McClements, D.J. Production of nanoparticles by anti-solvent precipitation for use in food systems. Trends Food Sci. Technol. 2013, 34, 109–123. [Google Scholar] [CrossRef]
- Muhammad, D.R.A.; Doost, A.S.; Gupta, V.; Bin Sintang, M.D.; Van de Walle, D.; Van der Meeren, P.; Dewettinck, K. Stability and functionality of xanthan gum–shellac nanoparticles for the encapsulation of cinnamon bark extract. Food Hydrocoll. 2020, 100, 105377. [Google Scholar] [CrossRef]
- González, A.; Martinez, M.L.; Paredes, A.; León, A.E.; Ribotta, P.D. Study of the preparation process and variation of wall components in chia (Salvia hispanica L.) oil microencapsulation. Powder Technol. 2016, 301, 868–875. [Google Scholar] [CrossRef] [Green Version]
- Joye, I.J.; Davidov-Pardo, G.; McClements, D. Encapsulation of resveratrol in biopolymer particles produced using liquid antisolvent precipitation. Part 2: Stability and functionality. Food Hydrocoll. 2015, 49, 127–134. [Google Scholar] [CrossRef]
- Doost, A.S.; Muhammad, D.R.A.; Stevens, C.V.; Dewettinck, K.; Van Der Meeren, P. Fabrication and characterization of quercetin loaded almond gum-shellac nanoparticles prepared by antisolvent precipitation. Food Hydrocoll. 2018, 83, 190–201. [Google Scholar] [CrossRef]
- Mourtzinos, I.; Salta, F.; Yannakopoulou, K.; Chiou, A.; Karathanos, V.T. Encapsulation of olive leaf extract in beta-cyclodextrin. J. Agric. Food Chem. 2007, 55, 8088–8094. [Google Scholar] [CrossRef]
- Paulo, F.; Santos, L. Encapsulation of the Antioxidant Tyrosol and Characterization of Loaded Microparticles: An Integrative Approach on the Study of the Polymer-Carriers and Loading Contents. Food Bioprocess Technol. 2020, 13, 764–785. [Google Scholar] [CrossRef]
- Pool, H.R.; Quintanar, D.; Figueroa, J.D.D.; Mano, C.M.; Bechara, E.; Godínez, L.A.; Mendoza, S. Antioxidant Effects of Quercetin and Catechin Encapsulated into PLGA Nanoparticles. J. Nanomater. 2012, 2012. [Google Scholar] [CrossRef]
- Bahreini, E.; Aghaiypour, K.; Abbasalipourkabir, R.; Mokarram, A.R.; Goodarzi, M.T.; Saidijam, M. Preparation and nanoencapsulation of l-asparaginase II in chitosan-tripolyphosphate nanoparticles and in vitro release study. Nanoscale Res. Lett. 2014, 9, 340. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Zhong, Q. Processes improving the dispersibility of spray-dried zein nanoparticles using sodium caseinate. Food Hydrocoll. 2014, 35, 358–366. [Google Scholar] [CrossRef]
- Shaygannia, S.; Eshaghi, M.R.; Fazel, M.; Hashemiravan, M. Phenolic compounds and antioxidant activities of lemon wastes affected by microencapsulation using coatings of Arabic, Persian, and basil seed gums. J. Food Meas. Charact. 2021, 15, 1452–1462. [Google Scholar] [CrossRef]
- Wang, J.; Hu, S.; Nie, S.; Yu, Q.; Xie, M. Reviews on Mechanisms ofIn VitroAntioxidant Activity of Polysaccharides. Oxidative Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Malsawmtluangi, C.; Thanzami, K.; Lalhlenmawia, H.; Selvan, V.; Palanisamy, S.; Kandasamy, R.; Pachuau, L. Physicochemical characteristics and antioxidant activity of Prunus cerasoides D. Don gum exudates. Int. J. Biol. Macromol. 2014, 69, 192–199. [Google Scholar] [CrossRef]
- Ahmed, M.; Akter, M.S.; Lee, J.-C.; Eun, J.-B. Encapsulation by spray drying of bioactive components, physicochemical and morphological properties from purple sweet potato. LWT 2010, 43, 1307–1312. [Google Scholar] [CrossRef]
- Aksoy, F.S.; Tekin-Cakmak, Z.H.; Karasu, S.; Aksoy, A.S. Oxidative stability of the salad dressing enriched by microencapsulated phenolic extracts from cold-pressed grape and pomegranate seed oil by-products evaluated using OXITEST. Food Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Waraho, T.; McClements, D.; Decker, E.A. Mechanisms of lipid oxidation in food dispersions. Trends Food Sci. Technol. 2011, 22, 3–13. [Google Scholar] [CrossRef]



| Sample | Olive Pomace Powder | Rocket Seed Gum | Chia Seed Gum |
|---|---|---|---|
| pH | 5.06 ± 0.1 | 6.24 ± 0.14 | 6.85 ± 0.15 |
| Carbonhydrates (% w/w) | 78.95 ± 0.84 | 57.49 ± 1.41 | 67.58 ± 1.11 |
| Fat (% w/w) | 9.21 ± 0.21 | 0.69 ± 0.04 | 1.23 ± 0.14 |
| Ash (% w/w) | 3.39 ± 0.29 | 8.26 ± 0.37 | 9.23 ± 0.35 |
| Moisture (% w/w) | 3.9 ± 0.04 | 10.5 ± 0.5 | 9.5 ± 0.5 |
| Protein (% w/w) | 4.55 ± 0.30 | 23.01 ± 0.55 | 12.46 ± 0.12 |
| Instrinct Viscosity (dL/g) | n.d. | 3.69 ± 0.4 | 19.13 ± 0.81 |
| Molecular Weight (Da) | n.d. | 6.82 ± 0.75 × 105 | 2.23 ± 0.12 × 106 |
| Phenolic Compounds | Retention Time (min) | Concentration (µg/g Dried OPE) |
|---|---|---|
| Gallic acid | 5.867 | 314.4 |
| Protocatechuic acid | 8.864 | 60.4 |
| Hydroxytyrosol | 9.3 | 2857.0 |
| Catechin | 12.360 | 48.8 |
| p-Hydroxybenzoic acid | 13.429 | 15.6 |
| Tyrosol | 14.3 | 358.8 |
| Syringic acid | 14.919 | 16.4 |
| Elagic acid | 20.495 | 134.4 |
| m-Coumaric acid | 22.623 | 15.2 |
| o-Coumaric acid | 25.082 | 2.8 |
| Myricetin | 26.950 | 348.4 |
| Quercetin | 33.083 | 217.2 |
| Kaempferol | 36.162 | 68.8 |
| Luteolin | 74.8 | 715.6 |
| Sample | Particle Size (nm) | PDI | Zeta Potential (mV) | EE (%) |
|---|---|---|---|---|
| RSGNP | 304.1 ± 4.49 D | 0.395 ± 0.01 C | −23.1 ± 0.85 A | |
| OPE-RSGNP | 318 ± 3.11 C | 0.514 ± 0.06 A | −22.6 ± 1.23 A | 82.86 ± 4.13 A |
| CSGNP | 425.26 ± 6.49 B | 0.485 ± 0.08 B | −28.1 ± 0.95 B | |
| OPE-CSGNP | 490 ± 8.67 A | 0.483 ± 0.10 B | −29.9 ± 2.57 B | 67.01 ± 4.29 B |
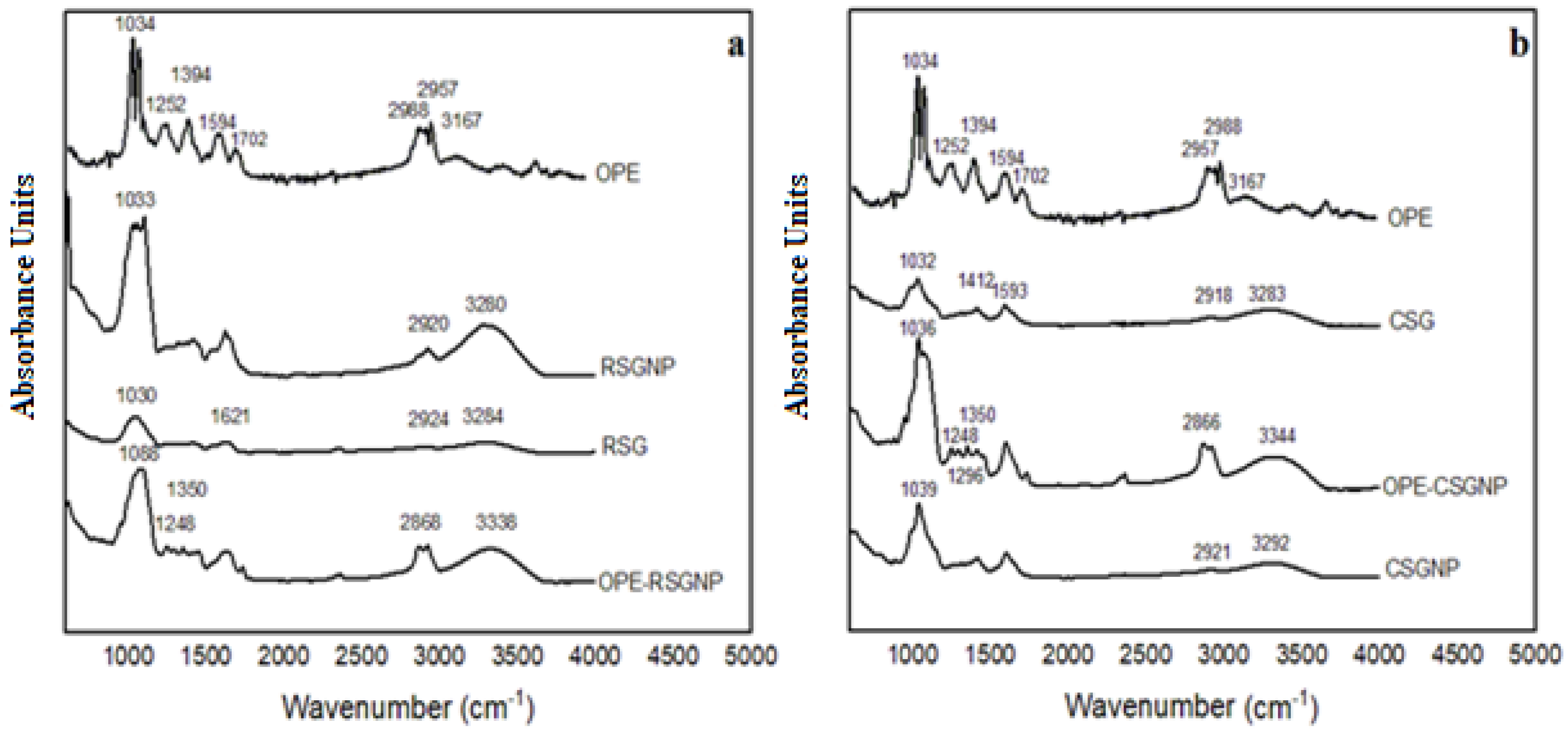
| FTIR Spectrum of | Compared with FTIR Bands of | Band (cm−1) Observed | Observed Shift after Encapsulation |
|---|---|---|---|
| OPE-RSGNP | OPE | 2988 and 2957 cm−1 C-H stretching, especially asymmetric and symmetric vibration | 2868 cm−1 |
| 3167 cm−1 corresponding to O-H hydroxyl group | 3338 cm−1 | ||
| 1594, 1702 cm−1 corresponding to C=O stretching of the carbonyl groups | - | ||
| 1104, 1074, 1034 cm−1 attributed to the C-O stretching of the ester groups | 1088 cm−1 | ||
| 1394 and 1252 cm−1 corresponding to stretching of C-O groups and deformation of O-H, respectively | 1350 cm−1 1248 cm−1 | ||
| RSGNP | 3280 cm−1 corresponding to O-H hydroxyl group | 3338 cm−1 | |
| 2920 cm−1 the bond between the C-H groups, namely the CH2 stretch | 2868 cm−1 | ||
| 1033 cm−1 shows the C-O-C bonds | 1088 cm−1 | ||
| OPE-CSGNP | OPE | 2988 and 2957 cm−1 C-H stretching especially asymmetric and symmetric vibration | 2866 cm−1 |
| 3167 cm−1 corresponding to O-H hydroxyl group | 3344 cm−1 | ||
| 1594, 1702 cm−1 corresponding to C=O stretching of the carbonyl groups | - | ||
| 1104, 1074, 1034 cm−1 attributed to the C-O stretching of the ester groups | 1071 cm−1 1036 cm−1 | ||
| 1394 and 1252 cm−1 corresponding to stretching of C-O groups and deformation of O-H, respectively | 1350 cm−1 1296 cm−1 1248 cm−1 | ||
| CSGNP | 3292 cm−1 corresponding to O-H hydroxyl group | 3344 cm−1 | |
| 2921 cm−1 the bond between the C-H groups, namely the CH2 stretch | 2866 cm−1 | ||
| 1039 shows the C-O-C bonds | 1071 cm−1 1036 cm−1 |
| Sample | Bioactive Properties of OPE and Gum Nanoparticles | Oxidative Stability of Pickering Emulsions | |
|---|---|---|---|
| TPC (mg GAE/g) | CUPRAC (mg TE/ g) | IP Value of | |
| OPE | 44.24 ± 0.22 | 254.56 ± 1.70 | 2.51 ± 0.02 |
| OPE-RSGNP | 27.92 ± 1.01 A | 235.28 ± 0.36 A | 4.39 ± 0.11 A |
| RSGNP | 17.08 ± 0.5 B | 148.49 ± 7.45 B | 4.10 ± 0.08 B |
| OPE-CSGNP | 19.52 ± 0.33 a | 212.07 ± 50 a | 3.23 ± 0.07 a |
| CSGNP | 10.25 ± 1.13 b | 135.28 ± 0.36 b | 2.59 ± 0.07 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akcicek, A.; Bozkurt, F.; Akgül, C.; Karasu, S. Encapsulation of Olive Pomace Extract in Rocket Seed Gum and Chia Seed Gum Nanoparticles: Characterization, Antioxidant Activity and Oxidative Stability. Foods 2021, 10, 1735. https://doi.org/10.3390/foods10081735
Akcicek A, Bozkurt F, Akgül C, Karasu S. Encapsulation of Olive Pomace Extract in Rocket Seed Gum and Chia Seed Gum Nanoparticles: Characterization, Antioxidant Activity and Oxidative Stability. Foods. 2021; 10(8):1735. https://doi.org/10.3390/foods10081735
Chicago/Turabian StyleAkcicek, Alican, Fatih Bozkurt, Cansu Akgül, and Salih Karasu. 2021. "Encapsulation of Olive Pomace Extract in Rocket Seed Gum and Chia Seed Gum Nanoparticles: Characterization, Antioxidant Activity and Oxidative Stability" Foods 10, no. 8: 1735. https://doi.org/10.3390/foods10081735






