Sugar-Free Milk Chocolate as a Carrier of Omega-3 Polyunsaturated Fatty Acids and Probiotics: A Potential Functional Food for the Diabetic Population
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Chemicals
2.2. Bacterial Strains’ Propagation, Microencapsulation, and Viability Assessment
2.3. Chocolate Preparation
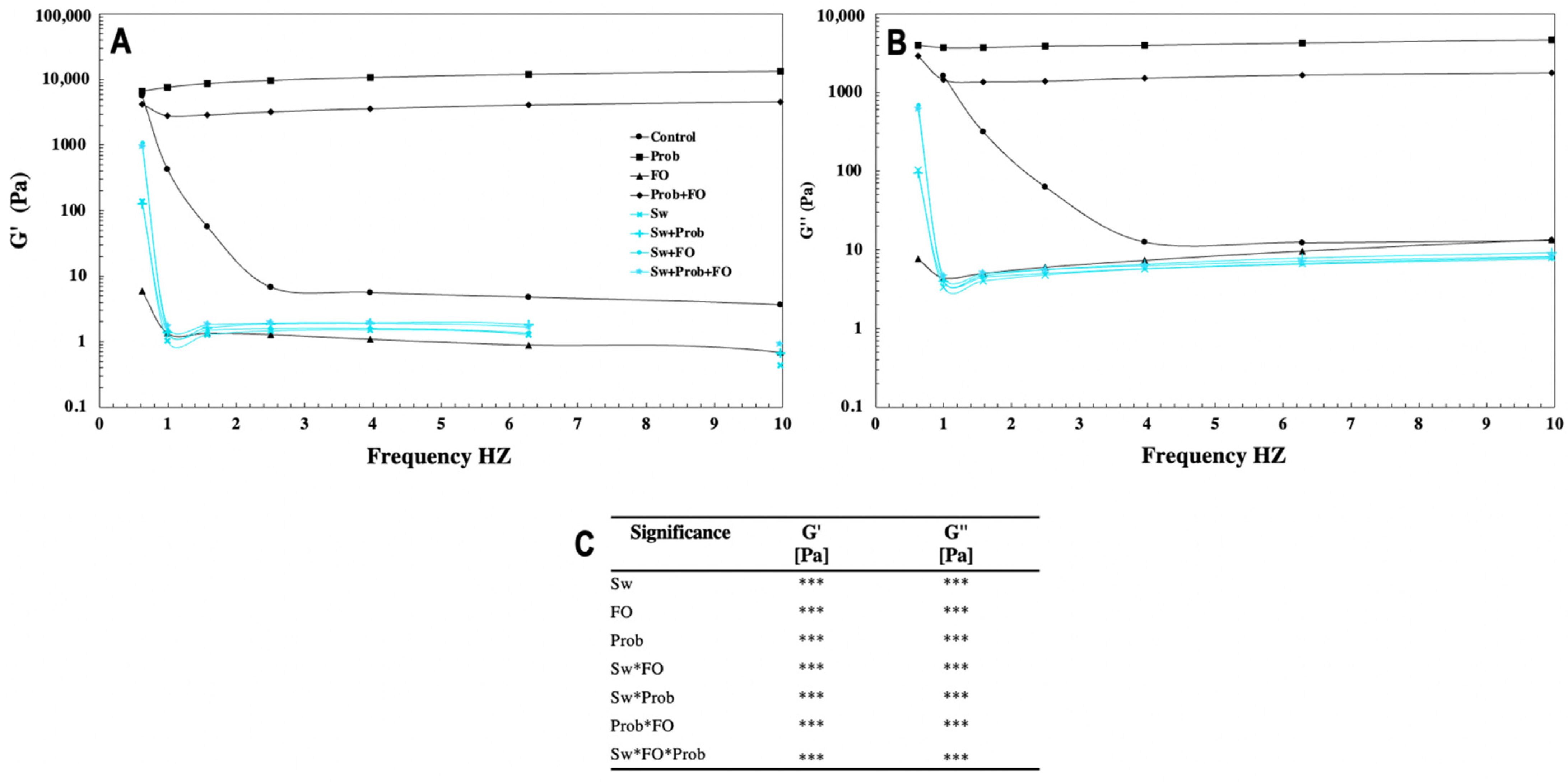
2.4. Water Activity, Color, Texture, and Rheological Determinations
2.5. Fatty Acid Methyl Esters (FAMEs) Profile
2.6. Sensory Evaluation
2.7. Statistical Analysis
3. Results and Discussion
3.1. Probiotics Viability
3.2. Physicochemical Properties of Sugar-Free Milk Chocolate Formulations with Added Probiotics and Fish Oil
3.2.1. Water Activity (aw)
3.2.2. Whiteness Index (WI)
3.2.3. Texture
3.3. Rheological Analysis: Shear Stress, Apparent Viscosity, and Frequency Sweep Test
3.3.1. Flow Behavior
3.3.2. Mechanical Properties
3.4. Fatty Acid Methyl Esters (FAMEs) Profile
3.5. Consumers’ Acceptability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stanhope, K.L. Sugar consumption, metabolic disease and obesity: The state of the controversy. Crit. Rev. Clin. Lab. Sci. 2016, 53, 52–67. [Google Scholar] [CrossRef]
- Gómez-Fernández, A.R.; Santacruz, A.; Jacobo-Velázquez, D.A. The complex relationship between metabolic syndrome and sweeteners. J. Food Sci. 2021, 86, 1511–1531. [Google Scholar] [CrossRef]
- Konar, N.; Toker, O.S.; Pirouzian, H.R.; Oba, S.; Polat, D.G.; Palabiyik, İ.; Polyrazoglu, E.S.; Sagdic, O. Enrichment of milk chocolate by using EPA and DHA originated from various origins: Effects on product quality. Sugar Tech 2018, 20, 745–755. [Google Scholar] [CrossRef]
- De Felice, S.L. The nutraceutical revolution: Its impact on food industry R&D. Trends Food Sci. Technol. 1995, 6, 59–61. [Google Scholar]
- Santini, A.; Novellino, E. Nutraceuticals-shedding light on the grey area between pharmaceuticals and foods. Expert Rev. Clin. Pharmacol. 2018, 11, 545–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skytte, U.P.; Kaylegian, K.E. Ingredients from milk. In Beckett’s Industrial Chocolate Manufacture and Use; Beckett, S.T., Fowler, M.S., Ziegler, G.R., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2017; pp. 102–134. [Google Scholar]
- Shah, A.B.; Jones, G.P.; Vasiljevic, T. Sucrose-free chocolate sweetened with Stevia rebaudiana extract and containing different bulking agents–effects on physicochemical and sensory properties. Int. J. Food Sci. Technol. 2010, 45, 1426–1435. [Google Scholar] [CrossRef]
- Carocho, M.; Morales, P.; Ferreira, I.C.F.R. Sweeteners as food additives in the XXI century: A review of what is known, and what is to come. Food Chem. Toxicol. 2017, 107, 302–317. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ojeda, F.J.; Plaza-Díaz, J.; Sáez-Lara, M.J.; Gil, A. Effects of sweeteners on the gut microbiota: A review of experimental studies and clinical trials. Adv. Nutr. 2019, 10, S31–S48. [Google Scholar] [CrossRef] [Green Version]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Philippaert, K.; Pironet, A.; Mesuere, M.; Sones, W.; Vermeiren, L.; Kerselaers, S.; Pinto, S.; Antoine, N.; Gysemans, C.; Laureys, J.; et al. Steviol glycosides enhance pancreatic beta-cell function and taste sensation by potentiation of TRPM5 channel activity. Nat. Commun. 2017, 8, 14733. [Google Scholar] [CrossRef] [PubMed]
- Rahmawaty, S.; Lyons-Wall, P.; Batterham, M.; Charlton, K.; Meyer, B.J. Food patterns of Australian children ages 9 to 13 y in relation to ω-3 long chain polyunsaturated intake. Nutrition 2014, 30, 169–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, H.; Geng, T.; Huang, T.; Zhao, Q. Fish oil supplementation and insulin sensitivity: A systematic review and meta-analysis. Lipids Health Dis. 2017, 16, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Interim Summary of Conclusions and Dietary Recommendations on Total Fat and Fatty Acids. Available online: https://www.who.int/nutrition/topics/FFA_summary_rec_conclusion.pdf?ua=1 (accessed on 28 June 2021).
- Albert, B.B.; Derraik, J.G.B.; Brennan, C.M.; Biggs, J.B.; Smith, G.C.; Garg, M.L.; Cameron-Smith, D.; Hofman, P.L.; Cutfield, W.S. Higher omega-3 index is associated with increased insulin sensitivity and more favorable metabolic profile in middle-aged overweight men. Sci. Rep. 2014, 4, 6697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Hage, R.; Hernandez-Sanabria, E.; Van de Wiele, T. Emerging trends in “smart probiotics”: Functional consideration for the development of novel health and industrial applications. Front. Microbiol. 2017, 8, 1889. [Google Scholar] [CrossRef] [PubMed]
- Guidelines for the Evaluation of Probiotics in Food. Available online: https://www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf (accessed on 28 June 2021).
- Sohag, M.S.U.; Paul, M.; Al-Bari, M.A.A.; Wahed, M.I.I.; Khan, M.R.I. Potential antidiabetic activities of probiotic strains, L. acidophilus and L. bulgaricus against fructose-fed hyperglycemic rats. Food Nutr. Sci. 2019, 10, 1419–1432. [Google Scholar]
- Stevenson, C.; Blaauw, R.; Fredericks, E.; Visser, J.; Roux, S. Randomized clinical trial: Effect of lactobacillus plantarum 299 v on symptoms of irritable bowel syndrome. Nutrition 2014, 30, 1151–1157. [Google Scholar] [CrossRef]
- Marcial-Coba, M.S.; Saaby, L.; Knøchel, S.; Nielsen, D.S. Dark chocolate as a stable carrier of microencapsulated Akkermansia muciniphila and Lactobacillus casei. FEMS Microbiol. Lett. 2019, 366, fny290. [Google Scholar] [CrossRef]
- Mirković, M.; Seratlić, S.; Kilcawley, K.; Mannion, D.; Mirković, N.; Radulović, Z. The sensory quality and volatile profile of dark chocolate enriched with encapsulated probiotic Lactobacillus plantarum bacteria. Sensors 2018, 18, 2570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glicerina, V.; Balestra, F.; Rosa, M.D.; Romani, S. Effect of manufacturing process on the microstructural and rheological properties of milk chocolate. J. Food Eng. 2015, 145, 45–50. [Google Scholar] [CrossRef]
- Gonçalves, E.V.; Lannes, S.C.d.S. Chocolate rheology. Food Sci. Technol. 2010, 30, 845–851. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, Y.; Gutiérrez, T. Evaluation of the sensory quality of chocolate. In Chocolate-Cocoa Byproducts Technology, Rheology, Styling, and Nutrition; Sira, E.P., Ed.; Nova: New York, NY, USA, 2015; pp. 167–189. [Google Scholar]
- Faccinetto-Beltrán, P.; Gómez-Fernández, A.R.; Orozco-Sánchez, N.E.; Pérez-Carrillo, E.; Marín-Obispo, L.M.; Hernández-Brenes, C.; Santacruz, A.; Jacobo-Velázquez, D.A. Physicochemical properties and sensory acceptability of a next-generation functional chocolate added with omega-3 polyunsaturated fatty acids and probiotics. Foods 2021, 10, 333. [Google Scholar] [CrossRef] [PubMed]
- Cikrikci, S.; Yucekutlu, M.; Mert, B.; Oztop, M.H. Physical characterization of low-calorie chocolate formulations. J. Food Meas. Charact. 2017, 11, 41–49. [Google Scholar] [CrossRef]
- Gómez-Fernández, A.; Faccinetto-Beltrán, P.; Orozco-Sánchez, N.; Pérez-Carrillo, E.; Santacruz, A.; Jacobo-Velázquez, D.A. Physicochemical properties and sensory acceptability of sugar-free dark chocolate formulations added with probiotics. Rev. Mex. Ing. Química 2021, 20, 697–709. [Google Scholar] [CrossRef]
- Kiumarsi, M.; Rafe, A.; Yeganehzad, S. Effect of different bulk sweeteners on the dynamic oscillatory and shear rheology of chocolate. Appl. Rheol. 2017, 27, 11–19. [Google Scholar]
- Torres-Moreno, M.; Torrescasana, E.; Salas-Salvadó, J.; Blanch, C. Nutritional composition and fatty acids profile in cocoa beans and chocolates with different geographical origin and processing conditions. Food Chem. 2015, 166, 125–132. [Google Scholar] [CrossRef]
- BAM. Chapter 5: Salmonella. Available online: https://www.fda.gov/food/laboratory-methods-food/bam-chapter-6-shigella (accessed on 28 June 2021).
- BAM. Chapter 4: Enumeration of Escherichia coli and the Coliform Bacteria. Available online: https://www.fda.gov/food/laboratory-methods-food/bam-chapter-4-enumeration-escherichia-coli-and-coliform-bacteria (accessed on 28 June 2021).
- NORMA Oficial Mexicana. NOM-186-SSA1/SCFI-2013, Cacao, Chocolate y Pruebas Similares, y Derivados del Cacao. Especificaciones Sanitarias. Denominación Comercial. Métodos de Prueba. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5332832&fecha=17/02/2014 (accessed on 28 June 2021).
- Yao, M.; Wu, J.; Li, B.; Xiao, H.; McClements, D.J.; Li, L. Microencapsulation of Lactobacillus salivarious Li01 for enhanced storage viability and targeted delivery to gut microbiota. Food Hydrocoll. 2017, 72, 228–236. [Google Scholar] [CrossRef]
- Ding, W.K.; Shah, N.P. Acid, bile, and heat tolerance of free and microencapsulated probiotic bacteria. J. Food Sci. 2007, 72, M446–M450. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Carranza, P.; López-Malo, A.; Jiménez, T. Microencapsulation quality and efficiency of Lactobacillus casei by spray drying using maltodextrin and vegetable extracts. J. Food Res. 2013, 3, 61–69. [Google Scholar] [CrossRef]
- Tárrega, A.; Rocafull, A.; Costell, E. Effect of blends of short and long-chain inulin on the rheological and sensory properties of prebiotic low-fat custards. LWT Food Sci. Technol. 2010, 43, 556–562. [Google Scholar] [CrossRef] [Green Version]
- Anekella, K.; Orsat, V. Optimization of microencapsulation of probiotics in raspberry juice by spray drying. LWT Food Sci. Technol. 2013, 50, 17–24. [Google Scholar] [CrossRef]
- Boza, Y.; Barbin, D.; Scamparini, A.R.P. Survival of Beijerinckia sp. microencapsulated in carbohydrates by spray-drying. J. Microencapsul. 2004, 21, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.P. Functional cultures and health benefits. Int. Dairy J. 2007, 17, 1262–1277. [Google Scholar] [CrossRef]
- Mandal, S.; Hati, S.; Puniya, A.K.; Singh, R.; Singh, K. Development of synbiotic milk chocolate using encapsulated Lactobacillus casei ncdc 298. J. Food Process. Preserv. 2013, 37, 1031–1037. [Google Scholar] [CrossRef]
- Konar, N.; Oba, S.; Toker, O.S.; Palabiyik, I.; Goktas, H.; Artik, N.; Sagdic, O. Rapid tempering of sucrose-free milk chocolates by β V seeding: Textural, rheological and melting properties. Eur. Food Res. Technol. 2017, 243, 1849–1860. [Google Scholar] [CrossRef]
- Silva, N.D.S.E.; Hernández, E.J.G.P.; Araújo, C.D.S.; Joele, M.R.S.P.; Lourenço, L.D.F.H. Development and optimization of biodegradable fish gelatin composite film added with buritic oil. CyTA J. Food 2018, 16, 340–349. [Google Scholar] [CrossRef]
- Toker, O.S.; Oba, S.; Palabiyik, I.; Pirouzian, H.R.; Konar, N.; Artik, N.; Sagdic, O. Alternative tempering of sugar-free dark chocolates by βv seeding: Sensorial, micro-structural and some physical properties and volatile profile. Int. J. Food Eng. 2019, 15, 20180067. [Google Scholar] [CrossRef]
- Hartel, R.W. Chocolate: Fat bloom during storage, the influence of structural elements. Manuf. Confect. 1999, 79, 89–99. [Google Scholar]
- Aidoo, R.P.; Afoakwa, E.O.; Dewettinck, K. Rheological properties, melting behaviours and physical quality characteristics of sugar-free chocolates processed using inulin/polydextrose bulking mixtures sweetened with stevia and thaumatin extracts. LWT Food Sci. Technol. 2015, 62, 592–597. [Google Scholar] [CrossRef]
- Rad, A.H.; Pirouzian, H.R.; Toker, O.S.; Konar, N. Application of simplex lattice mixture design for optimization of sucrose-free milk chocolate produced in a ball mill. LWT Food Sci. Technol. 2019, 115, 108435. [Google Scholar]
- Harter, R.W.; von Ele, J.H.; Hoberger, R. Fats, oils and emulsifiers. In Confectionary Science and Technology; Harter, R.W., von Ele, J.H., Hoberger, R., Eds.; Confectionery Science and Technology: Cham, Switzerland, 2018; pp. 85–124. [Google Scholar]
- Konar, N.; Palabiyik, I.; Toker, O.S.; Polat, D.G.; Kelleci, E.; Pirouzian, H.R.; Akcicek, A.; Sagdic, O. Conventional and sugar-free probiotic white chocolate: Effect of inulin DP on various quality properties and viability of probiotics. J. Funct. Foods 2018, 43, 206–213. [Google Scholar] [CrossRef]
- Afoakwa, E.O.; Paterson, A.; Fowler, M. Effects of particle size distribution and composition on dark chocolate’s rheological properties. Eur. Food Res. Technol. 2008, 226, 1259–1268. [Google Scholar] [CrossRef]
- Timms, R.E. Interactions between fats, bloom and rancidity. In Confectionery Fats Handbook, 1st ed.; Timms, R.E., Ed.; Woodhead Publishing: Cambridge, UK, 2012; pp. 255–294. [Google Scholar]
- Timms, R.E. Phase behavior of fats and their mixtures. Prog. Lipid Res. 1984, 23, 1–38. [Google Scholar] [CrossRef]
- Silva, M.P.; Tulini, F.L.; Marinho, J.F.U.; Mazzocato, M.C.; De Martinis, E.C.P.; Luccas, V.; Favaro-Trindade, C.S. Semisweet chocolate as a vehicle for the probiotics Lactobacillus acidophilus LA3 and Bifidobacterium animalis subsp. Lactis BLC1: Evaluation of chocolate stability and probiotic survival under in vitro simulated gastrointestinal conditions. LWT Food Sci. Technol. 2017, 75, 640–647. [Google Scholar]
- Pirouzian, H.R.; Konar, N.; Palabiyik, I.; Oba, S.; Toker, O.S. Pre-crystallization process in chocolate: Mechanism, importance and novel aspects. Food Chem. 2020, 321, 126718. [Google Scholar] [CrossRef] [PubMed]
- Foong, Y.J.; Lee, S.T.; Ramli, N.; Tan, Y.N.; Ayob, M.K. Incorporation of potential probiotic Lactobacillus plantarum isolated from fermented cocoa beans into dark chocolate: Bacterial viability and physicochemical properties analysis. J. Food Qual. 2013, 36, 164–171. [Google Scholar] [CrossRef]
- Toker, O.S.; Konar, N.; Palabiyik, I.; Pirouzian, H.R.; Oba, S.; Polat, D.G.; Poyrazoglu, E.S.; Sagdic, O. Formulation of dark chocolate as a carrier to deliver eicosapentaenoic and docosahexaenoic acids: Effects on product quality. Food Chem. 2018, 254, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Belščak-Cvitanović, A.; Komes, D.; Dujmović, M.; Karlović, S.; Biškić, M.; Brnčić, M.; Ježek, D. Physical, bioactive and sensory quality parameters of reduced sugar chocolates formulated with natural sweeteners as sucrose alternatives. Food Chem. 2015, 167, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Laličić-Petronijević, J.; Popov-Raljić, J.; Obradović, D.; Radulović, Z.; Paunović, D.; Petrušić, M.; Pezo, L. Viability of probiotic strains Lactobacillus acidophilus NCFM® and Bifidobacterium lactis HN019 and their impact on sensory and rheological properties of milk and dark chocolates during storage for 180 days. J. Funct. Food 2015, 15, 541–550. [Google Scholar] [CrossRef]
- Żyżelewicz, D.; Nebesny, E.; Motyl, I.; Libudzisz, Z. Effect of milk chocolate supplementation with lyophilised lactobacillus cells on its attributes. Czech J. Food Sci. 2010, 28, 392–406. [Google Scholar] [CrossRef] [Green Version]
- Glicerina, V.; Balestra, F.; Rosa, M.D.; Romani, S. Rheological, textural and calorimetric modifications of dark chocolate during process. J. Food Eng. 2013, 119, 173–179. [Google Scholar] [CrossRef]
- Afoakwa, E.O. Chocolate Science and Technology, 1st ed.; Wiley-Blackwell Publishers: Oxford, UK, 2016; pp. 54–101. [Google Scholar]
- Leung, K.S.; Galano, J.M.; Durand, T.; Lee, J.C.Y. Profiling of omega-polyunsaturated fatty acids and their oxidized products in salmon after different cooking methods. Antioxidants 2018, 7, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mozuraityte, R.; Kristinova, V.; Standal, I.B.; Carvajal, A.K.; Aursand, M. Oxidative Stability and Shelf Life of Fish Oil. In Oxidative Stability and Shelf Life of Foods Containing Oils and Fats; AOCS Press: Rockville, MD, USA, 2016; pp. 209–231. [Google Scholar]
- Goldfein, K.R.; Slavin, J.L. Why sugar is added to food: Food science 101. Compr. Rev. Food Sci. Food Saf. 2015, 14, 644–656. [Google Scholar] [CrossRef] [Green Version]
- Faraji, H.; Lindsay, R.C. Antioxidant protection of bulk fish oils by dispersed sugars and polyhydric alcohols. J. Agric. Food Chem. 2005, 53, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Lagast, S.; De Steur, H.; Schouteten, J.J.; Gellynck, X. A comparison of two low-calorie sweeteners and sugar in dark chocolate on sensory attributes and emotional conceptualisations. Int. J. Food Sci. Nutr. 2017, 69, 344–357. [Google Scholar] [CrossRef] [PubMed]
- Rad, A.H.; Pirouzian, H.R.; Konar, N.; Toker, O.S.; Polat, D.G. Effects of polyols on the quality characteristics of sucrose-free milk chocolate produced in a ball mill. RSC Adv. 2019, 9, 29676–29688. [Google Scholar] [CrossRef] [Green Version]

| Ingredients | % Percentage in Each Formulation (w/w) | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | Prob | FO | Prob + FO | Sw | Sw + Prob | Sw + FO | Sw + Prob + FO | |
| Alkalinized cocoa paste | 12.46 | 12.43 | 11.64 | 11.61 | 13.00 | 12.97 | 12.12 | 12.12 |
| Natural cocoa | 3.00 | 3.00 | 2.80 | 2.80 | 3.00 | 3.00 | 2.80 | 2.80 |
| Cocoa butter | 26.15 | 26.10 | 24.43 | 24.39 | 23.24 | 23.19 | 21.67 | 21.67 |
| Whole milk powder | 13.42 | 13.39 | 12.54 | 12.51 | 14.00 | 13.97 | 13.05 | 13.05 |
| Skim milk powder | 10.54 | 10.51 | 9.85 | 9.83 | 11.00 | 10.98 | 10.25 | 10.25 |
| Soy lecithin | 0.384 | 0.38 | 0.36 | 0.36 | 0.30 | 0.30 | 0.30 | 0.30 |
| PGPR | 0.192 | 0.19 | 0.18 | 0.18 | 0.20 | 0.20 | 0.19 | 0.19 |
| NaCl | 0.08 | 0.08 | 0.07 | 0.07 | 0.08 | 0.08 | 0.08 | 0.08 |
| Vanilla | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 |
| Sugar | 33.75 | 33.68 | 31.53 | 31.47 | - | - | - | - |
| Isomalt LMPF | - | - | - | - | 35.12 | 35.05 | 32.95 | 32.74 |
| Stevia | - | - | - | - | 0.03 | 0.03 | 0.03 | 0.03 |
| Probiotic | - | 0.21 | - | 0.21 | - | 0.21 | - | 0.21 |
| Fish oil | - | - | 6.57 | 6.55 | - | - | 6.54 | 6.54 |
| Sample | aw a | WI | Hardness b (N) | Fracturability b (N) |
|---|---|---|---|---|
| Control | 0.46 ± 0.02 a | 19.08 ± 0.99 de | 3072.8 ± 93.6 a | 2824.2 ± 117.5 b |
| Prob | 0.47 ± 0.01 a | 27.21 ± 0.29 a | 2170.6 ± 198.3 c | 2676.6 ± 129.8 b |
| FO | 0.45 ± 0.01 a | 20.73 ± 0.49 de | 1644.6 ± 103.9 d | 2834.2 ± 202.9 b |
| Prob + FO | 0.45 ± 0.01 a | 26.01 ± 0.26 ab | 1719.8 ± 176.3 d | 2823.8 ± 294.1 b |
| Sw | 0.41 ± 0.01 b | 24.68 ± 1.29 bc | 2709.2 ± 140.5 b | 3606.2 ± 96.8 a |
| Sw + Prob | 0.40 ± 0.01 b | 14.69 ± 1.41 f | 2599.0 ± 103.6 b | 3300.6 ± 101.9 a |
| Sw + FO | 0.45 ± 0.01 a | 18.95 ± 0.49 e | 1241.2 ± 47.7 e | 2031.6 ± 121.6 c |
| Sw + Prob + FO | 0.46 ± 0.01 a | 21.45 ± 1.47 cd | 1545.2 ± 44.5 de | 2454.2 ± 63.9 bc |
| Significance c | ||||
| Sw | ** | *** | NS | NS |
| FO | * | NS | *** | *** |
| Prob | NS | * | NS | NS |
| Sw*FO | *** | NS | NS | *** |
| Sw*Prob | NS | *** | * | NS |
| Prob*FO | NS | ** | *** | NS |
| Sw*FO*Prob | NS | *** | NS | NS |
| Fatty Acid | Chocolate Samples | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | Prob | FO | Prob + FO | Sw | Sw + Prob | Sw + FO | Sw + Prob + FO | |
| Octanoic acid (C8:0) | 46.55 ± 4.61 b | 55.47 ± 2.67 ab | 58.027 ± 7.67 ab | 56.19 ± 4.20 ab | 53.46 ± 4.78 ab | 62.021 ± 5.08 ab | 65.36 ± 8.81 a | 613.28 ± 6.05 ab |
| Decanoic acid (C10:0) | 10.48 ± 1.70 b | 12.84 ± 0.93 ab | 16.55 ± 3.33 ab | 14.20 ± 1.78 ab | 13.89 ± 1.61 ab | 16.02 ± 2.69 ab | 10.4792 ± 1.71 a | 104.79 ± 1.70 a |
| Lauric acid (C12:0) | 8.02 ± 0.94 c | 9.91 ± 0.75 bc | 14.33 ± 1.23 abc | 16.33 ± 3.34 ab | 12.41 ± 1.12 abc | 13.89 ± 3.61 abc | 17.80 ± 2.41 a | 165.47 ± 2.92 a |
| Myristic acid (C14:0) | 33.48 ± 0.76 c | 39.85 ± 0.31 c | 261.24 ± 14.08 b | 265.52 ± 30.74 b | 46.89 ± 1.38 c | 78.45 ± 32.45 c | 354.92 ± 26.85 a | 3208.28 ± 18.87 ab |
| Pentadecanoic acid (C15:0) | 9.22 ± 0.44 c | 10.53 ± 0.25 c | 28.57 ± 1.48 b | 29.23 ± 3.04 b | 11.79 ± 0.84 c | 13.51 ± 2.85 c | 38.37 ± 3.15 a | 365.43 ± 2.9 a |
| Palmitic acid (C16:0) | 5412.38 ± 61.76 bc | 6390.28 ± 63.55 a | 5061.02 ± 242.55 c | 4739.24 ± 326.71 c | 6117.75 ± 198.24 ab | 6237.88 ± 389.55 ab | 6098.85 ± 332.69 ab | 64,200.62 ± 440.60 a |
| Heptadecanoic acid (C17:0) | 43.37 ± 0.45 c | 49.97 ± 0.16 bc | 54.58 ± 3.12 b | 51.79 ± 3.55 bc | 48.62 ± 1.71 bc | 49.84 ± 5.11 bc | 67.13 ± 4.22 a | 674.77 ± 5.82 a |
| Stearic acid (C18:0) | 6684.21 ± 2.10 b | 7846.70 ± 79.76 a | 5617.02 ± 225.79 c | 5190.71 ± 335.91 c | 7476.05 ± 246.78 ab | 7566.89 ± 421.72 ab | 6785.18 ± 354.72 b | 72,410.12 ± 507.05 ab |
| Arachidic acid (C20:0) | 220.47 ± 2.10 b | 257.99 ± 2.45 a | 181.37 ± 7.51 c | 164.50 ± 10.85 c | 249.25 ± 8.49 ab | 251.35 ± 13.82 ab | 227.57 ± 11.71 ab | 2423.21 ± 16.91 ab |
| Behenic acid (C22:0) | 40.01 ± 0.16 bc | 46.55 ± 0.27 a | 35.98 ± 1.43 cd | 31.59 ± 2.16 d | 45.18 ± 1.22 ab | 46.05 ± 2.48 ab | 45.35 ± 2.64 ab | 471.32 ± 4.05 a |
| Lignoceric acid (C24:0) | 25.69 ± 0.30 bcd | 29.93 ± 3.52 abc | 23.82 ± 1.83 cd | 22.92 ± 1.19 c | 31.81 ± 2.09 ab | 30.36 ± 1.49 abc | 33.14 ± 2.44 a | 317.14 ± 3.39 ab |
| Myristoleic acid (C14:1) | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| Palmitoleic acid (C16:1) | 50.39 ± 1.30 c | 59.17 ± 0.00 c | 336.49 ± 18.50 b | 330.66 ± 36.51 b | 57.69 ± 1.96 c | 77.91 ± 23.23 c | 453.92 ± 30.49 a | 419.48 ± 24.94 a |
| Oleic Acid (C18:1) | 6329.61 ± 52.79 c | 7403.02 ± 0.07 a | 4967.76 ± 194.57 d | 4563.29 ± 265.40 d | 7118.32 ± 246.68 abc | 7211.03 ± 373.29 ab | 6473.06 ± 341.50 bc | 6854.78 ± 433.29 abc |
| Vaccenic acid (C18:1) | 65.78 ± 1.12 c | 76.13 ± 0.00 c | 129.99 ± 65.57 b | 124.2 ± 12.02 b | 72.46 ± 2.90 c | 77.29 ± 9.70 c | 168.56 ± 10.77 a | 165.24 ± 11.53 a |
| Eicosenoic acid (C20:1) | 190.24 ± 0.31 c | 13.11 ± 0.00 c | 18.511 ± 1.10 b | 17.89 ± 1.79 b | 12.57 ± 0.44 c | 14.46 ± 2.20 bc | 26.20 ± 2.11 a | 25.61 ± 2.05 a |
| Nervonic acid (C24:1) | N.D. | N.D. | 18.511 ± 1.10 a | 15.93 ± 1.48 a | N.D. | N.D. | 18.51 ± 3.11 a | 11.09 ± 1.88 b |
| Linoleic acid (C18:2) | 654.58 ± 5.59 b | 767.95 ± 8.11 a | 407.71 ± 19.88 c | 391.90 ± 19.86 c | 733.25 ± 21.76 ab | 730.29 ± 28.96 ab | 687.41 ± 40.22 ab | 736.66 ± 49.90 ab |
| Gamma Linolenic acid (C18:3) | N.D. | N.D. | 9.55 ± 1.49 a | 8.65 ± 0.75 a | N.D. | N.D. | 10.83 ± 1.45 a | 11.46 ± 2.28 a |
| Alpha Linolenic acid (C18:3) | 48.60 ± 0.66 c | 58.40 ± 0.94 c | 79.41 ± 4.60 b | 73.60 ± 6.67 b | 53.22 ± 1.51 c | 55.44 ± 3.47 c | 114.22 ± 7.76 a | 112.20 ± 6.96 a |
| Stearidionic acid (C18:4) | N.D. | N.D. | 90.37 ± 6.48 b | 87.67 ± 11.05 b | N.D. | N.D. | 114.66 ± 6.62 a | 109.23 ± 7.67 a |
| Eicosadienoic acid (C20:2) | N.D. | N.D. | 28.21 ± 2.38 a | 26.51 ± 2.89 a | N.D. | N.D. | 17.69 ± 2.24 b | 18.40 ± 2.98 b |
| Homo-gamma-linolenic acid (C20:3) | N.D. | N.D. | 9.012 ± 0.82 b | 8.86 ± 0.55 b | N.D. | N.D. | 10.63 ± 1.44 ab | 12.04 ± 1.39 a |
| Dihomogamma linolenic acid (C20:3) | N.D. | N.D. | 13.99 ± 1.85 a | 11.07 ± 1.20 a | N.D. | N.D. | 12.57 ± 1.47 a | 13.87 ± 2.02 a |
| Arachidonic acid (C20:4) | N.D. | N.D. | 22.92 ± 2.66 b | 22.75 ± 2.02 b | N.D. | N.D. | 31.18 ± 2.73 a | 28.57 ± 2.26 a |
| Eicosapentaenoic acid (C20:5) | N.D. | N.D. | 316.75 ± 20.57 b | 305.6 ± 35.34 b | N.D. | N.D. | 421.21 ± 32.36 a | 309.91 ± 22.28 a |
| Docosapentaenoic acid n-6 (C22:5) | N.D. | N.D. | 8.37 ± 20.57 b | 9.02 ± 0.66 b | N.D. | N.D. | 14.98 ± 2.19 a | 14.84 ± 0.87 a |
| Docosapentaenoic acid n-3 (C22:5) | N.D. | N.D. | 42.39 ± 6.66 b | 46.33 ± 5.10 b | N.D. | N.D. | 64.63 ± 5.93 a | 52.013 ± 7.22 ab |
| Docosahexaenoic acid (C22:6) | N.D. | N.D. | 351.83 ± 22.20 b | 341.07 ± 43.00 b | N.D. | N.D. | 463.481 ± 31.99 a | 436.57 ± 27.53 a |
| Total ω-3 | 48.61 ± 0.66 c | 58.40 ± 0.94 c | 894.76 ± 61.76 b | 865.35 ± 102.23 b | 53.23 ± 1.51 c | 55.44 ± 3.47 c | 1190.80 ± 86.05 a | 1114.80 ± 73.02 a |
| Total ω-6 | 654.58 ± 5.60 b | 767.95 ± 8.11 a | 548.78 ± 28.01 c | 467.70 ± 25.30 c | 733.25 ± 21.76 ab | 730.29 ± 28.96 ab | 772.74 ± 49.35 a | 821.99 ± 58.79 a |
| Saturated fatty acids (SFA) | 12,533.89 ± 130.45 bc | 14,750.05 ± 152.21 a | 11,353.24 ± 505.99 cd | 10,582.23 ± 716 d | 14,107.12 ± 460.99 ab | 14,366.42 ± 879.43 ab | 13,752.27 ± 746.68 ab | 145,029.57 ± 1010.38 a |
| Monounsaturated fatty acids (MUFA) | 6456.17 ± 54.99 b | 7551.44 ± 78.89 a | 5469.07 ± 221.59 c | 5051.99 ± 315.66 c | 7261.04 ± 251.89 ab | 7380.69 ± 407.77 ab | 7140.27 ± 387.76 ab | 7476.23 ± 473.488 a |
| Polyunsaturated fatty acids (PUFA) | 703.19 ± 6.25 c | 826.35 ± 8.75 c | 1443.54 ± 89.76 b | 1333.05 ± 125.75 b | 786.48 ± 23.26 c | 785.74 ± 32.41 c | 1963.54 ± 133.79 a | 1936.79 ± 131.78 a |
| Total fatty acids | 19,693.26 ± 191.51 bc | 23,127.85 ± 239.28 a | 18,255.87 ± 816.67 c | 16,967.28 ± 1155.56 c | 22,154.64 ± 735.78 ab | 22,532.86 ± 1319.25 ab | 22,856.09 ± 1266.02 a | 23,915.98 ± 1615.57 a |
| Sample | Appearance a | Flavor a | Texture a | Overall Acceptability a |
|---|---|---|---|---|
| Control | 8.25 ± 0.07 a | 7.07 ± 0.11 a | 7.49 ± 0.09 ab | 7.21 ± 0.09 a |
| Prob | 7.22 ± 0.14 bcd | 6.93 ± 0.18 a | 7.63 ± 0.46 a | 7.035 ± 0.15 ab |
| FO | 6.96 ± 0.17 d | 4.60 ± 0.21 d | 5.409 ± 0.20 d | 4.75 ± 0.21 e |
| Prob + FO | 7.02 ± 0.16 cd | 4.63 ± 0.20 d | 5.48 ± 0.20 d | 5.035 ± 0.21 e |
| Sw | 7.34 ± 0.13 bc | 6.39 ± 0.17 b | 6.97 ± 0.14 bc | 6.56 ± 0.17 c |
| Sw + Prob | 7.49 ± 0.13 b | 6.24 ± 0.17 b | 6.86 ± 0.14 c | 6.57 ± 0.15 bc |
| Sw + FO | 7.15 ± 0.15 bcd | 5.07 ± 0.21 cd | 6.66 ± 0.18 c | 5.69 ± 0.19 d |
| Sw + Prob + FO | 7.15 ± 0.15 bcd | 5.07 ± 0.20 c | 6.59 ± 0.17 c | 5.67 ± 0.19 d |
| Significance b | ||||
| Sw | NS | NS | NS | NS |
| FO | ** | *** | *** | *** |
| Prob | NS | NS | NS | NS |
| Sw*FO | NS | *** | *** | *** |
| Sw*Prob | NS | NS | NS | NS |
| Prob*FO | NS | NS | NS | NS |
| Sw*FO*Prob | NS | NS | NS | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Fernández, A.R.; Faccinetto-Beltrán, P.; Orozco-Sánchez, N.E.; Pérez-Carrillo, E.; Marín-Obispo, L.M.; Hernández-Brenes, C.; Santacruz, A.; Jacobo-Velázquez, D.A. Sugar-Free Milk Chocolate as a Carrier of Omega-3 Polyunsaturated Fatty Acids and Probiotics: A Potential Functional Food for the Diabetic Population. Foods 2021, 10, 1866. https://doi.org/10.3390/foods10081866
Gómez-Fernández AR, Faccinetto-Beltrán P, Orozco-Sánchez NE, Pérez-Carrillo E, Marín-Obispo LM, Hernández-Brenes C, Santacruz A, Jacobo-Velázquez DA. Sugar-Free Milk Chocolate as a Carrier of Omega-3 Polyunsaturated Fatty Acids and Probiotics: A Potential Functional Food for the Diabetic Population. Foods. 2021; 10(8):1866. https://doi.org/10.3390/foods10081866
Chicago/Turabian StyleGómez-Fernández, Andrea R., Paulinna Faccinetto-Beltrán, Norma E. Orozco-Sánchez, Esther Pérez-Carrillo, Luis Martín Marín-Obispo, Carmen Hernández-Brenes, Arlette Santacruz, and Daniel A. Jacobo-Velázquez. 2021. "Sugar-Free Milk Chocolate as a Carrier of Omega-3 Polyunsaturated Fatty Acids and Probiotics: A Potential Functional Food for the Diabetic Population" Foods 10, no. 8: 1866. https://doi.org/10.3390/foods10081866








