Application of 3D Printing in the Design of Functional Gluten-Free Dough
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Rosehip Powder Preparation
2.3. Dough Preparation
2.4. 3D Printing Assay
2.5. Baking and Postprocessing
2.6. Determinations
2.6.1. Rheological Properties of Dough
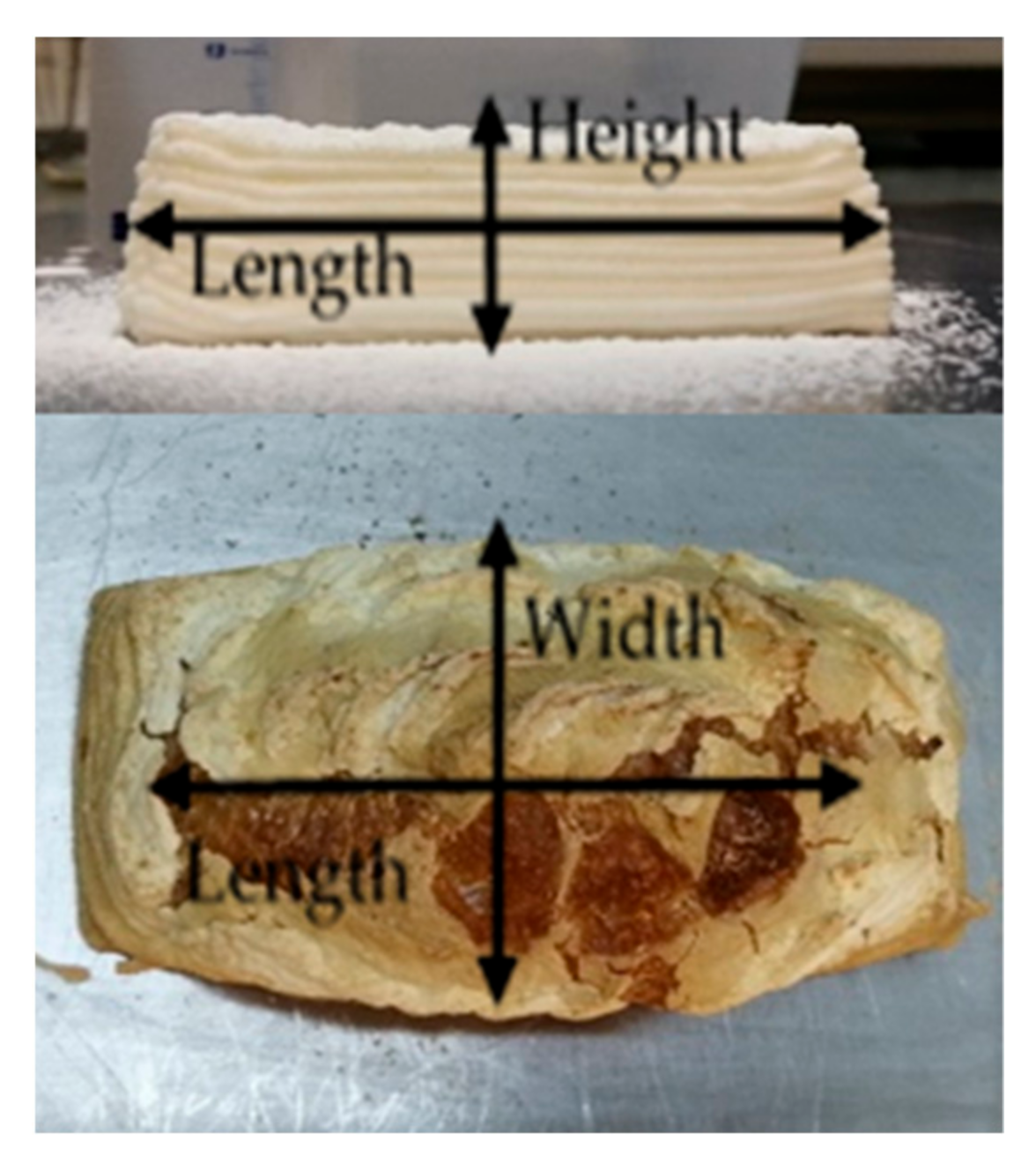
2.6.2. Image Analysis
2.6.3. Physico-Chemical Parameters
2.6.4. Colour Measurement
2.6.5. Textural Characterization
2.6.6. Centesimal Composition
2.6.7. Bioactive Compounds
2.7. Statistical Analysis
3. Results and Discussion
3.1. Rheological Characterisation of Dough
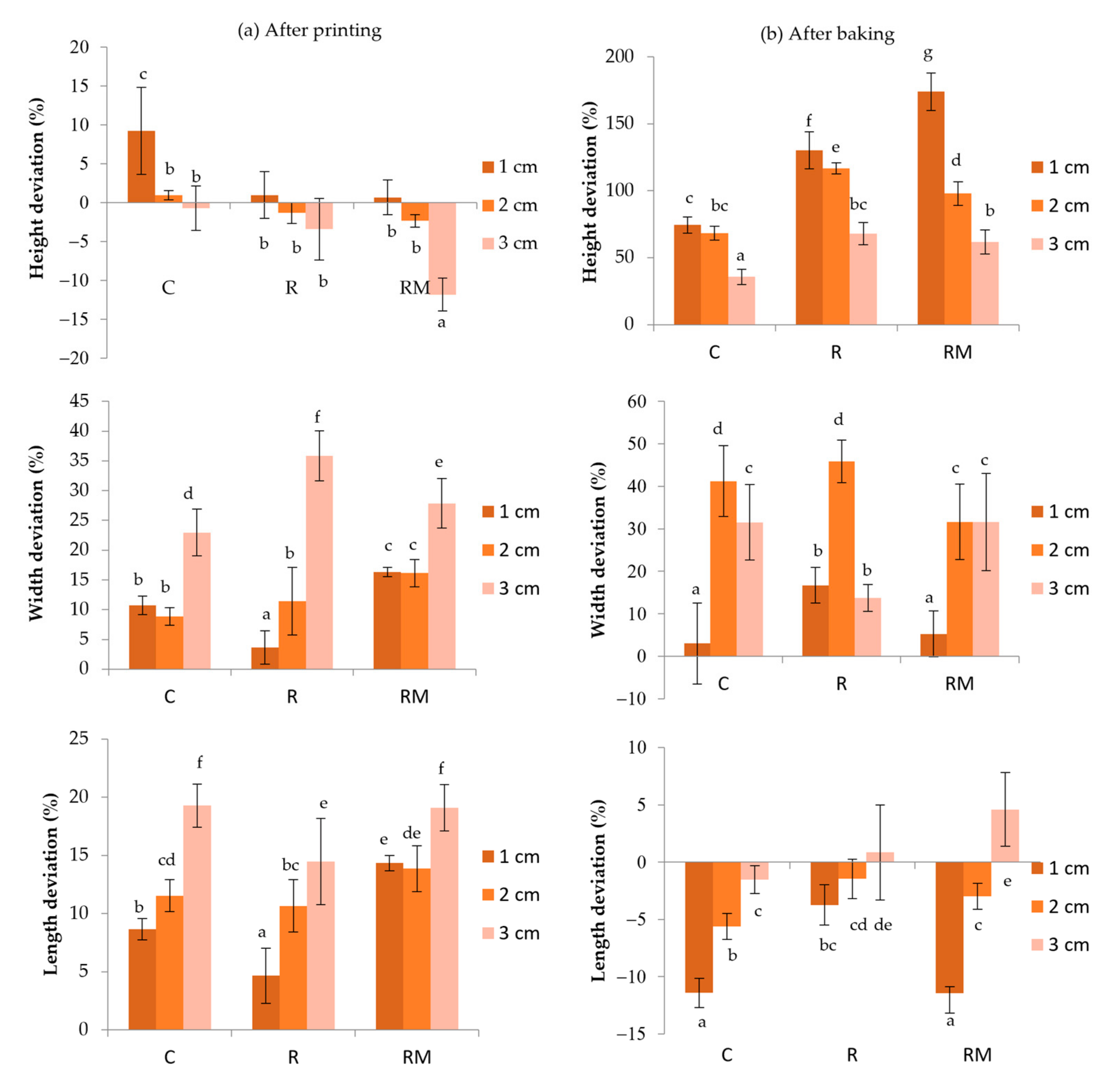
3.2. Image Analysis
3.3. Physicochemical Parameters
3.4. Colour Changes
3.5. Texture
3.6. Centesimal Composition
3.7. Bioactive Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Selvi, M.K.; Sowmya, B.; Kannan, T.; Latha, M.; Jena, I.; Arun Kumar, V.; Vijayaraj, P. Advances in personalized food and nutrition. In Research and Technological Advances in Food Science; Bhanu Prakash, Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 31–60. [Google Scholar] [CrossRef]
- Godoi, F.C.; Prakash, S.; Bhandari, B.R. 3d printing technologies applied for food design: Status and prospects. J. Food Eng. 2016, 179, 44–54. [Google Scholar] [CrossRef] [Green Version]
- Jagadiswaran, B.; Alagarasan, V.; Palanivelu, P.; Theagarajan, R.; Moses, J.A.; Anandharamakrishnan, C. Valorization of food industry waste and by-products using 3D printing: A study on the development of value-added functional cookies. Futur. Foods 2021, 4, 100036. [Google Scholar] [CrossRef]
- Hussain, S.; Arora, V.K.; Malakar, S. Formulation of protein-enriched 3D printable food matrix and evaluation of textural, rheological characteristics, and printing stability. J. Food Process. Preserv. 2021, 45, e15182. [Google Scholar] [CrossRef]
- Uribe-Wandurraga, Z.N.; Zhang, L.; Noort, M.W.J.; Schutyser, M.A.I.; García-Segovia, P.; Martínez-Monzó, J. Printability and Physicochemical Properties of Microalgae-Enriched 3D-Printed Snacks. Food Bioprocess Technol. 2020, 13, 2029–2042. [Google Scholar] [CrossRef]
- Derossi, A.; Caporizzi, R.; Paolillo, M.; Severini, C. Programmable texture properties of cereal-based snack mediated by 3D printing technology. J. Food Eng. 2020, 289, 110160. [Google Scholar] [CrossRef]
- Rahman, J.M.H.; Shiblee, M.N.I.; Ahmed, K.; Khosla, A.; Kawakami, M.; Furukawa, H. Rheological and mechanical properties of edible gel materials for 3D food printing technology. Heliyon 2020, 6, e05859. [Google Scholar] [CrossRef]
- García-Segovia, P.; García-Alcaraz, V.; Balasch-Parisi, S.; Martínez-Monzó, J. 3D printing of gels based on xanthan/konjac gums. Innov. Food Sci. Emerg. Technol. 2020, 64, 102343. [Google Scholar] [CrossRef]
- Nijdam, J.J.; Agarwal, D.; Schon, B.S. An experimental assessment of filament-extrusion models used in slicer software for 3D food-printing applications. J. Food Eng. 2022, 317, 110711. [Google Scholar] [CrossRef]
- Mantihal, S.; Prakash, S.; Bhandari, B. Texture-modified 3D printed dark chocolate: Sensory evaluation and consumer perception study. J. Texture Stud. 2019, 50, 386–399. [Google Scholar] [CrossRef] [Green Version]
- Caporizzi, R.; Derossi, A.; Severini, C. Cereal-Based and Insect-Enriched Printable Food: From Formulation to Postprocessing Treatments. Status and Perspectives; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128145647. [Google Scholar]
- Derossi, A.; Caporizzi, R.; Paolillo, M.; Oral, M.O.; Severini, C. Drawing the scientific landscape of 3D Food Printing. Maps and interpretation of the global information in the first 13 years of detailed experiments, from 2007 to 2020. Innov. Food Sci. Emerg. Technol. 2021, 70, 102689. [Google Scholar] [CrossRef]
- Granato, D.; Barba, F.J.; Bursać Kovačević, D.; Lorenzo, J.M.; Cruz, A.G.; Putnik, P. Functional Foods: Product Development, Technological Trends, Efficacy Testing, and Safety. Annu. Rev. Food Sci. Technol. 2020, 11, 93–118. [Google Scholar] [CrossRef] [Green Version]
- de la Calle, I.; Ros, G.; Peñalver, R.; Nieto, G. Celiac disease: Causes, pathology, and nutritional assessment of gluten-free diet. A review. Nutr. Hosp. 2020, 37, 1043–1051. [Google Scholar]
- Sharma, Y.; Hegde, R.V.; Venugopal, C. Health and nutrition from ornamentals. Int. J. Res. Ayurveda Pharm. 2011, 2, 375–382. [Google Scholar]
- Patel, S. Rose hip as an underutilized functional food: Evidence-based review. Trends Food Sci. Technol. 2017, 63, 29–38. [Google Scholar] [CrossRef]
- Fascella, G.; D’Angiolillo, F.; Mammano, M.M.; Amenta, M.; Romeo, F.V.; Rapisarda, P.; Ballistreri, G. Bioactive compounds and antioxidant activity of four rose hip species from spontaneous Sicilian flora. Food Chem. 2019, 289, 56–64. [Google Scholar] [CrossRef]
- Medveckiene, B.; Kulaitiene, J.; Jariene, E.; Vaitkevičiene, N.; Hallman, E. Carotenoids, polyphenols, and ascorbic acid in organic rosehips (Rosa spp.) cultivated in Lithuania. Appl. Sci. 2020, 10, 5337. [Google Scholar] [CrossRef]
- Goztepe, B.; Kayacan, S.; Bozkurt, F.; Tomas, M.; Sagdic, O.; Karasu, S. Drying kinetics, total bioactive compounds, antioxidant activity, phenolic profile, lycopene and β-carotene content and color quality of Rosehip dehydrated by different methods. Lebensm.-Wiss. Technol. 2022, 153, 112476. [Google Scholar] [CrossRef]
- Igual, M.; Chi, M.S.; Adriana, P.; Vodnar, D.C.; Mart, J. Effect on Nutritional and Functional Characteristics by Encapsulating Rose canina Powder in Enriched Corn Extrudates. Foods 2021, 10, 2401. [Google Scholar] [CrossRef]
- Igual, M.; Chiş, M.S.; Păucean, A.; Vodnar, D.C.; Muste, S.; Man, S.; Martínez-Monzó, J.; García-Segovia, P. Valorization of rose hip (Rosa canina) puree co-product in enriched corn extrudates. Foods 2021, 10, 2787. [Google Scholar] [CrossRef]
- Vossen, E.; Utrera, M.; De Smet, S.; Morcuende, D.; Estévez, M. Dog rose (Rosa canina L.) as a functional ingredient in porcine frankfurters without added sodium ascorbate and sodium nitrite. Meat Sci. 2012, 92, 451–457. [Google Scholar] [CrossRef]
- Champagne, C.P.; Fustier, P. Microencapsulation for the improved delivery of bioactive compounds into foods. Curr. Opin. Biotechnol. 2007, 18, 184–190. [Google Scholar] [CrossRef]
- Hedges, A.R.; Shieh, W.J.; Sikorski, C.T. Use of Cyclodextrins for Encapsulation in the Use and Treatment of Food Products. In Encapsulation and Controlled Release of Food Ingredients; American Chemical Society: Washington, DC, USA, 1995; pp. 60–71. [Google Scholar]
- Costa, A.M.M.; Nunes, J.C.; Lima, B.N.B.; Pedrosa, C.; Calado, V.; Torres, A.G.; Pierucci, A.P.T.R. Effective stabilization of CLA by microencapsulation in pea protein. Food Chem. 2015, 168, 157–166. [Google Scholar] [CrossRef] [Green Version]
- Renzetti, S.; Dal Bello, F.; Arendt, E.K. Microstructure, fundamental rheology and baking characteristics of batters and breads from different gluten-free flours treated with a microbial transglutaminase. J. Cereal Sci. 2008, 48, 33–45. [Google Scholar] [CrossRef]
- Sarabhai, S.; Tamilselvan, T.; Prabhasankar, P. Role of enzymes for improvement in gluten-free foxtail millet bread: It’s effect on quality, textural, rheological and pasting properties. Lebensm.-Wiss. Technol. 2021, 137, 4–10. [Google Scholar] [CrossRef]
- Lazaridou, A.; Duta, D.; Papageorgiou, M.; Belc, N.; Biliaderis, C.G. Effects of hydrocolloids on dough rheology and bread quality parameters in gluten-free formulations. J. Food Eng. 2007, 79, 1033–1047. [Google Scholar] [CrossRef]
- Sun, X.; Koksel, F.; Nickerson, M.T.; Scanlon, M.G. Modeling the viscoelastic behavior of wheat flour dough prepared from a wide range of formulations. Food Hydrocoll. 2020, 98, 105129. [Google Scholar] [CrossRef]
- Ahmed, J.; Ptaszek, P.; Basu, S. Food Rheology: Scientific Development and Importance to Food Industry; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; ISBN 9780081004326. [Google Scholar]
- Masbernat, L.; Berland, S.; Leverrier, C.; Moulin, G.; Michon, C.; Almeida, G. Structuring wheat dough using a thermomechanical process, from liquid food to 3D-printable food material. J. Food Eng. 2021, 310, 110696. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC; Internatio.; Association of Analytical Communities: Gaithersburg, MD, USA, 2020. [Google Scholar]
- Bourne, M.C. Food Texture and Viscosity: Concept and Measurement, 2nd ed.; Academinc Press: Geneva, NY, USA, 2002; ISBN 0.120119062-S. [Google Scholar]
- Mariotti, F.; Tomé, D.; Mirand, P.P. Converting nitrogen into protein—Beyond 6.25 and Jones’ factors. Crit. Rev. Food Sci. Nutr. 2008, 48, 177–184. [Google Scholar] [CrossRef]
- Ohemeng-Ntiamoah, J.; Datta, T. Evaluating analytical methods for the characterization of lipids, proteins and carbohydrates in organic substrates for anaerobic co-digestion. Bioresour. Technol. 2018, 247, 697–704. [Google Scholar] [CrossRef]
- Morris, D. Quantitative Determination of Carbohydrates With Dreywood’s Anthrone Reagent. Science 1948, 107, 254–255. [Google Scholar] [CrossRef]
- García-Segovia, P.; Igual, M.; Martínez-Monzó, J. Beetroot microencapsulation with pea protein using spray drying: Physicochemical, structural and functional properties. Appl. Sci. 2021, 11, 6658. [Google Scholar] [CrossRef]
- Igual, M.; Cebadera, L.; Cámara, R.M.A.; Agudelo, C.; Martínez-Navarrete, N.; Cámara, M. Novel ingredients based on grapefruit freeze-dried formulations: Nutritional and bioactive value. Foods 2019, 8, 506. [Google Scholar] [CrossRef] [Green Version]
- Olives Barba, A.I.; Cámara Hurtado, M.; Sánchez Mata, M.C.; Fernández Ruiz, V.; López Sáenz De Tejada, M. Application of a UV-vis detection-HPLC method for a rapid determination of lycopene and β-carotene in vegetables. Food Chem. 2006, 95, 328–336. [Google Scholar] [CrossRef]
- Zhang, L.; Noort, M.; van Bommel, K. Towards the Creation of Personalized Bakery Products Using 3D Food Printing, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Andersson, S.C.; Rumpunen, K.; Johansson, E.; Olsson, M.E. Carotenoid content and composition in rose hips (Rosa spp.) during ripening, determination of suitable maturity marker and implications for health promoting food products. Food Chem. 2011, 128, 689–696. [Google Scholar] [CrossRef]
- Millar, K.A.; Barry-Ryan, C.; Burke, R.; McCarthy, S.; Gallagher, E. Dough properties and baking characteristics of white bread, as affected by addition of raw, germinated and toasted pea flour. Innov. Food Sci. Emerg. Technol. 2019, 56, 102189. [Google Scholar] [CrossRef]
- Gujral, H.S.; Guardiola, I.; Carbonell, J.V.; Rosell, C.M. Effect of cyclodextrinase on dough rheology and bread quality from rice flour. J. Agric. Food Chem. 2003, 51, 3814–3818. [Google Scholar] [CrossRef]
- Zhang, L.; Lou, Y.; Schutyser, M.A.I. 3D printing of cereal-based food structures containing probiotics. Food Struct. 2018, 18, 14–22. [Google Scholar] [CrossRef]
- Kim, H.W.; Bae, H.; Park, H.J. Classification of the printability of selected food for 3D printing: Development of an assessment method using hydrocolloids as reference material. J. Food Eng. 2017, 215, 23–32. [Google Scholar] [CrossRef]
- Horstmann, S.W.; Axel, C.; Arendt, E.K. Water absorption as a prediction tool for the application of hydrocolloids in potato starch-based bread. Food Hydrocoll. 2018, 81, 129–138. [Google Scholar] [CrossRef]
- Horstmann, S.W.; Atzler, J.J.; Heitmann, M.; Zannini, E.; Lynch, K.M.; Arendt, E.K. A comparative study of gluten-free sprouts in the gluten-free bread-making process. Eur. Food Res. Technol. 2019, 245, 617–629. [Google Scholar] [CrossRef]
- Rosell, C.M.; Rojas, J.A.; Benedito de Barber, C. Influence of hydrocolloids on dough rheology and bread quality. Food Hydrocoll. 2001, 15, 75–81. [Google Scholar] [CrossRef]
- Nijdam, J.J.; LeCorre-Bordes, D.; Delvart, A.; Schon, B.S. A rheological test to assess the ability of food inks to form dimensionally stable 3D food structures. J. Food Eng. 2021, 291, 110235. [Google Scholar] [CrossRef]
- Gamonpilas, C.; Pongjaruvat, W.; Fuongfuchat, A.; Methacanon, P.; Seetapan, N.; Thamjedsada, N. Physicochemical and rheological characteristics of commercial chili sauces as thickened by modified starch or modified starch/xanthan mixture. J. Food Eng. 2011, 105, 233–240. [Google Scholar] [CrossRef]
- Bodart, M.; de Peñaranda, R.; Deneyer, A.; Flamant, G. Photometry and colorimetry characterisation of materials in daylighting evaluation tools. Build. Environ. 2008, 43, 2046–2058. [Google Scholar] [CrossRef]
- Kazaz, S.; Baydar, H.; Erbas, S. Variations in chemical compositions of Rosa damascena Mill, and Rosa canina L. Fruits. Czech J. Food Sci. 2009, 27, 178–184. [Google Scholar] [CrossRef] [Green Version]
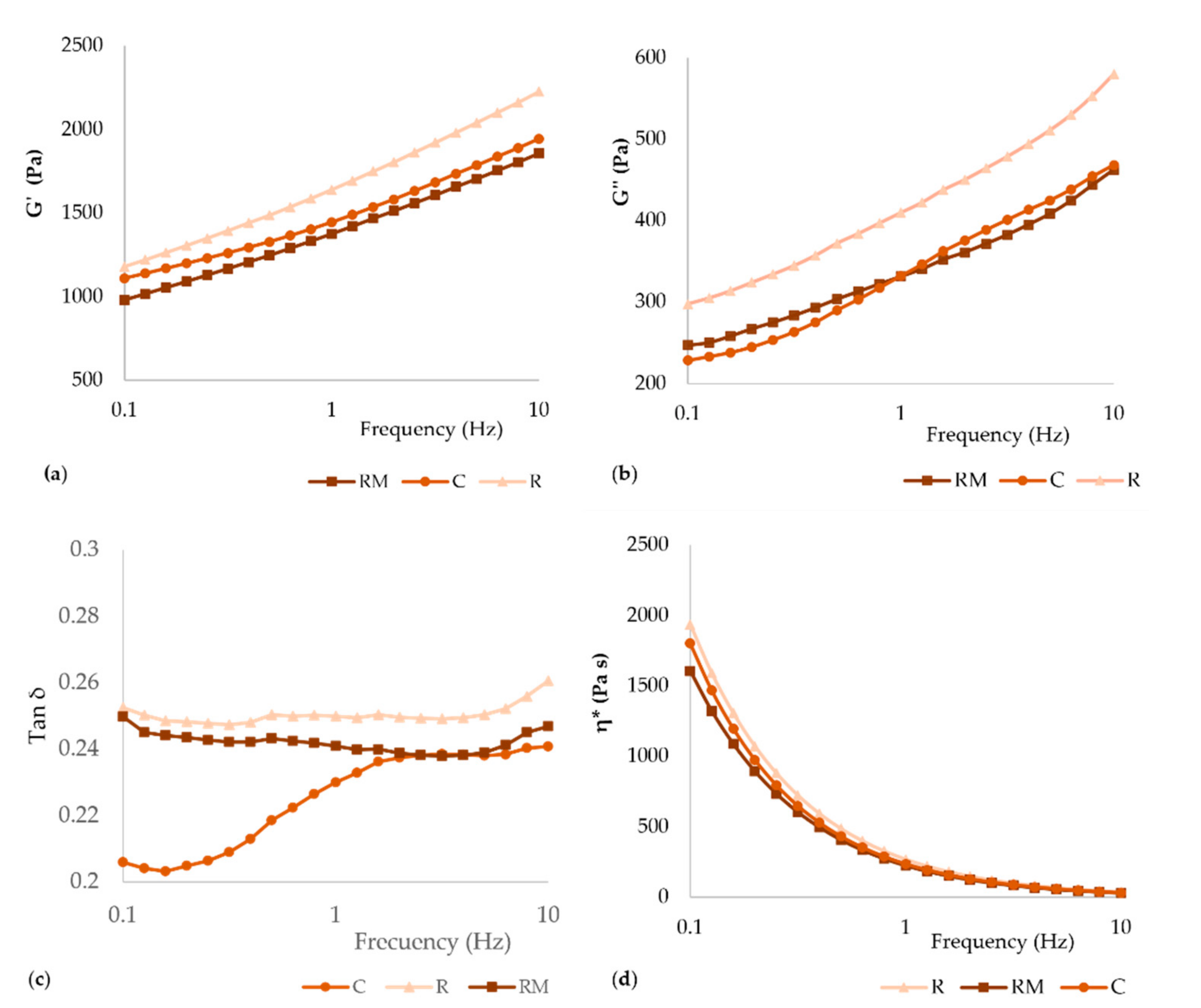

| Dough | G′ (Pa) | G″ (Pa) | tan δ | η* (Pa s) |
|---|---|---|---|---|
| C | 1408 ± 64 a | 324 ± 14 a | 0.2300 ± 0.0003 a | 230 ± 10 a |
| R | 1639 ± 88 b | 410 ± 19 b | 0.2499 ± 0.0018 c | 269 ± 14 b |
| RM | 1409 ± 92 a | 339 ± 21 a | 0.2408 ± 0.0011 b | 231 ± 15 a |
| Sample | aw | Moisture (g/100 g Sample) |
|---|---|---|
| CD | - | 58.9 ± 0.2 a |
| RD | - | 58.62 ± 0.13 a |
| RMD | - | 57.1 ± 0.7 b |
| 1CB | 0.8850 ± 0.0017 a | 26.6 ± 0.7 c |
| 1RB | 0.906 ± 0.002 b | 35.434 ± 0.014 e |
| 1RMB | 0.924 ± 0.003 e | 39.6 ± 0.4 g |
| 2CB | 0.918 ± 0.0012 d,e | 31.6 ± 0.4 d |
| 2RB | 0.919 ± 0.007 d,e | 40.4 ± 0.2 g |
| 2RMB | 0.908 ± 0.012 b,c | 38.0 ± 0.8 f |
| 3CB | 0.913 ± 0.009 b,c,d | 36.1 ± 0.9 e |
| 3RB | 0.918 ± 0.003 c,d,e | 40.5 ± 0.8 g |
| 3RMB | 0.926 ± 0.007 e | 39.78 ± 0.04 g |
| Sample | L* | a* | b* | h | C | ΔE |
|---|---|---|---|---|---|---|
| CD | 81.221 ± 0.019 c | −1.7 ± 0.2 a | 9 ± 0.2 a | 100.5 ± 0.3 c | 9 ± 0 a | - |
| RD | 64.1 ± 0.3 a | 14.1 ± 0.4 c | 29.2 ± 0.4 c | 64.2 ± 0.3 a | 32.4 ± 0.6 c | 30.8 ± 0.6 b |
| RMD | 66.6 ± 0.2 b | 10.6 ± 0.3 b | 24.7 ± 0.3 b | 66.7 ± 0.5 b | 26.9 ± 0.3 b | 24.7 ± 0.3 a |
| 1C Ct | 59 ± 8 c,d | 7.1 ± 1.8 b | 25 ± 6 c,d | 73 ± 7 d | 26 ± 5 b,c | - |
| 1R Ct | 60 ± 7 c,d | 7.7 ± 0.8 b,c | 24 ± 2 c,d | 72 ± 3 d | 25.5 ± 1.9 b,c | 11 ± 6 a |
| 1RM Ct | 65 ± 5 d | 5.3 ± 1.4 a | 20 ± 3 a,b,c | 75 ± 4 d | 21 ± 3 a,b | 11 ± 8 a |
| 2C Ct | 64 ± 3 d | 7.4 ± 0.8 b,c | 28 ± 2 d | 75.3 ± 1.6 d | 29 ± 2 c | - |
| 2B Ct | 47 ± 8 a,b | 10.4 ± 1.5 e | 17 ± 8 a,b | 55 ± 12 a,b | 20 ± 7 a,b | 21 ± 10 b |
| 2RM Ct | 54 ± 7 b,c | 8.6 ± 1.5 c,d | 22 ± 5 b,c,d | 68 ± 4 c,d | 24 ± 5 a,b,c | 13 ± 8 a |
| 3C Ct | 62 ± 5 d | 6.9 ± 1.2 b | 27 ± 4 d | 75 ± 3 d | 28 ± 4 c | - |
| 3R Ct | 46 ± 9 a | 10 ± 2 d,e | 15 ± 10 a | 48 ± 9 a | 19 ± 9 a | 21 ± 10 b |
| 3RM Ct | 50 ± 10 a,b | 8.8 ± 1.6 c,d | 19 ± 9 a,b,c | 59 ± 19 b,c | 22 ± 8 a,b | 16 ± 14 a,b |
| 1C Cb | 50 ± 4 b | 1.4 ± 0.9 a | 7.5 ± 0.9 a | 79 ± 7 d | 7.7 ± 0.9 a | - |
| 1R Cb | 47.0 ± 1.3 a | 12.4 ± 1.2 e | 23.1 ± 1.6 d | 61.8 ± 1.3 c | 26.3 ± 1.8 e | 20 ± 2 e |
| !RM Cb | 45 ± 5 a | 8.1 ± 0.7 b | 14 ± 3 b | 59 ± 5 b,c | 16 ± 2 b | 12 ± 3 a |
| 2C Cb | 56 ± 3 c | 0.6 ± 0.9 a | 9 ± 2 a | 86 ± 4 e | 9 ± 2 a | - |
| 2R Cb | 44 ± 3 a | 11.7 ± 1.2 e | 17 ± 4 c | 54 ± 4 a | 20 ± 3 c | 18 ± 3 d,e |
| 2RM Cb | 47 ± 3 a | 9 ± 1.2 b,c | 15 ± 3 b,c | 59 ± 2 b,c | 18 ± 4 b,c | 15 ± 3 b,c |
| 3C Cb | 55.0 ± 0.8 c | 1 ± 2 a | 9 ± 4 a | 85 ± 6 e | 9 ± 4 a | - |
| 3R Cb | 44.1 ± 1.5 a | 10.3 ± 1.5 d | 16 ± 2 b,c | 57 ± 3 a,b | 19 ± 3 c | 17 ± 3 c,d |
| 3RM Cb | 47 ± 2 a | 9.6 ± 0.8 c,d | 16 ± 2 b,c | 59 ± 2 b,c | 18 ± 2 b,c | 14 ± 3 a,b |
| Sample | H (N) | A (N·s) | C | S | Ch (N) | R | P (N) |
|---|---|---|---|---|---|---|---|
| 1CB | 0.64 ± 0.12 a,b | −0.009 ± 0.008 a | 0.59 ± 0.06 a | 0.65 ± 0.12 a | 0.25 ± 0.09 a | 0.32 ± 0.04 a | 3.9 ± 0.6 f |
| 1RB | 0.42.6 ± 0.012 a | −0.06 ± 0.05 a | 0.73 ± 0.16 b,c | 0.92 ± 0.05 b,c | 0.29 ± 0.08 a | 0.38 ± 0.06 b | 2.9 ± 1.3 e |
| 1RMB | 1.19 ± 0.21 a,b,c | −0.012 ± 0.017 a | 0.84 ± 0.03 d | 0.924 ± 0.014 c | 0.93 ± 0.17 b,c | 0.525 ± 0.009 c | 2.4 ± 0.5 d,e |
| 2CB | 2.18 ± 0.26 d,e | −0.09 ± 0.19 a | 0.69 ± 0.06 a,b | 0.88 ± 0.03 b,c | 1.4 ± 0.2 c,d | 0.36 ± 0.04 a,b | 2.4 ± 0.6 d,e |
| 2RB | 1.33 ± 0.31 b,c | −0.04 ± 0.03 a | 0.82 ± 0.04 c,d | 0.93 ± 0.02 c | 1.03 ± 0.23 b,c | 0.49 ± 0.04 c | 2.2 ± 0.8 c,d |
| 2RMB | 0.93 ± 0.11 a,b | 0 ± 0 a | 0.818 ± 0.019 c,d | 0.93 ± 0.04 c | 0.71 ± 0.11 b | 0.5 ± 0.02 c | 1.8 ± 0.7 b,c |
| 3CB | 2.71 ± 0.91 e | −0.02 ± 0.02 a | 0.71 ± 0.04 b | 0.85 ± 0.04 b | 1.6 ± 0.4 d | 0.38 ± 0.04 b | 1.6 ± 0.2 b |
| 3RB | 1.85 ± 0.65 c,d | −0.006 ± 0.008 a | 0.84 ± 0.03 c,d | 0.959 ± 0.003 c | 1.5 ± 0.6 d | 0.508 ± 0.013 c | 1.2 ± 0.4 a,b |
| 3RMB | 1.38 ± 0.14 b,c,d | −0.02 ± 0.03 a | 0.88 ± 0.03 d | 0.9555 ± 0.0009 c | 1.15 ± 0.08 b,c,d | 0.5427 ± 0.0002 c | 0.74 ± 0.18 a |
| Sample | Available Carbohydrates | Fiber | Fat | Protein | Ashes |
|---|---|---|---|---|---|
| 1CB | 61 ± 2 d | 4.9 ± 1.2 b | 3.66 ± 0.03 f | 0.74 ± 0.06 c | 2.94 ± 0.12 b,c |
| 1RB | 42.98 ± 0.19 a | 14.5 ± 0.3 e | 3.5 ± 0.2 e,f | 0.72 ± 0.07 c | 2.89 ± 0.12 b |
| 1RMB | 48.6 ± 1.8 b | 5.4 ± 1.2 b | 3.31 ± 0.16 e | 0.58 ± 0.05 a,b | 2.51 ± 0.07 a |
| 2CB | 54.9 ± 1.3 c | 7.9 ± 0.6 c | 2.54 ± 0.12 c,d | 0.57 ± 0.04 a,b | 2.48 ± 0.12 a |
| 2RB | 41.1 ± 0.6 a | 12.88 ± 0.12 e | 2.42 ± 0.05 c | 0.61 ± 0.06 b | 2.63 ± 0.18 a |
| 2RMB | 53.75 ± 0.06 c | 2.3 ± 0.6 a | 2.74 ± 0.03 d | 0.551 ± 0.007 a,b | 2.67 ± 0.12 a |
| 3CB | 54 ± 2 c | 3.8 ± 1.2 a,b | 1.94 ± 0.15 a,b | 0.50 ± 0.04 a | 3.156 ± 0.009 c |
| 3RB | 43.0 ± 1.2 a | 10.4 ± 0.2 d | 2.12 ± 0.04 b | 0.590 ± 0.004 a,b | 3.499 ± 0.006 d |
| 3RMB | 49.9 ± 0.6 b | 4.3 ± 0.6 b | 1.71 ± 0.05 a | 0.55 ± 0.04 a,b | 3.788 ± 0.008 e |
| Sample | TP | TC | AC (Teq) |
|---|---|---|---|
| CD | 27.0 ± 0.9 a | 0.32 ± 0.02 a | 75 ± 5 b,c |
| RD | 145 ± 4 f | 15.33 ± 0.09 h | 136 ± 6 f |
| RMD | 77.39 ± 0.04 d,e | 7.23 ± 0.08 e | 92.0 ± 1.8 d |
| 1CB | 26.7 ± 1.2 a | 0.32 ± 0.04 a | 59 ± 2 a |
| 1RB | 46.5 ± 0.7 b | 7.998 ± 0.018 g | 106.1 ± 1.5 e |
| 1RMB | 48 ± 3 b | 5.22 ± 0.02 d | 79 ± 3 c |
| 2CB | 24.9 ± 1.2 a | 0.32 ± 0.02 a | 57.9 ± 0.3 a |
| 2RB | 77 ± 2 c,d,e | 7.776 ± 0.013 f | 112 ± 3 e |
| 2RMB | 71.9 ± 0.9 c | 4.55 ± 0.03 b | 74 ± 4 b,c |
| 3CB | 27.0 ± 0.4 a | 0.383 ± 0.012 a | 66.2 ± 1.8 a,b |
| 3RB | 81 ± 3 e | 7.72 ± 0.02 f | 113.5 ± 1.2 e |
| 3RMB | 73 ± 4 c,d | 4.81 ± 0.08 c | 71 ± 9 b,c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matas, A.; Igual, M.; García-Segovia, P.; Martínez-Monzó, J. Application of 3D Printing in the Design of Functional Gluten-Free Dough. Foods 2022, 11, 1555. https://doi.org/10.3390/foods11111555
Matas A, Igual M, García-Segovia P, Martínez-Monzó J. Application of 3D Printing in the Design of Functional Gluten-Free Dough. Foods. 2022; 11(11):1555. https://doi.org/10.3390/foods11111555
Chicago/Turabian StyleMatas, Adrián, Marta Igual, Purificación García-Segovia, and Javier Martínez-Monzó. 2022. "Application of 3D Printing in the Design of Functional Gluten-Free Dough" Foods 11, no. 11: 1555. https://doi.org/10.3390/foods11111555







