Digital PCR-Based Characterization of a g10evo-epsps Gene-Specific Matrix Reference Material for Its Food and Feed Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Plant Material
2.2. Primers and Probes
2.3. Isolation and Assessment of Genomic DNA
2.4. PCR Assays
2.5. Homogeneity Assessment
2.6. Stability Assessment
2.7. Co-Laboratory Study for Characterization
3. Results and Discussion
3.1. Plant Materials
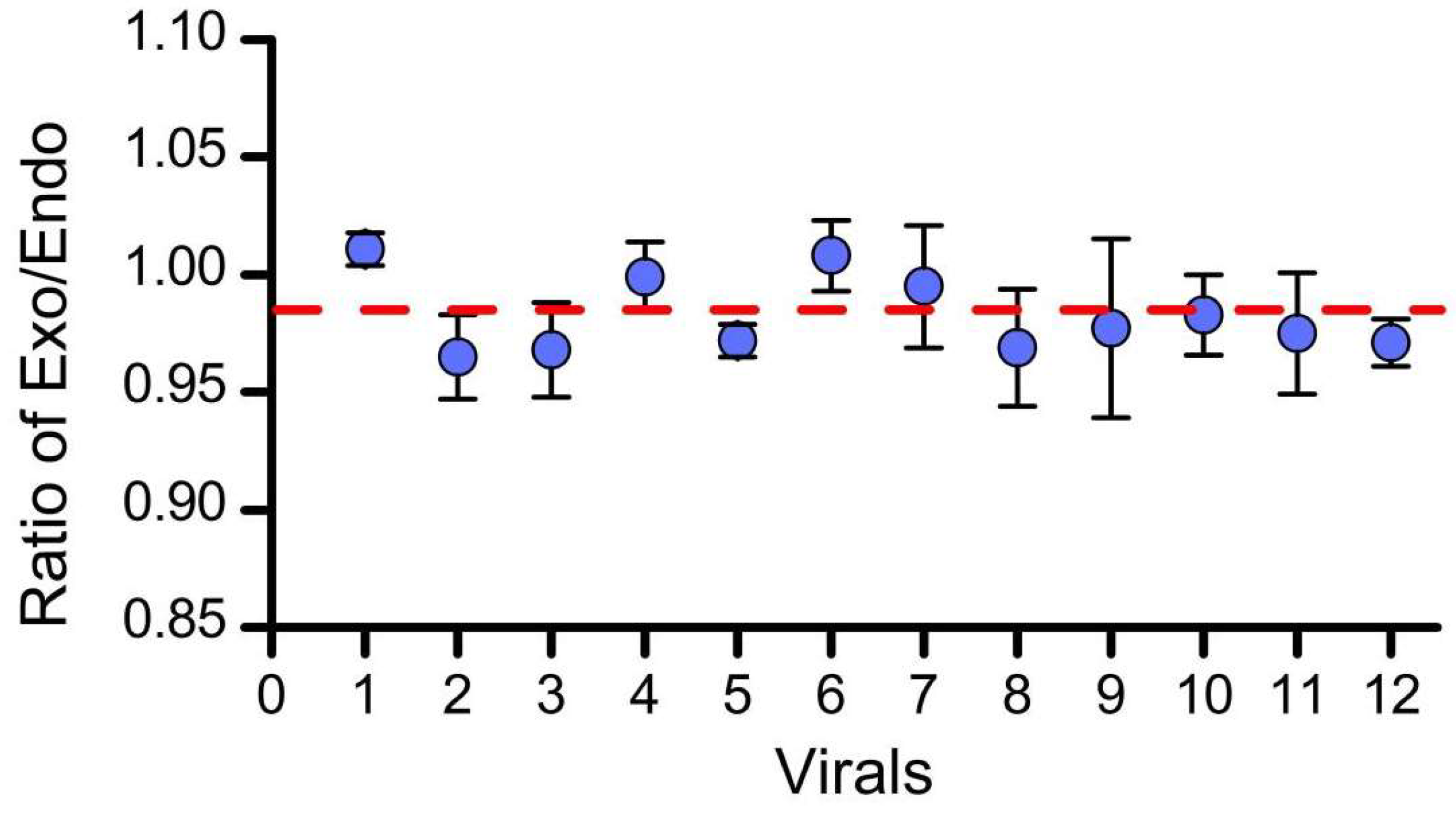
3.2. Homogeneity Assessment
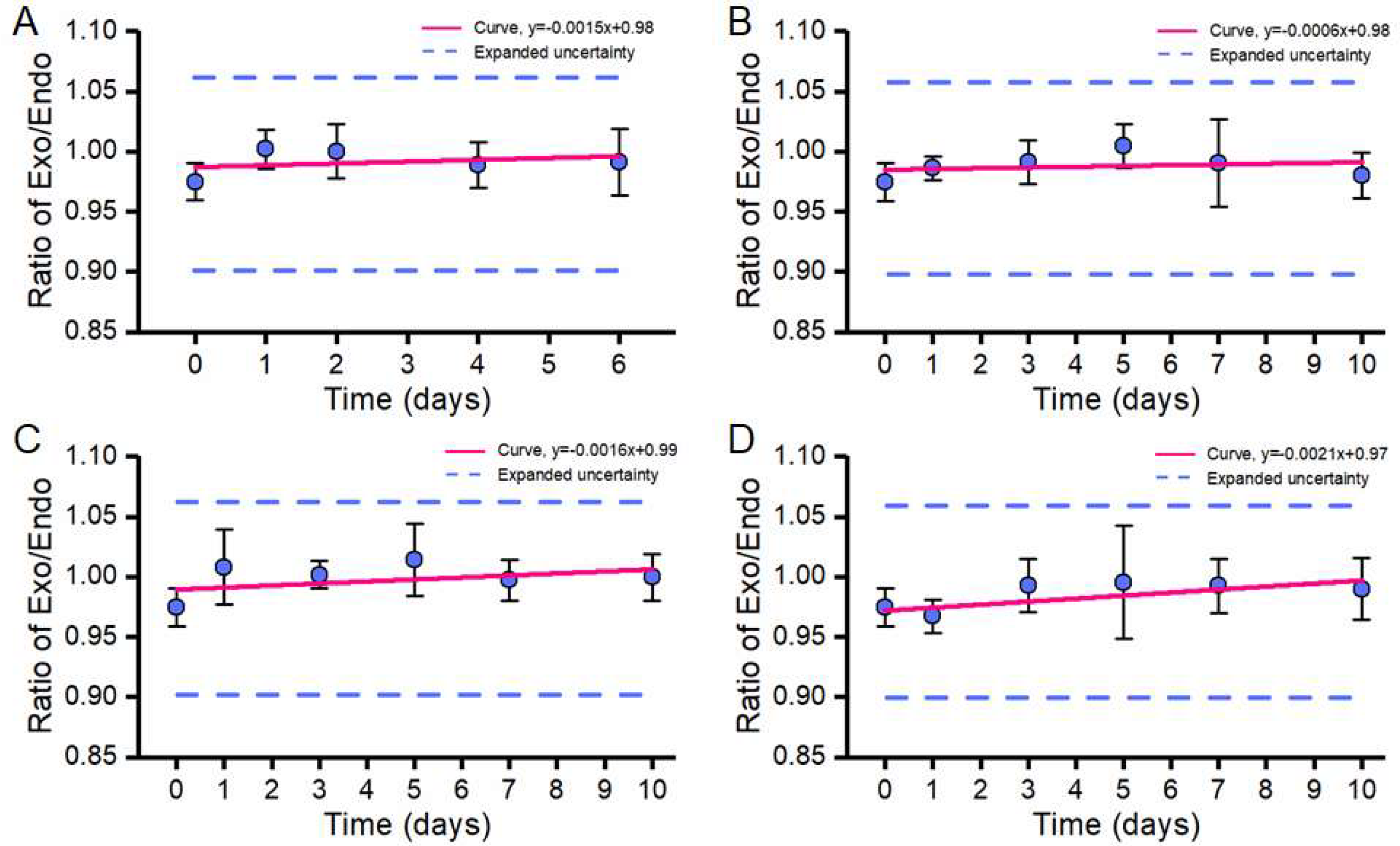
3.3. Stability Assessment
3.3.1. Short-Term Stability Test
3.3.2. Long-Term Stability Test
3.4. Inter-Laboratory Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marmiroli, N.; Maestri, E.; Gullì, M.; Malcevschi, A.; Peano, C.; Bordoni, R.; De Bellis, G. Methods for detection of GMOs in food and feed. Anal. Bioanal. Chem. 2008, 392, 369. [Google Scholar] [CrossRef] [PubMed]
- Gruère, G.P.; Rao, S. A review of international labeling policies of genetically modified food to evaluate India’s proposed rule. AgBioForum 2007, 10, 51–64. [Google Scholar]
- An, N.; Li, K.; Zhang, Y.; Wen, T.; Liu, W.; Liu, G.; Li, L.; Jin, W. A multiplex and regenerable surface plasmon resonance (MR-SPR) biosensor for DNA detection of genetically modified organisms. Talanta 2021, 231, 122361. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Paniagua López, M.; Manzanares-Palenzuela, C.L.; López-Ruiz, B. Biosensors for GMO Testing: Nearly 25 Years of Research. Crit. Rev. Anal. Chem. 2018, 48, 391–405. [Google Scholar] [CrossRef]
- Verginelli, D.; Paternò, A.; De Marchis, M.L.; Quarchioni, C.; Vinciguerra, D.; Bonini, P.; Peddis, S.; Fusco, C.; Misto, M.; Marfoglia, C.; et al. Development and comparative study of a pat/bar real-time PCR assay for integrating the screening strategy of a GMO testing laboratory. J. Sci. Food Agric. 2020, 100, 2121–2129. [Google Scholar] [CrossRef] [Green Version]
- Kamle, M.; Kumar, P.; Patra, J.K.; Bajpai, V.K. Current perspectives on genetically modified crops and detection methods. 3 Biotech 2017, 7, 219. [Google Scholar] [CrossRef]
- Funke, T.; Han, H.; Healy-Fried, M.L.; Fischer, M.; Schönbrunn, E. Molecular basis for the herbicide resistance of Roundup Ready crops. Proc. Natl. Acad. Sci. USA 2006, 103, 13010–13015. [Google Scholar] [CrossRef] [Green Version]
- ISO/TC334; Reference Materials—Selected Terms and Definitions. ISO Guide 30:2015. ISO: Geneva, Switzerland, 2015.
- Vogelstein, B.; Kinzler, K.W. Digital PCR. Proc. Natl. Acad. Sci. USA 1999, 96, 9236–9241. [Google Scholar] [CrossRef] [Green Version]
- JCGM 200:2012 International Vocabulary of Metrology–Basic and General Concepts and Associated Terms (VIM); Joint Committee for Guides in Metrology: Sèvres, France, 2012; Available online: https://www.bipm.org/en/publications/guides/vim.html (accessed on 12 February 2022).
- Burke, D.G.; Dong, L.; Bhat, S.; Forbes-Smith, M.; Fu, S.; Pinheiro, L.; Jing, W.; Emslie, K.R. Digital Polymerase Chain Reaction Measured pUC19 Marker as Calibrant for HPLC Measurement of DNA Quantity. Anal. Chem. 2013, 85, 1657–1664. [Google Scholar] [CrossRef]
- Whale, A.S.; Jones, G.M.; Pavšič, J.; Dreo, T.; Redshaw, N.; Akyürek, S.; Akgöz, M.; Divieto, C.; Sassi, M.P.; He, H.-J.; et al. Assessment of Digital PCR as a Primary Reference Measurement Procedure to Support Advances in Precision Medicine. Clin. Chem. 2018, 64, 1296–1307. [Google Scholar] [CrossRef] [Green Version]
- Chaouachi, M.; Bérard, A.; Saïd, K. Relative quantification in seed GMO analysis: State of art and bottlenecks. Transgenic Res. 2013, 22, 461–476. [Google Scholar] [CrossRef]
- Holst-Jensen, A.; De Loose, M.; Van den Eede, G. Coherence between Legal Requirements and Approaches for Detection of Genetically Modified Organisms (GMOs) and Their Derived Products. J. Agric. Food Chem. 2006, 54, 2799–2809. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, Y.; Li, X.; Wang, Y.; Zhang, L.; Li, Y.; Wu, G. Developing a matrix reference material for screening of transgenic rice. Anal. Bioanal. Chem. 2015, 407, 9153–9163. [Google Scholar] [CrossRef]
- Wu, Y.; Li, J.; Li, X.; Zhai, S.; Gao, H.; Li, Y.; Zhang, X.; Wu, G. Development and strategy of reference materials for the DNA-based detection of genetically modified organisms. Anal. Bioanal. Chem. 2019, 411, 1729–1744. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, L.; Yang, H.; Li, X.; Zhang, X.; Xu, J.; Zhang, D.; Jin, W.; Yang, L. Development of certified matrix-based reference material as a calibrator for genetically modified rice G6H1 analysis. J. Agric. Food Chem. 2018, 66, 3708–3715. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, L.; Zhang, L.; Zhang, X.; Li, X.; Zhai, S.; Gao, H.; Li, Y.; Wu, G.; Wu, Y. Development of a certified genomic DNA reference material for detection and quantification of genetically modified rice KMD. Anal. Bioanal. Chem. 2020, 412, 7007–7016. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.; Li, L.; Li, X.; Zhang, X.; Zhai, S.; Gao, H.; Li, Y.; Wu, G.; Wu, Y. Development of genomic dna certified reference materials for genetically modified rice Kefeng 6. ACS Omega 2020, 5, 21602–21609. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, X.; Wan, Y.; Jin, W. Development of a novel reference plasmid for accurate quantification of genetically modified kefeng6 rice dna in food and feed samples. BioMed Res. Int. 2013, 2013, 134675. [Google Scholar] [CrossRef]
- Wu, Y.; Li, J.; Wang, Y.; Li, X.; Li, Y.; Zhu, L.; Li, J.; Wu, G. Development and application of a general plasmid reference material for GMO screening. Plasmid 2016, 87-88, 28–36. [Google Scholar] [CrossRef]
- Shen, Z.; Lin, C.; Xu, X.; Li, X. Highly Resistant Glyphosate Mutant Gene and Its Improvement Method and Application. CN201110009329.0, 7 August 2013. [Google Scholar]
- ISO 21570:1-103; Foodstuffs—Methods of Analysis for the Detection of Genetically Modified Organisms and Derived Products—Quantitative Nucleic Acid-Based Methods. ISO: Geneva, Switzerland, 2005.
- Ministry of Agriculture Announcement No. 2630-15-2017; Detection of Genetically Modified Plants and Derived Products—Qualitative PCR Method for Herbicide-Tolerant Soybean SHZD32-1 and Its Derivates. Ministry of Agriculture: Beijing, China, 2017.
- Ministry of Agriculture Announcement No. 2122-16-2007; Detection of Genetically Modified Plants and Derived Products—Qualitative PCR Method for Insect-Resistant Maize MON810 and Its Derivates. Ministry of Agriculture: Beijing, China, 2007.
- Ministry of Agriculture Announcement No. 2122-8-2014; Detection of Genetically Modified Plants and Derived Products—Quantitative PCR Method for Insect-Resistant Rice TT51-1 and Its Derivates. Ministry of Agriculture: Beijing, China, 2014.
- ISO/Guide 35:2017(en); Reference Material-Guidance for Characterization and Assessment of Homogeneity and Stability. ISO: Geneva, Switzerland, 2017. Available online: https://www.iso.org/obp/ui/#iso:std:iso:guide:35:ed-4:v1:en (accessed on 12 February 2022).
- Chen, X.; Ji, Y.; Li, K.; Wang, X.; Peng, C.; Xu, X.; Pei, X.; Xu, J.; Li, L. Development of a duck genomic reference material by digital pcr platforms for the detection of meat adulteration. Foods 2021, 10, 1890. [Google Scholar] [CrossRef] [PubMed]

| Crop | Event Name | Pilot Test | Environment Release | Productive Trial | Safety Certificate |
|---|---|---|---|---|---|
| Soybean | Zuts33 | + | |||
| Shzd32-1 | + | ||||
| CAL16 | + | ||||
| Corn | RF125 | + | |||
| SK12-6 | + | ||||
| GAB-3 | + | ||||
| PO-3 | + | ||||
| ZmX-9 | + | ||||
| G3X-1 | + | ||||
| Rice | OsX-1 | + | |||
| AIL-3 | + | ||||
| Cotton | GV-1 | + |
| Crop | Primers | Sequences (5′-3′) | Amplicon Size (bp) |
|---|---|---|---|
| g10evo-epsps | F4 | TTACCGTGAGAGGTGGTAGACCT | 90 |
| R4 | GTGGTATCACCCTCAGCGAAG | ||
| P4 | FAM-TTCCTTCACCGACGCC-MGB | ||
| Lectin | F | CCAGCTTCGCCGCTTCCTTC | 74 |
| R | GAAGGCAAGCCCATCTGCAAGCC | ||
| P | FAM-CTTCACCTTCTATGCCCCTGACAC-TAMRA |
| Sample | Resource | Q | f | S2 | F | F0.05 (11, 24) | Result |
|---|---|---|---|---|---|---|---|
| g10evo- epsps | between- vials | 8.71 × 10−3 | 11 | 7.92 × 10−4 | 1.86 | 2.18 | F < F0.05 (11, 24) |
| within-vial | 1.02 × 10−2 | 24 | 4.26 × 10−4 |
| No. | Mean | SD | RSD |
|---|---|---|---|
| 1 | 0.970 | 0.01 | 0.99% |
| 2 | 1.000 | 0.02 | 1.72% |
| 3 | 1.009 | 0.01 | 1.07% |
| 4 | 0.968 | 0.02 | 1.73% |
| 5 | 0.968 | 0.02 | 2.47% |
| 6 | 0.977 | 0.03 | 3.11% |
| 7 | 0.960 | 0.02 | 2.26% |
| 8 | 0.974 | 0.01 | 1.41% |
| Mean | 0.978 | ||
| SD | 0.018 | ||
| RSD | 1.845% | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Yu, H.; Wang, P.; Peng, C.; Wang, X.; Xu, X.; Xu, J.; Liang, J.; Li, L. Digital PCR-Based Characterization of a g10evo-epsps Gene-Specific Matrix Reference Material for Its Food and Feed Detection. Foods 2022, 11, 1888. https://doi.org/10.3390/foods11131888
Chen X, Yu H, Wang P, Peng C, Wang X, Xu X, Xu J, Liang J, Li L. Digital PCR-Based Characterization of a g10evo-epsps Gene-Specific Matrix Reference Material for Its Food and Feed Detection. Foods. 2022; 11(13):1888. https://doi.org/10.3390/foods11131888
Chicago/Turabian StyleChen, Xiaoyun, Huiru Yu, Pengfei Wang, Cheng Peng, Xiaofu Wang, Xiaoli Xu, Junfeng Xu, Jingang Liang, and Liang Li. 2022. "Digital PCR-Based Characterization of a g10evo-epsps Gene-Specific Matrix Reference Material for Its Food and Feed Detection" Foods 11, no. 13: 1888. https://doi.org/10.3390/foods11131888
APA StyleChen, X., Yu, H., Wang, P., Peng, C., Wang, X., Xu, X., Xu, J., Liang, J., & Li, L. (2022). Digital PCR-Based Characterization of a g10evo-epsps Gene-Specific Matrix Reference Material for Its Food and Feed Detection. Foods, 11(13), 1888. https://doi.org/10.3390/foods11131888







