Sustainable Development and Storage Stability of Orange By-Products Extract Using Natural Deep Eutectic Solvents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Chemical and Reagents
2.3. COSMOtherm Simulation
2.4. Preparation of Natural Deep Eutectic Solvents
2.5. NADES Characterization
2.6. Extraction Procedure
2.7. Total Polyphenol Content by UV–Vis Spectroscopy
2.8. Total Flavonoid Content by UV–Vis Spectroscopy
2.9. Optimization Process
2.10. TPC Stability
2.11. Statistical Analysis
3. Results
3.1. NADES Characterization
3.2. COSMOtherm Simulation and Model Validation
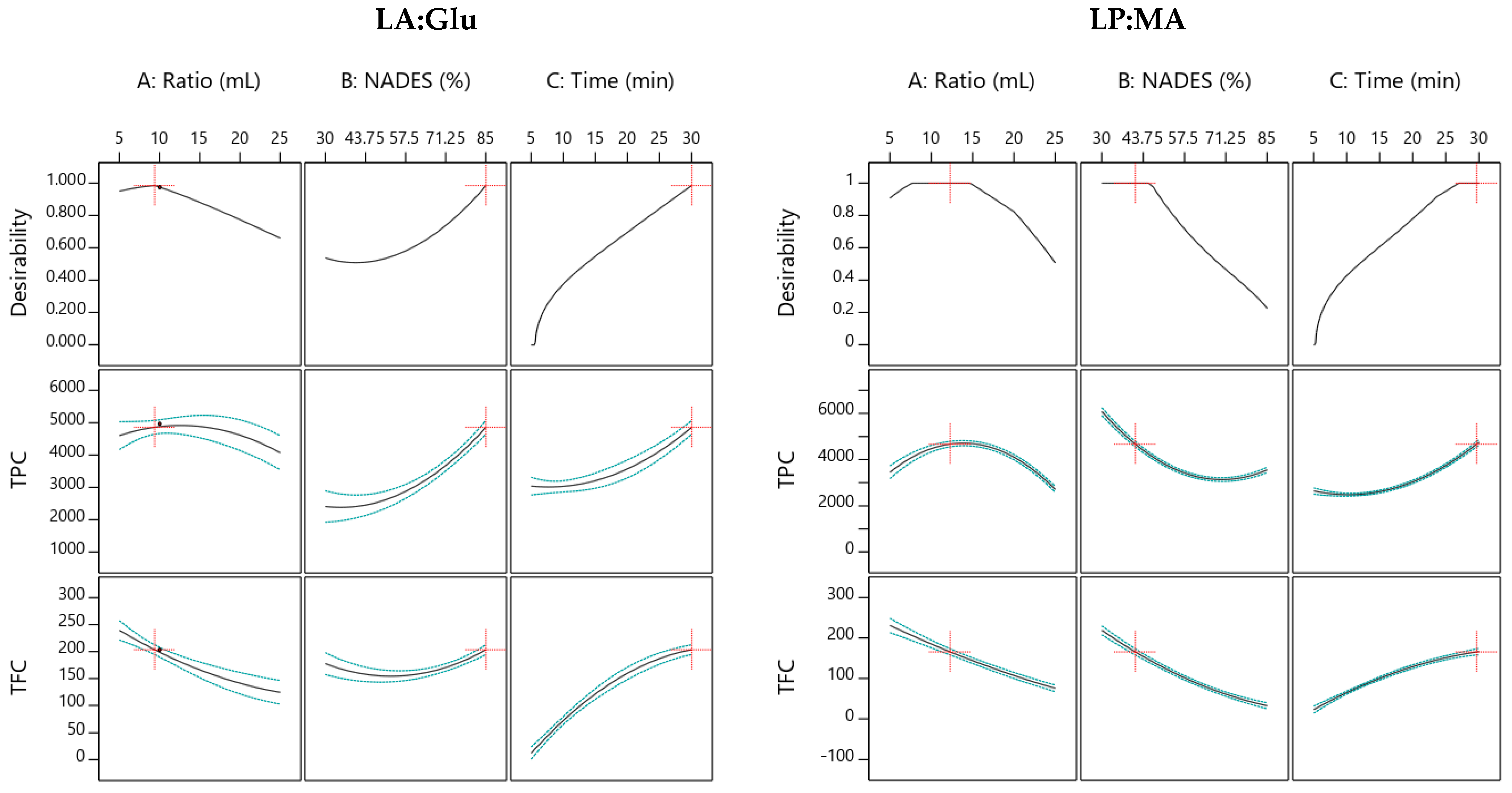
3.3. Optimization of Extraction Conditions by Response Surface Methodology
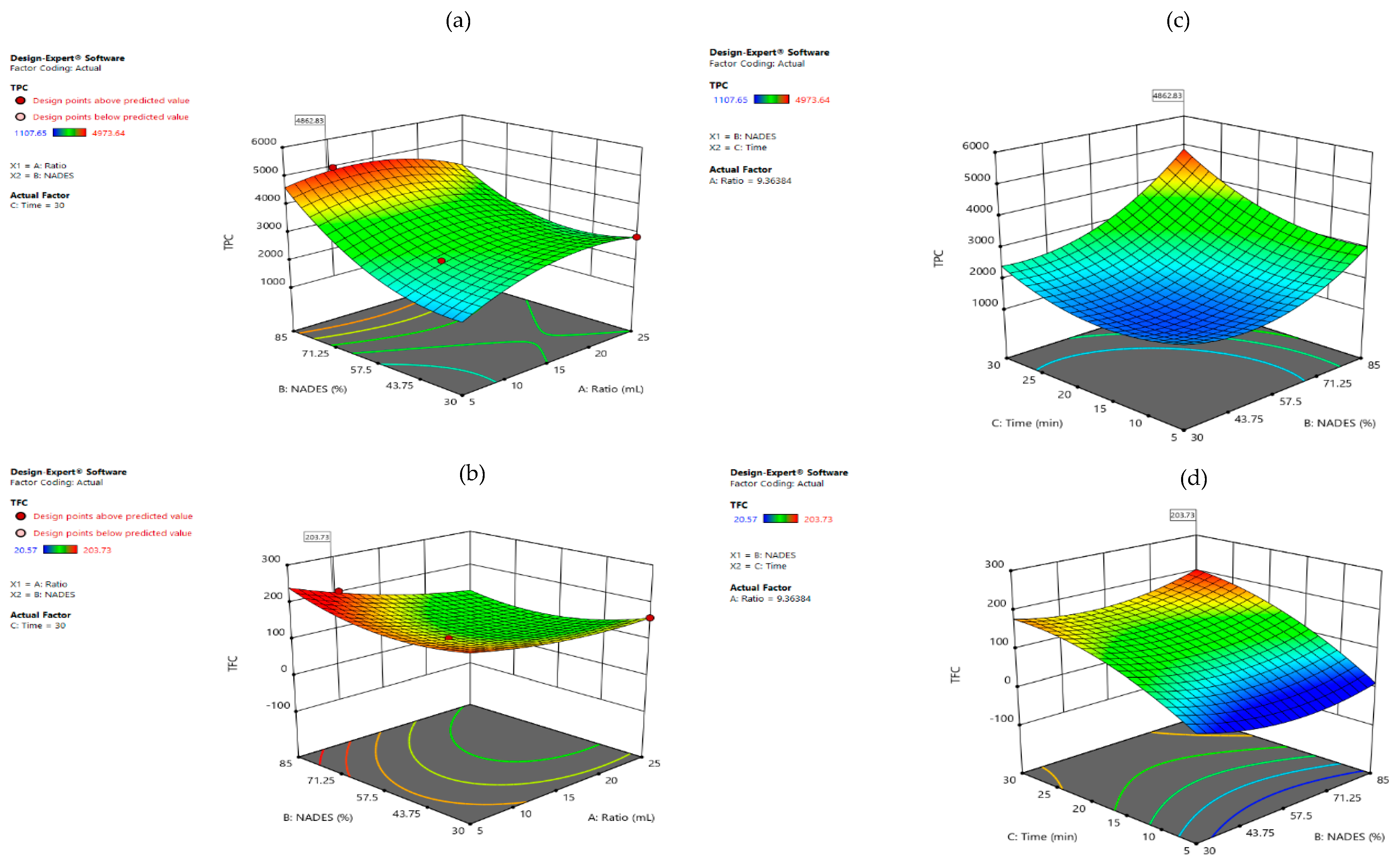
3.3.1. Lactic Acid: Glucose
545 × X12 399.44 × X22 + 579.71 × X32
15.89 × X12 + 18.62 × X22 − 34.19 × X32
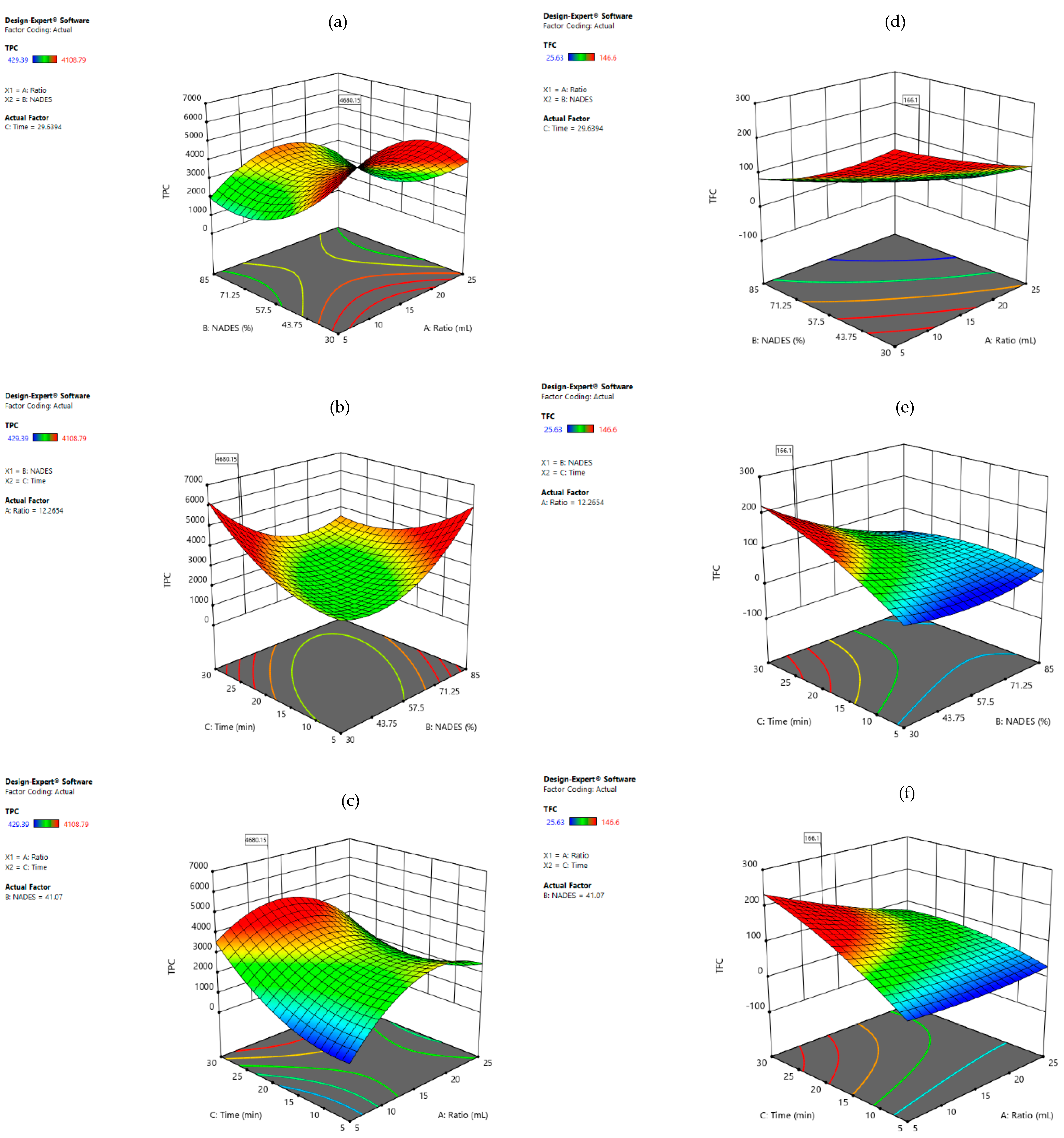
3.3.2. L-Proline: Malic Acid
718.06 × X1X3 − 1019.80 × X2X3 − 1610.97 × X12 + 739.78 × X22 + 908.10 × X32
− 34.59 × X2X3 + 9.51 × X12 + 12.80 × X22 − 20.81 × X32
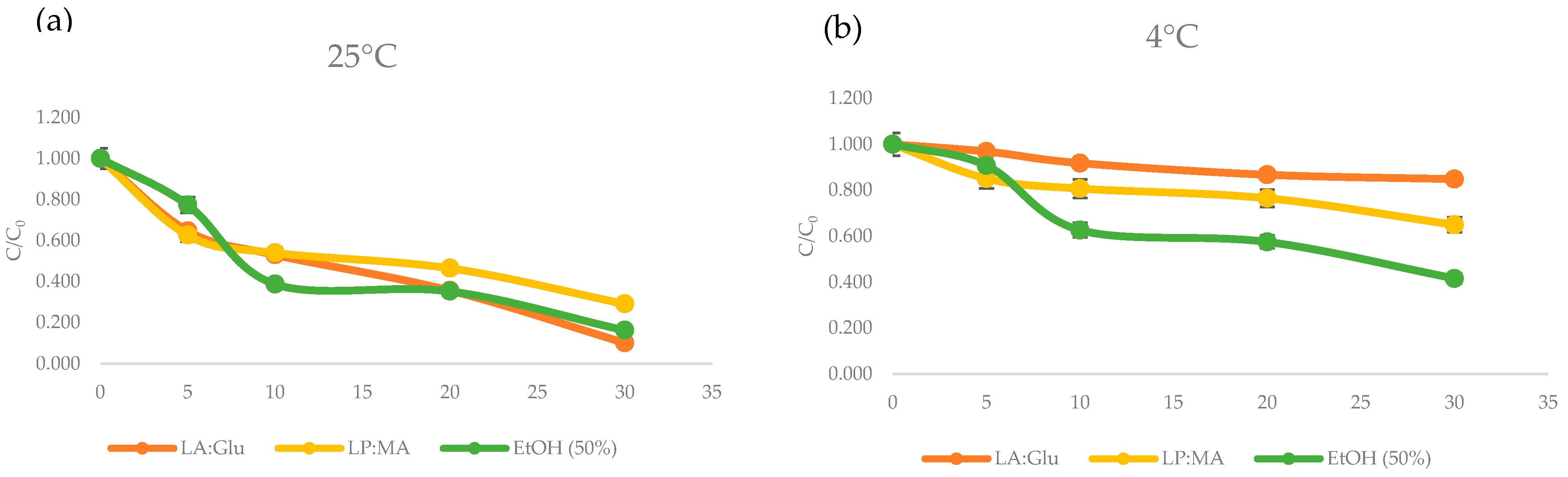
3.4. TPC Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Montenegro-Landívar, M.F.; Tapia-Quirós, P.; Vecino, X.; Reig, M.; Valderrama, C.; Granados, M.; Cortina, J.L.; Saurina, J. Fruit and vegetable processing wastes as natural sources of antioxidant-rich extracts: Evaluation of advanced extraction technologies by surface response methodology. J. Environ. Chem. Eng. 2021, 9, 105330. [Google Scholar] [CrossRef]
- Matharu, A.S.; de Melo, E.M.; Houghton, J.A. Opportunity for high value-added chemicals from food supply chain wastes. Bioresour. Technol. 2016, 215, 123–130. [Google Scholar] [CrossRef]
- Murador, D.C.; De Souza Mesquita, L.M.; Neves, B.V.; Braga, A.R.C.; Martins, P.L.G.; Zepka, L.Q.; De Rosso, V.V. Bioaccessibility and cellular uptake by Caco-2 cells of carotenoids and chlorophylls from orange peels: A comparison between conventional and ionic liquid mediated extractions. Food Chem. 2021, 339, 127818. [Google Scholar] [CrossRef]
- Tajaldini, M.; Samadi, F.; Khosravi, A.; Ghasemnejad, A.; Asadi, J. Protective and anticancer effects of orange peel extract and naringin in doxorubicin treated esophageal cancer stem cell xenograft tumor mouse model. Biomed. Pharmacother. 2020, 121, 109594. [Google Scholar] [CrossRef] [PubMed]
- Anticona, M.; Blesa, J.; Frigola, A.; Esteve, M.J. High Biological Value Compounds Extraction from Citrus Waste with Non-Conventional Methods. Foods 2020, 9, 811. [Google Scholar] [CrossRef] [PubMed]
- Panić, M.; Drakula, S.; Cravotto, G.; Verpoorte, R.; Hruškar, M.; Redovniković, I.; Radošević, K. Biological activity and sensory evaluation of cocoa by-products NADES extracts used in food fortification. Innov. Food Sci. Emerg. Technol. 2020, 66, 102514. [Google Scholar] [CrossRef]
- Ozturk, B.; Parkinson, C.; Gonzalez-Miquel, M. Extraction of polyphenolic antioxidants from orange peel waste using deep eutectic solvents. Sep. Purif. Technol. 2018, 206, 1–13. [Google Scholar] [CrossRef]
- Yara-Varón, E.; Fabiano-Tixier, A.S.; Balcells, M.; Canela-Garayoa, R.; Bily, A.; Chemat, F. Is it possible to substitute hexane with green solvents for the extraction of carotenoids? A theoretical versus experimental solubility study. RSC Adv. 2016, 6, 27750–27759. [Google Scholar] [CrossRef]
- Chemat, F.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Strube, J.; Uhlenbrock, L.; Gunjevic, V.; Cravotto, G. Green extraction of natural products. Origins, current status, and future challenges. Trends Anal. Chem. 2019, 118, 248–263. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef]
- Bosiljkov, T.; Dujmić, F.; Cvjetko Bubalo, M.; Hribar, J.; Vidrih, R.; Brnčić, M.; Zlatic, E.; Radojčić Redovniković, I.; Jokić, S. Natural deep eutectic solvents and ultrasound-assisted extraction: Green approaches for extraction of wine lees anthocyanins. Food Bioprod. Process. 2017, 102, 195–203. [Google Scholar] [CrossRef]
- Manurung, R.; Siregar, A.G.A. Performance of menthol based deep eutectic solvents in the extraction of carotenoids from crude palm oil. Int. J. Geomate 2019, 19, 131–137. [Google Scholar] [CrossRef]
- Martins, N.; Ferreira, I.C.F.R. Wastes and by-products: Upcoming sources of carotenoids for biotechnological purposes and health-related applications. Trends Food Sci. Technol. 2017, 62, 33–48. [Google Scholar] [CrossRef]
- Mitar, A.; Panić, M.; Kardum, J.P.; Halambek, J.; Sander, A.; Kučan, K.Z.; Redovniković, I.R.; Radošević, K. Physicochemical Properties, Cytotoxicity, and Antioxidative Activity of Natural Deep Eutectic Solvents Containing Organic Acid. Chem. Biochem. Eng. Q 2019, 33, 1454. [Google Scholar] [CrossRef]
- Jokić, S.; Molnar, M.; Cikoš, A.M.; Jakovljević, M.; Šafranko, S.; Jerković, I. Separation of selected bioactive compounds from orange peel using the sequence of supercritical CO2 extraction and ultrasound solvent extraction: Optimization of limonene and hesperidin content. Sep. Sci. Technol. 2020, 55, 2799–2811. [Google Scholar] [CrossRef]
- Panić, M.; Gunjević, V.; Radošević, K.; Bubalo, M.C.; Ganić, K.K.; Redovniković, I.R. Cosmotherm as an effective tool for selection of deep eutectic solvents based ready-to-use extracts from Graševina grape pomace. Molecules 2021, 26, 4722. [Google Scholar] [CrossRef]
- Zurob, E.; Cabezas, R.; Villarroel, E.; Rosas, N.; Merlet, G.; Quijada-Maldonado, E.; Romero, J.; Plaza, A. Design of natural deep eutectic solvents for the ultrasound-assisted extraction of hydroxytyrosol from olive leaves supported by COSMO-RS. Sep. Sci. Technol 2020, 248, 117054. [Google Scholar] [CrossRef]
- Panić, M.; Gunjević, V.; Cravotto, G.; Redovniković, I.R. Enabling technologies for the extraction of grape-pomace anthocyanins using natural deep eutectic solvents in up-to-half-litre batches extraction of grape-pomace anthocyanins using NADES. Food Chem. 2019, 300, 125185. [Google Scholar] [CrossRef]
- Panić, M.; Andlar, M.; Tišma, M.; Rezić, T.; Šibalić, D.; Cvjetko Bubalo, M.; Radojčić Redovniković, I. Natural deep eutectic solvent as a unique solvent for valorisation of orange peel waste by the integrated biorefinery approach. Waste Manag. 2021, 120, 340–350. [Google Scholar] [CrossRef]
- Zeisel, S.H. Dietary choline deficiency causes DNA strand breaks and alters epigenetic marks on DNA and histones. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2012, 733, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Panić, M.; Stojković, M.R.; Kraljić, K.; Škevin, D.; Redovniković, I.R.; Srček, V.G.; Radošević, K. Ready-to-use green polyphenolic extracts from food by-products. Food Chem. 2019, 283, 061. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Cvjetko Bubalo, M.; Vidović, S.; Redovniković, I.R.; Jokić, S. New perspective in extraction of plant biologically active compounds by green solvents. In Food and Bioproducts Processing. Food Bioprod. Process. 2018, 109, 52–73. [Google Scholar] [CrossRef]
- Doldolova, K.; Bener, M.; Lalikoğlu, M.; Aşçı, Y.S.; Arat, R.; Apak, R. Optimization and modeling of microwave-assisted extraction of curcumin and antioxidant compounds from turmeric by using natural deep eutectic solvents. Food Chem. 2021, 353, 129337. [Google Scholar] [CrossRef]
- Huang, Y.; Feng, F.; Jiang, J.; Qiao, Y.; Wu, T.; Voglmeir, J.; Chen, Z.G. Green and efficient extraction of rutin from tartary buckwheat hull by using natural deep eutectic solvents. Food Chem. 2017, 221, 1400–1405. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.J.; Verpoorte, R.; Choi, Y. Natural Deep Eutectic Solvents as a New Extraction Media for Phenolic Metabolites in Carthamus tinctorius L. Anal. Chem. 2013, 85, 6272–6278. [Google Scholar] [CrossRef]
- Boukroufa, M.; Boutekedjiret, C.; Petigny, L.; Rakotomanomana, N.; Chemat, F. Bio-refinery of orange peels waste: A new concept based on integrated green and solvent free extraction processes using ultrasound and microwave techniques to obtain essential oil, polyphenols, and pectin. Ultrason. Sonochem. 2015, 24, 72–79. [Google Scholar] [CrossRef]
- Radošević, K.; Ćurko, N.; Gaurina Srček, V.; Cvjetko Bubalo, M.; Tomašević, M.; Kovačević Ganić, K.; Radojčić Redovniković, I. Natural deep eutectic solvents as beneficial extractants for enhancement of plant extracts bioactivity. LWT Food Sci. Technol. 2016, 73, 45–51. [Google Scholar] [CrossRef]
- Grillo, G.; Gunjević, V.; Radošević, K.; Redovniković, I.R.; Cravotto, G. Deep Eutectic Solvents and Nonconventional Technologies for Blueberry-Peel Extraction: Kinetics, Anthocyanin Stability, and Antiproliferative Activity. Antioxidants 2020, 9, 1069. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.T.; Pauletto, R.; da Silva Cavalheiro, S.; Bochi, V.C.; Rodrigues, E.; Weber, J.; de Silva, C.; Morisso, F.D.P.; Barcia, M.T.; Emanuelli, T. Natural deep eutectic solvents as a biocompatible tool for the extraction of blueberry anthocyanins. J. Food Compos. Anal. 2020, 89, 103470. [Google Scholar] [CrossRef]
- SCOGS U.S. Food and Drug Administration. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/?set=SCOGS&sort=Sortsubstance&order=ASC&startrow=1&type=basic&search=LACTICACID (accessed on 30 June 2022).
- Saharan, R.; Kumar, S.; Khokra, S.L.; Singh, S.; Tiwari, A.; Tiwari, V.; Sahoo, B.M.; Kumar, M. A Comprehensive Review on Therapeutic Potentials of Natural Cyclic Peptides. Curr. Nutr. Food. Sci. 2022, 18, 53509. [Google Scholar] [CrossRef]
- Ozturk, B.; Winterburn, J.; Gonzalez-Miquel, M. Orange peel waste valorisation through limonene extraction using bio-based solvents. Biochem. Eng. J. 2019, 151, 107298. [Google Scholar] [CrossRef]
- Pal, C.B.T.; Jadeja, G.C. Microwave-assisted deep eutectic solvent extraction of phenolic antioxidants from onion (Allium cepa L.) peel: A Box–Behnken design approach for optimization. J. Food Sci. Technol. 2019, 56, 4211–4223. [Google Scholar] [CrossRef]
- Gullón, P.; Gullón, B.; Romaní, A.; Rocchetti, G.; Lorenzo, J.M. Smart advanced solvents for bioactive compounds recovery from agri-food by-products: A review. Trends Food Sci. Technol. 2020, 101, 182–197. [Google Scholar] [CrossRef]
- Pinelo, M.; Rubilar, M.; Jerez, M.; Sineiro, J.; Núñez, M.J. Effect of Solvent, Temperature, and Solvent-to-Solid Ratio on the Total Phenolic Content and Antiradical Activity of Extracts from Different Components of Grape Pomace. J. Agric. Food Chem. 2005, 53, 2111–2117. [Google Scholar] [CrossRef]
- Teslic, N.; Santos, F.; Oliveira, F.; Stupar, A.; Pojic, M.; Mandic, A.; Pavlic, B.; Kljakic, A.C.; Duarte, A.R.C.; Paiva, A.; et al. Simultaneous Hydrolysis of Ellagitannins and Extraction of Ellagic Acid from Defatted Raspberry Seeds Using Natural Deep Eutectic Solvents (NADES). Antioxidants 2022, 11, 254. [Google Scholar] [CrossRef]
- Prabowo, W.C.; Agustina, R.; Nur, Y.; Hidayati, R.; Rahmawati, D.; Arifuddin, M. Green and Optimum Extraction of Total Polyphenols Content from Mitragyna speciosa Korth. Havil Leaves using Microwave-Assisted Natural Deep Eutectic Solvent Extraction. Pharmacogn. J. 2022, 14, 29–38. [Google Scholar] [CrossRef]
- Dai, Y.; Rozema, E.; Verpoorte, R.; Choi, Y.H. Application of natural deep eutectic solvents to the extraction of anthocyanins from Catharanthus roseus with high extractability and stability replacing conventional organic solvents. J. Chromatogr. A 2016, 1434, 50–56. [Google Scholar] [CrossRef]
- Rebocho, S.; Mano, F.; Cassel, E.; Anacleto, B.; do Rosário Bronze, M.; Paiva, A.; Duarte, A.R.C. Fractionated extraction of polyphenols from mate tea leaves using a combination of hydrophobic/hydrophilic NADES. Curr. Res. Nutr. Food Sci. 2022, 5, 571–580. [Google Scholar] [CrossRef]


| Acronym | Hydrogen Bond Acceptor | Hydrogen Bond Donor | Molar Ratio | pH | Polarity (kcal/mol) |
|---|---|---|---|---|---|
| ChChl: Glu | Choline chloride | Glucose | 2:1 | 4.14 ± 0.02 | 49.79 ± 0.27 |
| ChChl: Fruc | Choline chloride | Fructose | 1.9:1 | 4.35 ± 0.01 | 51.26 ± 0.30 |
| ChChl: Xy | Choline chloride | Xylose | 2:1 | 4.26 ± 0.01 | 49.21 ± 0.10 |
| ChChl: Gly | Choline chloride | Glycerol | 1:2 | 5.19 ± 0.00 | 49.00 ± 0.17 |
| ChChl: MA | Choline chloride | Malic acid | 1:1 | 1.62 ± 0.03 | 47.85 ± 0.05 |
| ChChl: TA | Choline chloride | Tartaric acid | 2:1 | 1.54 ± 0.02 | 47.98 ± 0.01 |
| ChChl: LA | Choline chloride | Lactic acid | 1:3 | 1.43 ± 0.02 | 47.97 ± 0.01 |
| ChChl: CA | Choline chloride | Citric acid | 2:1 | 1.45 ± 0.02 | 47.81 ± 0.00 |
| ChChl: LP: MA | Choline chloride | L-Proline: Malic acid | 1:1:1 | 2.11 ± 0.00 | 48.87 ± 0.01 |
| LA: Glu | Lactic acid | Glucose | 5:1 | 1.16 ± 0.04 | 47.89 ± 0.04 |
| MA: Glu | Malic acid | Glucose | 1:1 | 1.34 ± 0.02 | 47.57 ± 0.00 |
| LP: MA | L-Proline | Malic acid | 1:1 | 2.22 ± 0.01 | 48.30 ± 0.03 |
| Bet: CA | Betaine | Citric acid | 1:1 | 2.36 ± 0.00 | 47.97 ± 0.02 |
| Bet: MA | Betaine | Malic acid | 1:1 | 2.08 ± 0.01 | 48.79 ± 0.02 |
| Acronym | ln(γ) 85% NADES | ln(γ) 75% NADES | ln(γ) 50% NADES | ln(γ) 40% NADES | ln(γ) 30% NADES |
|---|---|---|---|---|---|
| EtOH | −1.063 | ||||
| LA: Glu | −4.52 | −4.02 | −3.66 | −3.31 | −2.24 |
| LP: MA | −4.73 | −3.78 | −3.28 | −2.83 | −1.71 |
| MA: Glu | −4.81 | −3.67 | −2.94 | −2.31 | −0.93 |
| ChChl: LA | −2.15 | −1.88 | −1.60 | −1.29 | −0.28 |
| Bet: MA | −3.60 | −2.12 | −1.20 | −0.55 | 0.47 |
| ChChl: LP: MA | −1.44 | −1.11 | −0.75 | −0.30 | 0.61 |
| ChChl: Xyl | −2.49 | −1.73 | −0.87 | 0.00 | 1.78 |
| Bet: CA | −0.53 | −0.26 | −0.12 | 0.06 | 0.70 |
| ChChl: MA | −1.16 | −0.60 | −0.07 | 0.44 | 1.31 |
| ChChl: TA | −2.14 | −1.10 | −0.33 | 0.48 | 1.74 |
| ChChl: Glu | −2.24 | −1.35 | −0.34 | 0.53 | 2.04 |
| ChChl:CA | −1.52 | −0.66 | 0.02 | 0.75 | 1.93 |
| ChChl: Gly | −0.60 | −0.21 | 0.28 | 0.77 | 1.62 |
| ChChl: Fruc | −1.01 | −0.51 | 0.11 | 0.82 | 1.89 |
| Independent Variable | Level | |||||
|---|---|---|---|---|---|---|
| −∞ | −1 | 0 | +1 | +∞ | ||
| Solid:liquid ratio | X1 | 5 | 10 | 15 | 20 | 25 |
| NADES (%, v/v) | X2 | 30 | 40 | 50 | 75 | 85 |
| Extraction time | X3 | 5 | 10 | 15 | 20 | 30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Urios, C.; Viñas-Ospino, A.; Puchades-Colera, P.; López-Malo, D.; Frígola, A.; Esteve, M.J.; Blesa, J. Sustainable Development and Storage Stability of Orange By-Products Extract Using Natural Deep Eutectic Solvents. Foods 2022, 11, 2457. https://doi.org/10.3390/foods11162457
Gómez-Urios C, Viñas-Ospino A, Puchades-Colera P, López-Malo D, Frígola A, Esteve MJ, Blesa J. Sustainable Development and Storage Stability of Orange By-Products Extract Using Natural Deep Eutectic Solvents. Foods. 2022; 11(16):2457. https://doi.org/10.3390/foods11162457
Chicago/Turabian StyleGómez-Urios, Clara, Adriana Viñas-Ospino, Pablo Puchades-Colera, Daniel López-Malo, Ana Frígola, María José Esteve, and Jesús Blesa. 2022. "Sustainable Development and Storage Stability of Orange By-Products Extract Using Natural Deep Eutectic Solvents" Foods 11, no. 16: 2457. https://doi.org/10.3390/foods11162457







