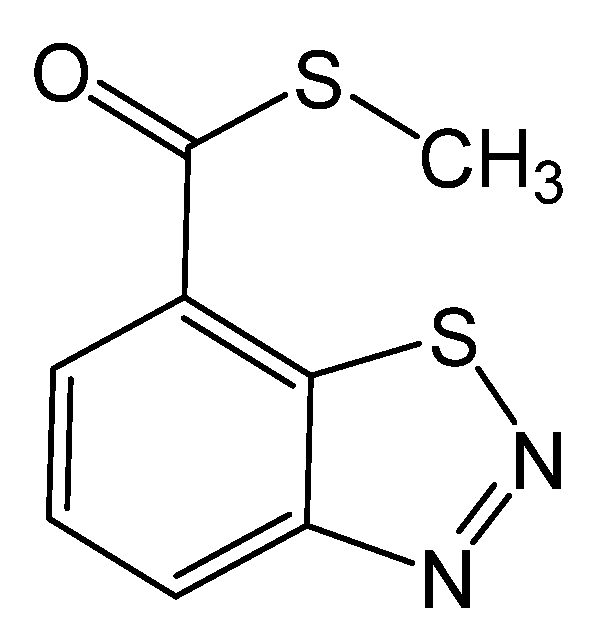
Pre-Harvest Benzothiadiazole Spraying Promotes the Cumulation of Phenolic Compounds in Grapes
Abstract
:1. Introduction
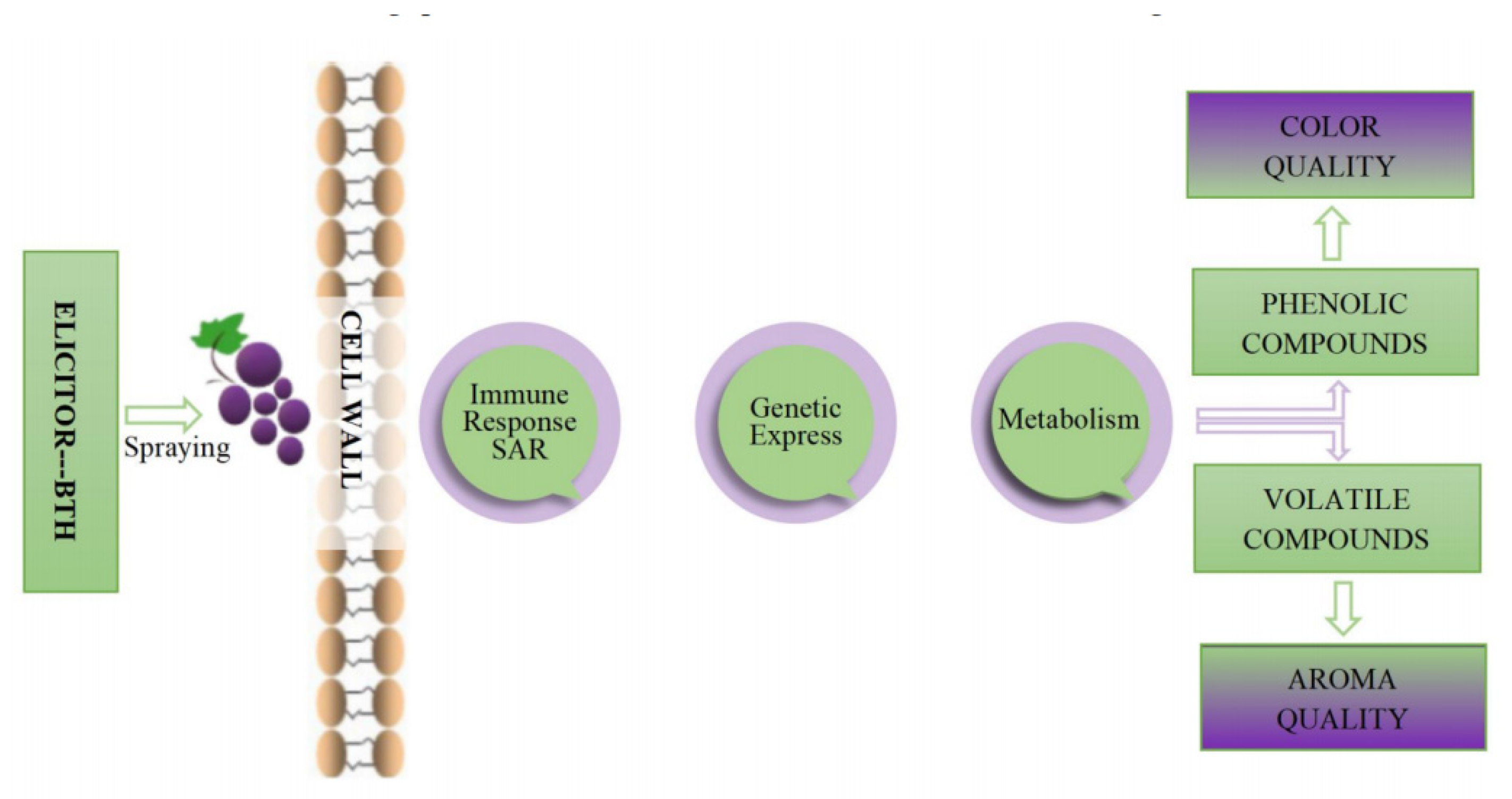
2. BTH Treatment Promotes the Cumulation of Phenolic Substances in Grapes
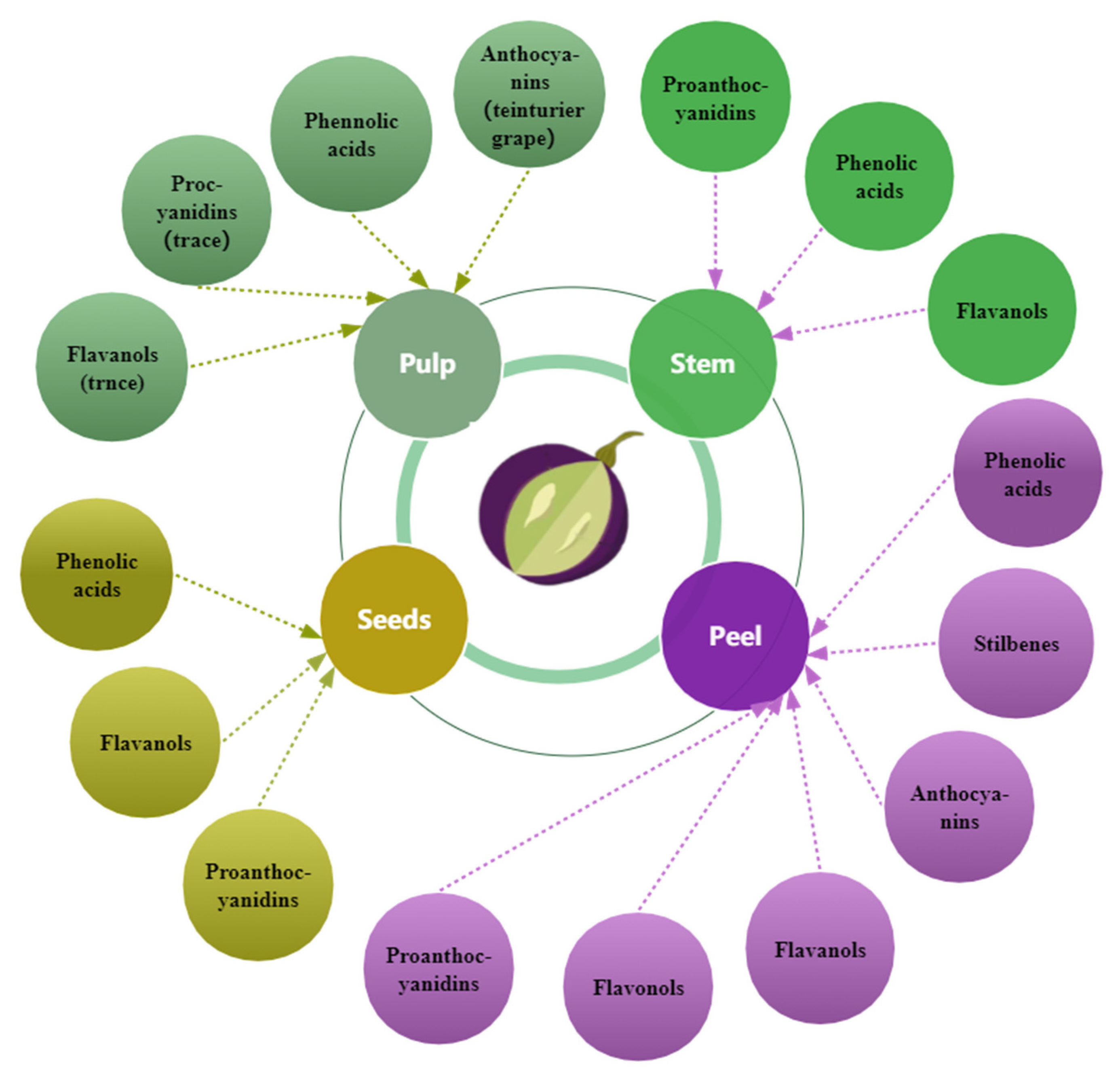
2.1. The Composition of Grape Phenols
2.2. Effect of BTH on the Composition of Grape Phenols
2.2.1. Effect on Anthocyanins
2.2.2. Effect on Flavonols
2.2.3. Effect on Proanthocyanidins (Tannins)
2.2.4. Effect on Stilbenes
2.2.5. Effect on Color Quality
3. Effect of BTH on Phenols Metabolism
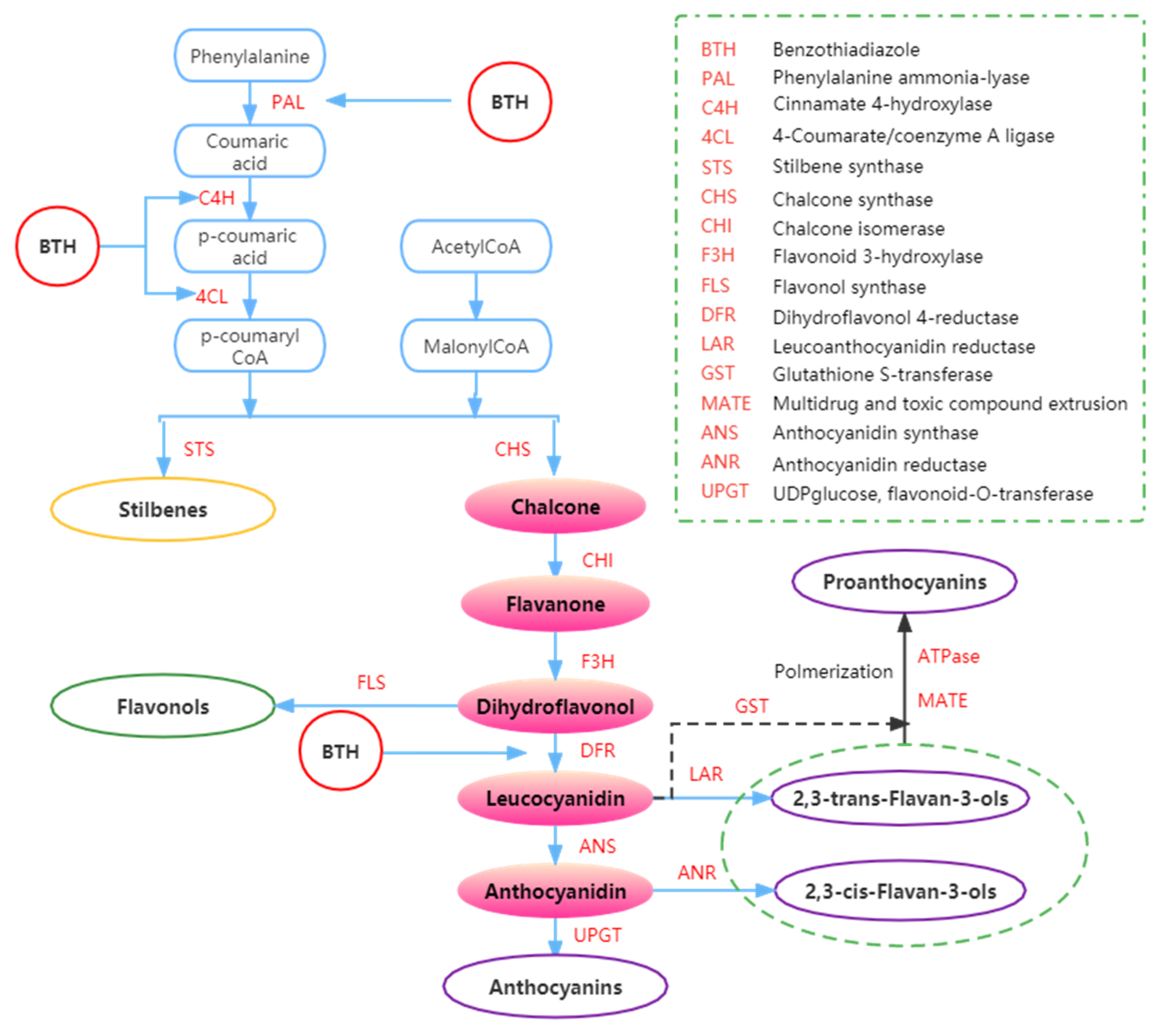
3.1. Synthesis of Plant Phenols
3.2. Influence of BTH
4. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ojeda, H.; Andary, C.; Kraeva, E.; Carbonneau, A.; Deloire, A. Influence of Pre- and Postveraison Water Deficit on Synthesis and Concentration of Skin Phenolic Compounds during Berry Growth of Vitis vinifera cv. Shiraz. Am. J. Enol. Vitic. 2002, 53, 261–267. [Google Scholar]
- Vitalini, S.; Ruggiero, A.; Rapparini, F.; Neri, L.; Tonni, M.; Iriti, M. The application of chitosan and benzothiadiazole in vineyard (Vitis vinifera L. cv Groppello Gentile) changes the aromatic profile and sensory attributes of wine. Food Chem. 2014, 162, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Ilc, T.; Werck-Reichhart, D.; Navrot, N. Meta-Analysis of the Core Aroma Components of Grape and Wine Aroma. Front. Plant Sci. 2016, 7, 1472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salifu, R.; Chen, C.; Sam, F.E.; Jiang, Y. Application of Elicitors in Grapevine Defense: Impact on Volatile Compounds. Horticulturae 2022, 8, 451. [Google Scholar] [CrossRef]
- Feng, H.; Yuan, F.; Skinkis, P.A.; Qian, M.C. Influence of Cluster Zone Leaf Removal on Pinot Noir Grape Chemical and Volatile Composition. Food Chem. 2015, 173, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Sivilotti, P.; Falchi, R.; Herrera, J.C.; Škvarč, B.; Butinar, L.; Sternad Lemut, M.; Bubola, M.; Sabbatini, P.; Lisjak, K.; Vanzo, A. Combined Effects of Early Season Leaf Removal and Climatic Conditions on Aroma Precursors in Sauvignon Blanc Grapes. J. Agric. Food Chem. 2017, 65, 8426–8434. [Google Scholar] [CrossRef]
- Lacroux, F.; Tregoat, O.; Leeuwen, C.V.; Pons, A.; Tominaga, T.; Lavigne-Cruège, V.; Dubourdieu, D. Effect of foliar nitrogen and sulphur application on aromatic expression. J. Int. Sci. Vigne Vin 2008, 42, 125–132. [Google Scholar]
- Giordano, M.; Zecca, O.; Belviso, S.; Reinotti, M.; Gerbi, V.; Rolle, L. Volatile Fingerprint and Physico-Mechanical Properties of ‘Muscat Blanc’ Grapes Grown in Mountain Area: A First Evidence of the Influence of Water Regimes. IJFS 2013, 3, 329–338. [Google Scholar]
- Savoi, S.; Wong, D.C.J.; Arapitsas, P.; Miculan, M.; Bucchetti, B.; Peterlunger, E.; Fait, A.; Mattivi, F.; Castellarin, S.D. Transcriptome and Metabolite Profiling Reveals That Prolonged Drought Modulates the Phenylpropanoid and Terpenoid Pathway in White Grapes (Vitis vinifera L.). BMC Plant Biol. 2016, 16, 67. [Google Scholar] [CrossRef] [Green Version]
- Iriti, M.; Rossoni, M.; Borgo, M.; Ferrara, L.; Faoro, F. Induction of Resistance to Gray Mold with Benzothiadiazole Modifies Amino Acid Profile and Increases Proanthocyanidins in Grape: Primary versus Secondary Metabolism. J. Agric. Food Chem. 2005, 53, 9133–9139. [Google Scholar] [CrossRef]
- Martínez-Gil, A.M.; Garde-Cerdán, T.; Martínez, L.; Alonso, G.L.; Salinas, M.R. Effect of Oak Extract Application to Verdejo Grapevines on Grape and Wine Aroma. J. Agric. Food Chem. 2011, 59, 3253–3263. [Google Scholar] [CrossRef]
- Martínez-Gil, A.M.; Garde-Cerdán, T.; Zalacain, A.; Pardo-García, A.I.; Salinas, M.R. Applications of an Oak Extract on Petit Verdot Grapevines. Influence on Grape and Wine Volatile Compounds. Food Chem. 2012, 132, 1836–1845. [Google Scholar] [CrossRef]
- Martínez-Gil, A.M.; Pardo-García, A.I.; Zalacain, A.; Alonso, G.L.; Salinas, M.R. Lavandin Hydrolat Applications to Petit Verdot Vineyards and Their Impact on Their Wine Aroma Compounds. Food Res. Int. 2013, 53, 391–402. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, B.; Jiang, Y.; Wang, X.; Lyu, Z.; Han, L.; Zhang, X. Effects of Ultra-High Pressure Treatment on Phenolic Compounds in Cabernet Gernischet Grape. Food Ferment. Ind. 2022. [Google Scholar] [CrossRef]
- Kok, D. Influences of Pre- and Post-Veraison Cluster Thinning Treatments on Grape Composition Variables and Monoterpene Levels of Vitis vinifera L. cv. Sauvignon Blanc. J. Food Agric. Environ. 2011, 9, 22–26. [Google Scholar]
- Salifu, R.; Jiang, Y.; Ba, L.; Zhang, Z.; Feng, L.; Li, J. Influence of Benzothiadiazole on the Amino Acids and Aroma Compositions of ‘Cabernet Gernischt’ Grapes (Vitis vinifera L.). Horticulturae 2022, 8, 812. [Google Scholar] [CrossRef]
- Caboni, P.; Cabras, P. Pesticides’ Influence on Wine Fermentation. Adv. Food Nutr. Res. 2010, 59, 43–62. [Google Scholar] [CrossRef]
- Thakur, M.; Sohal, B.S. Role of Elicitors in Inducing Resistance in Plants against Pathogen Infection: A Review. ISRN Biochem. 2013, 13, 10. [Google Scholar] [CrossRef] [Green Version]
- Delaunois, B.; Farace, G.; Jeandet, P.; Clément, C.; Baillieul, F.; Dorey, S.; Cordelier, S. Elicitors as Alternative Strategy to Pesticides in Grapevine? Current Knowledge on Their Mode of Action from Controlled Conditions to Vineyard. Environ Sci. Pollut. Res. 2014, 21, 4837–4846. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Portu, J.; Santamaría, P.; López, R.; Garde-Cerdán, T. Effects on Grape Amino Acid Concentration through Foliar Application of Three Different Elicitors. Food Res. Int. 2017, 99, 688–692. [Google Scholar] [CrossRef]
- Perazzolli, M.; Dagostin, S.; Ferrari, A.; Elad, Y.; Pertot, I. Induction of Systemic Resistance against Plasmopara viticola in Grapevine by Trichoderma harzianum T39 and Benzothiadiazole. Biol. Control. 2008, 47, 228–234. [Google Scholar] [CrossRef]
- Wang, K.; Liao, Y.; Cao, S.; Di, H.; Zheng, Y. Postharvest Biology and Technology Effects of Benzothiadiazole on Disease Resistance and Soluble Sugar Accumulation in Grape Berries and Its Possible Cellular Mechanisms Involved. Postharvest Biol. Technol. 2015, 102, 51–60. [Google Scholar] [CrossRef]
- Gómez-Plaza, E.; Bautista-Ortín, A.B.; Ruiz-García, Y.; José, I.; Gil-Muñoz, R. Effect of Elicitors on the Evolution of Grape Phenolic Compounds during the Ripening Period. J. Sci. Food Agric. 2017, 97, 977–983. [Google Scholar] [CrossRef]
- Li, Y.; Héloir, M.C.; Zhang, X.; Geissler, M.; Trouvelot, S.; Jacquens, L.; Henkel, M.; Su, X.; Fang, X.; Wang, Q.; et al. Surfactin and Fengycin Contribute to the Protection of a Bacillus Subtilis Strain against Grape Downy Mildew by Both Direct Effect and Defence Stimulation. Mol. Plant Pathol. 2019, 20, 1037–1050. [Google Scholar] [CrossRef]
- Ochoa-Velasco, C.E.; Avila-Sosa, R.; Navarro-Cruz, A.R.; López-Malo, A.; Palou, E. Chapter 9—Biotic and Abiotic Factors to Increase Bioactive Compounds in Fruits and Vegetables. In Handbook of Food Bioengineering; Grumezescu, A.M., Holban, A.M.B.T.-F.B., Eds.; Academic Press: New York, NY, USA, 2017; pp. 317–349. ISBN 978-0-12-811413-1. [Google Scholar]
- Vuolo, M.M.; Lima, V.S.; Maróstica Junior, M.R. Chapter 2—Phenolic Compounds: Structure, Classification, and Antioxidant Power; Campos, M.R.S., Ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 33–50. ISBN 978-0-12-814774-0. [Google Scholar]
- Machado, T.; Portugal, I.; Padilha, C.; Padilha, F.F.; Li Ma, M. New Trends in the Use of Enzymes for the Recovery of Polyphenols in Grape Byproducts. J. Food Biochem. 2021, 45, e13712. [Google Scholar] [CrossRef]
- He, Y.; Li, J.; Mi, L.; Chen, Y.; Hu, Y.; Wang, B.; Wang, Y.; Jiang, Y. Effects of PH, temperature and SO2 on Pyranoanthocyanins (Vitisins) in Red Wine by Response Surface Methodology. Food Ferment. Ind. 2016, 42, 115–120. [Google Scholar]
- Crupi, P.; Alba, V.; Masi, G.; Caputo, A.R.; Tarricone, L. Effect of Two Exogenous Plant Growth Regulators on the Color and Quality Parameters of Seedless Table Grape Berries. Food Res. Int. 2019, 126, 108667. [Google Scholar] [CrossRef]
- Paladines-Quezada, D.F.; Moreno-Olivares, J.D.; Fernández-Fernández, J.I.; Bleda-Sánchez, J.A.; Gil-Muñoz, R. Application of Elicitors at Two Maturation Stages of Vitis vinifera L. cv Monastrell: Changes in Skin Cell Walls. Chemistry 2022, 4, 98–111. [Google Scholar] [CrossRef]
- Brown, J.C.; Jiang, X. Activities of Muscadine Grape Skin and Polyphenolic Constituents against Helicobacter pylori. J. Appl. Microbiol. 2013, 114, 982–991. [Google Scholar] [CrossRef] [Green Version]
- Weaver, S.R.; Rendeiro, C.; Mcgettrick, H.M.; Philp, A.; Lucas, S.J.E. Fine Wine or Sour Grapes? A Systematic Review and Meta-Analysis of the Impact of Red Wine Polyphenols on Vascular Health. Eur. J. Nutr. 2021, 60, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Paladines-Quezada, D.F.; Fern, I.; Moreno-Olivares, J.D.; Bleda-Sánchez, J.A.G.C.; Mart, A.; Gil-Muñoz, R. Application of Elicitors in Two Ripening Periods of Vitis vinifera L. cv Monastrell: Influence on Anthocyanin. Molecules 2021, 26, 1689. [Google Scholar] [CrossRef]
- Leifert, W.R.; Abeywardena, M.Y. Cardioprotective Actions of Grape Polyphenols. Nutr. Res. 2008, 28, 729–737. [Google Scholar] [CrossRef]
- Barros, A.; Gironés-Vilaplana, A.; Teixeira, A.; Collado-González, J.; Moreno, D.A.; Gil-Izquierdo, A.; Rosa, E.; Domínguez-Perles, R. Evaluation of Grape (Vitis vinifera L.) Stems from Portuguese Varieties as a Resource of (Poly) Phenolic Compounds: A Comparative Study. Food Res. Int. 2014, 65, 375–384. [Google Scholar] [CrossRef]
- Colombo, F.; Di Lorenzo, C.; Regazzoni, L.; Fumagalli, M.; Sangiovanni, E.; Peres de Sousa, L.; Bavaresco, L.; Tomasi, D.; Bosso, A.; Aldini, G.; et al. Phenolic Profiles and Anti-Inflammatory Activities of Sixteen Table Grape (Vitis vinifera L.) Varieties. Food Funct. 2019, 10, 1797–1807. [Google Scholar] [CrossRef]
- Tian, Q.; Xu, Z.; Sun, X.; Deavila, J.; Du, M.; Zhu, M. Grape Pomace Inhibits Colon Carcinogenesis by Suppressing Cell Proliferation and Inducing Epigenetic Modifications. J. Nutr. Biochem. 2020, 84, 108443. [Google Scholar] [CrossRef]
- Martins, I.M.; Macedo, G.A.; Macedo, J.A. Biotransformed Grape Pomace as a Potential Source of Anti-Inflammatory Polyphenolics: Effects in Caco-2 Cells. Food Biosci. 2020, 35, 100607. [Google Scholar] [CrossRef]
- Silva, V.; Igrejas, G.; Falco, V.; Santos, T.P.; Torres, C.; Oliveira, A.M.P.; Pereira, J.E.; Amaral, J.S.; Poeta, P. Chemical Composition, Antioxidant and Antimicrobial Activity of Phenolic Compounds Extracted from Wine Industry By-Products. Food Control 2018, 92, 516–522. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Navarro, J.; Izquierdo-Cañas, P.M.; Mena-Morales, A.; Martínez-Gascueña, J.; Chacón-Vozmediano, J.L.; García-Romero, E.; Hermosín-Gutiérrez, I.; Gómez-Alonso, S. Phenolic Compounds Profile of Different Berry Parts from Novel Vitis vinifera L. Red Grape Genotypes and Tempranillo Using HPLC-DAD-ESI-MS/MS: A Varietal Differentiation Tool. Food Chem. 2019, 295, 350–360. [Google Scholar] [CrossRef]
- Xia, E.; Deng, G.; Guo, Y.; Li, H. Biological Activities of Polyphenols from Grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef]
- Cosme, F.; Pinto, T.; Vilela, A. Phenolic Compounds and Antioxidant Activity in Grape Juices: A Chemical and Sensory View. Beverages 2018, 4, 22. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-García, Y.; Gómez-Plaza, E. Elicitors: A Tool for Improving Fruit Phenolic Content. Agriculture 2013, 3, 33–52. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-García, Y.; Gil-Muñoz, R.; López-Roca, J.M.; Martínez-Cutillas, A.; Romero-Cascales, I.; Gómez-Plaza, E. Increasing the Phenolic Compound Content of Grapes by Preharvest Application of Abscisic Acid and a Combination of Methyl Jasmonate and Benzothiadiazole. J. Agric. Food Chem. 2013, 61, 3978–3983. [Google Scholar] [CrossRef] [PubMed]
- Portu, J.; Santamaría, P.; López-Alfaro, I.; López, R.; Garde-Cerdán, T. Methyl Jasmonate Foliar Application to Tempranillo Vineyard Improved Grape and Wine Phenolic Content. J. Agric. Food Chem. 2015, 63, 2328–2337. [Google Scholar] [CrossRef] [PubMed]
- Paladines-Quezada, D.F.; Moreno-Olivares, J.D.; Fern, I. Elicitors and Pre-Fermentative Cold Maceration: Effects on Polyphenol Concentration in Monastrell Grapes and Wines. Biomolecules 2019, 9, 671. [Google Scholar] [CrossRef] [Green Version]
- Paladines-Quezada, D.F.; Moreno-Olivares, J.D.; Fernández-Fernández, J.I. Influence of methyl jasmonate and benzothiadiazole on the composition of grape skin cell walls and wines. Food Chem. 2019, 277, 691–697. [Google Scholar] [CrossRef]
- Zehra, A.; Anant, N.; Meena, M.; Swapnil, P. Current Research in Microbial Sciences Efficiency of Microbial Bio-Agents as Elicitors in Plant Defense Mechanism under Biotic Stress: A Review. Curr. Res. Microb. Sci. 2021, 2, 100054. [Google Scholar] [CrossRef]
- Gómez-Plaza, E.; Mestre-Ortuño, L.; Ruiz-García, Y.; Fernández-Fernández, J.I.; López-Roca, J.M. Effect of Benzothiadiazole and Methyl Jasmonate on the Volatile Compound Composition of Vitis vinifera L. Monastrell Grapes and Wines. Am. J. Enol. Vitic. 2012, 3, 394–401. [Google Scholar] [CrossRef]
- Miliordos, D.; Tsiknia, M.; Kontoudakis, N.; Dimopoulou, M.; Bouyioukos, C.; Kotseridis, Y. Impact of Application of Abscisic Acid, Benzothiadiazole and Chitosan on Berry Quality Characteristics and Plant Associated Microbial Communities of Vitis vinifera L Var. Mouhtaro Plants 2021, 13, 5802. [Google Scholar] [CrossRef]
- Durrant, W.E.; Dong, X. Systemic acquired resistance. Annu. Rev. Phytopathol. 2004, 42, 185–209. [Google Scholar] [CrossRef]
- Iriti, M.; Rossoni, M.; Borgo, M.; Faoro, F. Benzothiadiazole Enhances Resveratrol and Anthocyanin Biosynthesis in Grapevine, Meanwhile Improving Resistance to Botrytis cinerea. J. Agric. Food Chem. 2004, 52, 4406–4413. [Google Scholar] [CrossRef]
- Fernandez-Marin, M.I.; Guerrero, F.; Puertas, B. Impact of preharvest and postharvest treatment combinations on increase of stilbene content in grape. J. Int. Sci. Vigne Vin 2013, 47, 203–212. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Li, J.; Wang, Y.; Wang, B.; Zhang, G.; Zhang, R.; Jiang, Y. Effect of Postharvest Benzothiadiazole Treatment on Membrane Phospholipid Metabolism of Thick-Skinned Muskmelon. Food Sci. 2018, 39, 165–172. [Google Scholar] [CrossRef]
- Wang, B.; Li, J.X.; Li, J.; Wang, Y.; Hu, Y.; Jiang, Y. Effect of Postharvest Benzothiadiazole Treatment on Aroma Esters Derived from Amino Acid Metabolism and Metabolic Mechanism in ‘Yujinxiang’ Melon. Food Sci. 2018, 39, 212–220. [Google Scholar]
- Wang, Y.; Li, J.X.; Li, J.; Hao, L.; Hu, Y.; Wang, B.; Jiang, Y. Effect of Postharvest BTH Treatment on Aroma Monoterpenes and Key Metabolic Enzymes in ‘Yujinxiang’ Melon. Food Sci. 2019, 40, 214–221. [Google Scholar]
- Cipollini, D.; Purrington, C.B.; Bergelson, J. Cost of Induced Responses in Plants. Basic Appl. Ecol. 2003, 4, 79–89. [Google Scholar] [CrossRef] [Green Version]
- Iriti, M.; Faoro, F. Does Benzothiadiazole-Induced Resistance Increase Fitness Cost in Bean? J. Plant Pathol. 2003, 85, 265–270. [Google Scholar]
- Fumagalli, F.; Rossoni, M.; Iriti, M.; Gennaro, A.D.; Faoro, F.; Borroni, E.; Borgo, M.; Scienza, A.; Sala, A.; Folco, G. From Field to Health: A Simple Way to Increase the Nutraceutical Content of Grape as Shown by NO-Dependent Vascular Relaxation. J. Agric. Food Chem. 2006, 54, 5344–5349. [Google Scholar] [CrossRef]
- Gil-Muñoz, R.; Fernández-Fernández, J.I.; Crespo-Villegas, O.; Garde-Cerdán, T. Elicitors Used as a Tool to Increase Stilbenes in Grapes and Wines. Food Res. Int. 2017, 98, 34–39. [Google Scholar] [CrossRef]
- Gil-Muñoz, R.; Bautista-Ortín, A.B.; Ruiz-García, Y. Improving Phenolic and Chromatic Characteristics of Monastrell, Merlot and Syrah Wines by Using Methyl Jasmonate and Benzothiadiazole. Oeno One 2017, 51, 17–27. [Google Scholar]
- Flamini, R.; Mattivi, F.; Rosso, M.D.; Arapitsas, P. Advanced Knowledge of Three Important Classes of Grape Phenolics: Anthocyanins, Stilbenes and Flavonols. Int. J. Mol. Sci. 2013, 14, 19651–19669. [Google Scholar] [CrossRef]
- Biniari, K.; Xenaki, M.; Daskalakis, I.; Rusjan, D.; Bouza, D.; Stavrakaki, M. Polyphenolic Compounds and Antioxidants of Skin and Berry Grapes of Greek Vitis vinifera Cultivars in Relation to Climate Conditions. Food Chem. 2020, 307, 5518. [Google Scholar] [CrossRef] [PubMed]
- Myrtsi, E.D.; Koulocheri, S.D.; Iliopoulos, V.; Haroutounian, S.A. High-Throughput Quantification of 32 Bioactive Antioxidant Phenolic Compounds in Grapes, Wines and Vinification Byproducts by LC–MS/MS. Antioxidants 2021, 10, 1174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Cai, J.; Duan, C.; Reeves, M.J.; He, F. A Review of Polyphenolics in Oak Woods. Int. J. Mol. Sci. 2015, 16, 6978–7014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeb, A. Phenolic Antioxidants in Foods: Chemistry, Biochemistry and Analysis, 1st ed.; Springer Nature Switzerland AG: Cham, Switzerland, 2021; ISBN 3030747670. [Google Scholar]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, J.; Silva, C.L.; Castilho, P.C.; Câmara, J.S. An Attractive, Sensitive and High-Throughput Strategy Based on Microextraction by Packed Sorbent Followed by UHPLC-PDA Analysis for Quantification of Hydroxybenzoic and Hydroxycinnamic Acids in Wines. Microchem. J. 2013, 106, 129–138. [Google Scholar] [CrossRef]
- Wollgast, J.; Anklam, E. Review on Polyphenols in Theobroma Cacao: Changes in Composition during the Manufacture of Chocolate and Methodology for Identification and Quantification. Food Res. Int. 2000, 33, 423–447. [Google Scholar] [CrossRef]
- Villamor, R.R.; Ross, C.F. Wine Matrix Compounds Affect Perception of Wine Aromas. Annu. Rev. Food Sci. Technol. 2013, 4, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Lesschaeve, I.; Noble, A.C. Polyphenols: Factors Influencing Their Sensory Properties and Their Effects on Food and Beverage Preferences. Am. J. Clin. Nutr. 2005, 81, 330S–335S. [Google Scholar] [CrossRef] [Green Version]
- Harbertson, J.F.; Hodgins, R.E.; Thurston, L.; Schaffer, L.J.; Reid, M.; Landon, J.L.; Ross, C.F.; Adams, D.O. Variability of Tannin Concentration in Red Wines. Am. J. Enol. Vitic. 2008, 59, 210–214. [Google Scholar]
- Kennedy, J.A.; Matthews, M.A.; Waterhouse, A.L. Effect of Maturity and Vine Water Status on Grape Skin and Wine Flavonoids. Am. J. Enol. Vitic. 2002, 53, 268–274. [Google Scholar]
- Downey, M.O.; Dokoozlian, N.K.; Krstic, M.P. Cultural Practice and Environmental Impacts on the Flavonoid Composition of Grapes and Wine: A Review of Recent Research. Am. J. Enol. Vitic. 2006, 57, 257–268. [Google Scholar]
- Cohen, S.D.; Kennedy, A.J. Plant Metabolism and the Environment: Implications for Managing Phenolics. Crit. Rev. Food Sci. Nutr. 2010, 50, 620–643. [Google Scholar] [CrossRef] [PubMed]
- Cantos, E.; Espín, J.C.; Fernández, M.J.; Oliva, J.; Tomás-Barberán, F.A. Postharvest UV-C-Irradiated Grapes as a Potential Source for Producing Stilbene-Enriched Red Wines. J. Agric. Food Chem. 2003, 51, 1208–1214. [Google Scholar] [CrossRef] [PubMed]
- Monagas, M.; Gómez-Cordovés, C.; Bartolomé, B.; Laureano, O.; Ricardo da Silva, J.M. Monomeric, Oligomeric, and Polymeric Flavan-3-Ol Composition of Wines and Grapes from Vitis vinifera L. Cv. Graciano, Tempranillo, and Cabernet Sauvignon. J. Agric. Food Chem. 2003, 51, 6475–6481. [Google Scholar] [CrossRef] [PubMed]
- Rombaldi, C.V.; Bergamasqui, M.; Lucchetta, L.; Zanuzo, M.; Silva, J.A. Vineyard Yield and Grape Quality in Two Different Cultivation Systems. Rev. Bras. Frutic. 2020, 26, 89–91. [Google Scholar] [CrossRef]
- Bruno, G.L.; Sparapano, L. Effects of Three Esca-Associated Fungi on Vitis vinifera L.: V. Changes in the Chemical and Biological Profile of Xylem Sap from Diseased Cv. Sangiovese Vines. Physiol. Mol. Plant Pathol. 2007, 71, 210–229. [Google Scholar] [CrossRef]
- Pantelić, M.M.; Dabić Zagorac, D.Č.; Davidović, S.M.; Todić, S.R.; Bešlić, Z.S.; Gašić, U.M.; Tešić, Ž.L.; Natić, M.M. Identification and Quantification of Phenolic Compounds in Berry Skin, Pulp, and Seeds in 13 Grapevine Varieties Grown in Serbia. Food Chem. 2016, 211, 243–252. [Google Scholar] [CrossRef]
- Beckers, G.J.M.; Spoel, S.H. Fine-Tuning Plant Defence Signalling: Salicylate versus Jasmonate. Plant Biol. 2006, 8, 1–10. [Google Scholar] [CrossRef]
- Paladines-Quezada, D.F.; Fernández-Fernández, J.I.; Bautista-Ortín, A.B.; Gómez-Plaza, E.; Bleda-Sánchez, J.A.; Gil-Muñoz, R. Influence of the use of elicitors over the composition of cell wall grapes. In Proceedings of the In Vino Analytica Scientia, Salamanca, Spain, 17–20 July 2017; pp. 2–3. [Google Scholar]
- Mhetre, V.; Patel, V.B.; Singh, S.K.; Verma, M.K. Effect of New Generation Bio-Regulators on Anthocyanins and Berry Quality of Grape Cv. Beauty Seedless Effect of New Generation Bio-Regulators on Anthocyanins and Berry Quality of Grape Cv. Beauty Seedless. Indian J. Agric. Sci. 2021, 91, 116–119. [Google Scholar]
- Li, Z.; Xue, S.; Xu, X.; Wang, B.; Zheng, X.; Li, B.; Xie, P.; Bi, Y.; Prusky, D. Postharvest Biology and Technology Preharvest Multiple Sprays with Chitosan Accelerate the Deposition of Suberin Poly Phenolic at Wound Sites of Harvested Muskmelons. Postharvest Biol. Technol. 2021, 179, 111565. [Google Scholar] [CrossRef]
- Makris, D.P.; Kallithraka, S.; Kefalas, P. Flavonols in Grapes, Grape Products and Wines: Burden, Profile and Influential Parameters. J. Food Compos. Anal. 2006, 19, 396–404. [Google Scholar] [CrossRef]
- Mattivi, F.; Guzzon, R.; Vrhovsek, U.; Stefanini, M.; Velasco, R. Metabolite Profiling of Grape: Flavonols and Anthocyanins. J. Agric. Food Chem. 2006, 54, 7692–7702. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-García, Y.; Romero-Cascales, I.; Bautista-Ortín, A.B.; Gil-Muñoz, R.; Martínez-Cutillas, A. Increasing Bioactive Phenolic Compounds in Grapes: Response of Six Monastrell Grape Clones to Benzothiadiazole and Methyl Jasmonate Treatments. Am. J. Enol. Vitic. 2013, 64, 459–465. [Google Scholar] [CrossRef]
- Ruiz-García, Y.; Romero-Cascales, I.; Gil-Muñoz, R.; Ignacio, J. Improving Grape Phenolic Content and Wine Chromatic Characteristics through the Use of Two Different Elicitors: Methyl Jasmonate versus Benzothiadiazole. J. Agric. Food Chem. 2012, 60, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Pezet, R.; Viret, O.; Perret, C.; Tabacchi, R. Latency of Botrytis Cinerea Pers.: Fr. and Biochemical Studies During Growth and Ripening of Two Grape Berry Cultivars, Respectively Susceptible and Resistant to Grey Mould. J. Phytopathol. 2003, 151, 208–214. [Google Scholar] [CrossRef]
- Boss, P.K.; Davies, C.; Robinson, S.P. Analysis of the Expression of Anthocyanin Pathway Genes in Developing Vitis vinifera L. Cv Shiraz Grape Berries and the Implications for Pathway Regulation. Plant Physiol. 1996, 111, 1059–1066. [Google Scholar] [CrossRef] [Green Version]
- Ortega-Regules, A.; Romero-Cascales, I.; Ros-García, J.M.; López-Roca, J.M.; Gómez-Plaza, E. A First Approach towards the Relationship between Grape Skin Cell-Wall Composition and Anthocyanin Extractability. Anal. Chim. Acta 2006, 563, 26–32. [Google Scholar] [CrossRef]
- Medina-Plaza, C.; Dokoozlian, N.; Ponangi, R.P.; Blair, T.; Block, D.E.; Oberholster, A. Correlation between Skin Cell Wall Composition and Polyphenol Extractability of Pinot Noir and Cabernet Sauvignon Grapes. Am. J. Enol. Vitic. 2021, 72, 328–337. [Google Scholar] [CrossRef]
- Shahidi, F.; Yeo, J. Insoluble-Bound Phenolics in Food. Molecules 2016, 21, 1216. [Google Scholar] [CrossRef]
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; Silva-james, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards Integral Utilization of Grape Pomace from Winemaking Process: A Review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef]
- Singh, R.; Rastogi, S.; Dwivedi, U.N. Phenylpropanoid Metabolism in Ripening Fruits. Compr. Rev. Food Sci. Food Saf. 2010, 9, 398–416. [Google Scholar] [CrossRef] [PubMed]
- Hichri, I.; Barrieu, F.; Bogs, J.; Kappel, C.; Delrot, S.; Lauvergeat, V. Recent Advances in the Transcriptional Regulation of the Flavonoid Biosynthetic Pathway. J. Exp. Bot. 2011, 62, 2465–2483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, D.; Huang, T.; Tian, B. Advances in Biosynthesis and Biological Functions of Proanthocyanidins in Horticultural Plants. Foods 2020, 9, 1774. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Mir, Z.A.; Tyagi, A.; Mehari, H.; Meena, R.P.; Bhat, J.A.; Yadav, P.; Papalou, P.; Rawat, S.; Grover, A. Overexpression of NPR1 in Brassica juncea Confers Broad Spectrum Resistance to Fungal Pathogens. Front. Plant Sci. 2017, 8, 1693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romera, F.J.; García, M.J.; Lucena, C.; Martínez-Medina, A.; Aparicio, M.A.; Ramos, J.; Alcántara, E.; Angulo, M.; Pérez-Vicente, R. Induced Systemic Resistance (ISR) and Fe Deficiency Responses in Dicot Plants. Front. Plant Sci. 2019, 10, 287. [Google Scholar] [CrossRef] [Green Version]
- Gacnik, S.; Veberic, R.; Marinovic, S.; Halbwirth, H.; Mikulic-, M. Scientia Horticulturae Effect of Pre-Harvest Treatments with Salicylic and Methyl Salicylic Acid on the Chemical Profile and Activity of Some Phenylpropanoid Pathway Related Enzymes in Apple Leaves. Sci. Hortic. 2021, 277, 109794. [Google Scholar] [CrossRef]
- Xu, T.; Jiang, Y.; Li, J.; Zhang, N. Effect of BTH Treatment on Physiological Characteristics and Sensory Quality of ‘Yujinxiang ’ Muskmelon. Sci. Technol. Food Ind. 2014, 35, 315–318. [Google Scholar]
- Bektas, Y.; Eulgem, T. Synthetic Plant Defense Elicitors. Front. Plant Sci. 2015, 5, 804. [Google Scholar] [CrossRef]
- Ge, Y.; Tang, Q.; Li, C.; Duan, B.; Li, X.; Wei, M. LWT—Food Science and Technology Acibenzolar-S-Methyl Treatment Enhances Antioxidant Ability and Phenylpropanoid Pathway of Blueberries during Low Temperature Storage. LWT—Food Sci. Technol. 2019, 110, 48–53. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, X.; Bi, Y.; Zhou, X.; Zhou, W.; Ge, Y. Inhibition of postharvest volatile compounds release by preharvest acibenzolar-S-methyl (BTH) treatment on muskmelons (cv. Yindi). Trans. CSAE 2007, 23, 243–247. [Google Scholar]
- Torres, M.A. ROS in Biotic Interactions. Physiol. Plant. 2010, 138, 414–429. [Google Scholar] [CrossRef] [PubMed]
- Verhagen, B.W.M.; Trotel-Aziz, P.; Couderchet, M.; Höfte, M.; Aziz, A. Pseudomonas Spp.-Induced Systemic Resistance to Botrytis Cinerea Is Associated with Induction and Priming of Defence Responses in Grapevine. J. Exp. Bot. 2010, 61, 249–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belchí-Navarro, S.; Almagro, L.; Sabater-Jara, A.B.; Fernández-Pérez, F.; Bru, R.; Pedreño, M.A. Early Signaling Events in Grapevine Cells Elicited with Cyclodextrins and Methyl Jasmonate. Plant Physiol. Biochem. PPB 2013, 62, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Rolland, F.; Baena-Gonzalez, E.; Sheen, J. Sugar Sensing and Signaling in Plants: Conserved and Novel Mechanisms. Annu. Rev. Plant Biol. 2006, 57, 675–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeandet, P.; Bessis, R.A.; Gautheron, B. The Production of Resveratrol (3,5,4′-Trihydroxystilbene) by Grape Berries in Different Developmental Stages. Am. J. Enol. Vitic. 1991, 42, 41–46. [Google Scholar]
- Davies, C.; Robinson, S.P. Sugar Accumulation in Grape Berries. Cloning of Two Putative Vacuolar Invertase CDNAs and Their Expression in Grapevine Tissues. Plant Physiol. 1996, 111, 275–283. [Google Scholar] [CrossRef]
- Cao, S.; Hu, Z.; Zheng, Y.; Yang, Z.; Lu, B. Effect of BTH on Antioxidant Enzymes, Radical-Scavenging Activity and Decay in Strawberry Fruit. Food Chem. 2011, 125, 145–149. [Google Scholar] [CrossRef]
- Repka, V.; Fischerová, I.; Šilhárová, K. Methyl Jasmonate Is a Potent Elicitor of Multiple Defense Responses in Grapevine Leaves and Cell-Suspension Cultures. Biol. Plant 2004, 48, 273–283. [Google Scholar] [CrossRef]
- Gozzo, F. Systemic Acquired Resistance in Crop Protection: From Nature to a Chemical Approach. J. Agric. Food Chem. 2003, 51, 4487–4503. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, W.; Bi, Y.; Luo, Y. Postharvest BTH Treatment Induces Resistance of Peach (Prunus Persica L. Cv. Jiubao) Fruit to Infection by Penicillium Expansum and Enhances Activity of Fruit Defense Mechanisms. Postharvest Biol. Technol. 2005, 35, 263–269. [Google Scholar] [CrossRef]
- Zhu, X.; Cao, J.; Wang, Q.; Jiang, W. Postharvest Infiltration of BTH Reduces Infection of Mango Fruits (Mangifera Indica L. Cv. Tainong) by Colletotrichum gloeosporioides and Enhances Resistance Inducing Compounds. J. Phytopathol. 2008, 156, 68–74. [Google Scholar] [CrossRef]
- Lin, J.; Gong, D.; Zhu, S.; Zhang, L.; Zhang, L. Expression of PPO and POD Genes and Contents of Polyphenolic Compounds in Harvested Mango Fruits in Relation to Benzothiadiazole-Induced Defense against Anthracnose. Sci. Hortic. 2011, 130, 85–89. [Google Scholar] [CrossRef]
- Zhu, S.J.; Zhang, Z.W.; Xu, J.W.; Ma, L.Y.; Tang, W.L.; Liu, D.J. Effect of BTH treatment on storability and activity of related enzymes of harvested loquat fruit. Acta Hortic. 2007, 750, 445–450. [Google Scholar] [CrossRef]
- Ullah, C.; Tsai, C.-J.; Unsicker, S.B.; Xue, L.; Reichelt, M.; Gershenzon, J.; Hammerbacher, A. Salicylic Acid Activates Poplar Defense against the Biotrophic Rust Fungus Melampsora larici-populina via Increased Biosynthesis of Catechin and Proanthocyanidins. New Phytol. 2019, 221, 960–975. [Google Scholar] [CrossRef] [Green Version]
- Felicijan, M.; Kristl, J.; Krajnc, A.U. Pre-Treatment with Salicylic Acid Induces Phenolic Responses of Norway Spruce (Picea Abies) Bark to Bark Beetle (Ips Typographus) Attack. Trees-Struct. Funct. 2016, 30, 2117–2129. [Google Scholar] [CrossRef]

| Research Area | Subclasses | Reference |
|---|---|---|
| Variety | Monastrell | [23,30,33,44,46,47,61,82,87,88] |
| Merlot | [10,23,47,52,61,88] | |
| Syrah | [23,49,53,61,88] | |
| Cabernet Sauvignon | [47,82] | |
| Groppello | [2] | |
| Beauty Seedless | [83] | |
| Cabernet Gernischt | [16] | |
| Phenols | Anthocyanin | [10,23,30,33,44,46,52,61] |
| Flavonols | [23,33,44,46,87,88] | |
| Proanthocyanidins (Tannins) | [10,23,50,61,88] | |
| Stilbenes | [22,52,53,87] | |
| Metabolism | Enzymes | [10,22,23,50,52] |
| Hydrogen dioxide and ROS | [23,30] | |
| Color | Color intensity | [16,23,44,47,61,88] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Sam, F.E.; Li, J.; Bi, Y.; Ma, T.; Zhang, B. Pre-Harvest Benzothiadiazole Spraying Promotes the Cumulation of Phenolic Compounds in Grapes. Foods 2022, 11, 3345. https://doi.org/10.3390/foods11213345
Jiang Y, Sam FE, Li J, Bi Y, Ma T, Zhang B. Pre-Harvest Benzothiadiazole Spraying Promotes the Cumulation of Phenolic Compounds in Grapes. Foods. 2022; 11(21):3345. https://doi.org/10.3390/foods11213345
Chicago/Turabian StyleJiang, Yumei, Faisal Eudes Sam, Jixin Li, Yang Bi, Tengzhen Ma, and Bo Zhang. 2022. "Pre-Harvest Benzothiadiazole Spraying Promotes the Cumulation of Phenolic Compounds in Grapes" Foods 11, no. 21: 3345. https://doi.org/10.3390/foods11213345








