Bacterial Cellulose Nanofibril-Based Pickering Emulsions: Recent Trends and Applications in the Food Industry
Abstract
:1. Introduction
2. Formation and Stabilization Mechanism of Pickering Emulsions
3. Classification and Production of BC-Based Nanoparticles as Pickering Stabilizers
3.1. Bacterial Cellulose Nanocrystals (BCNCs)
3.2. Bacterial Cellulose Nanofibrils (BCNFs)
3.3. BCNF-Based Complex Nanoparticles
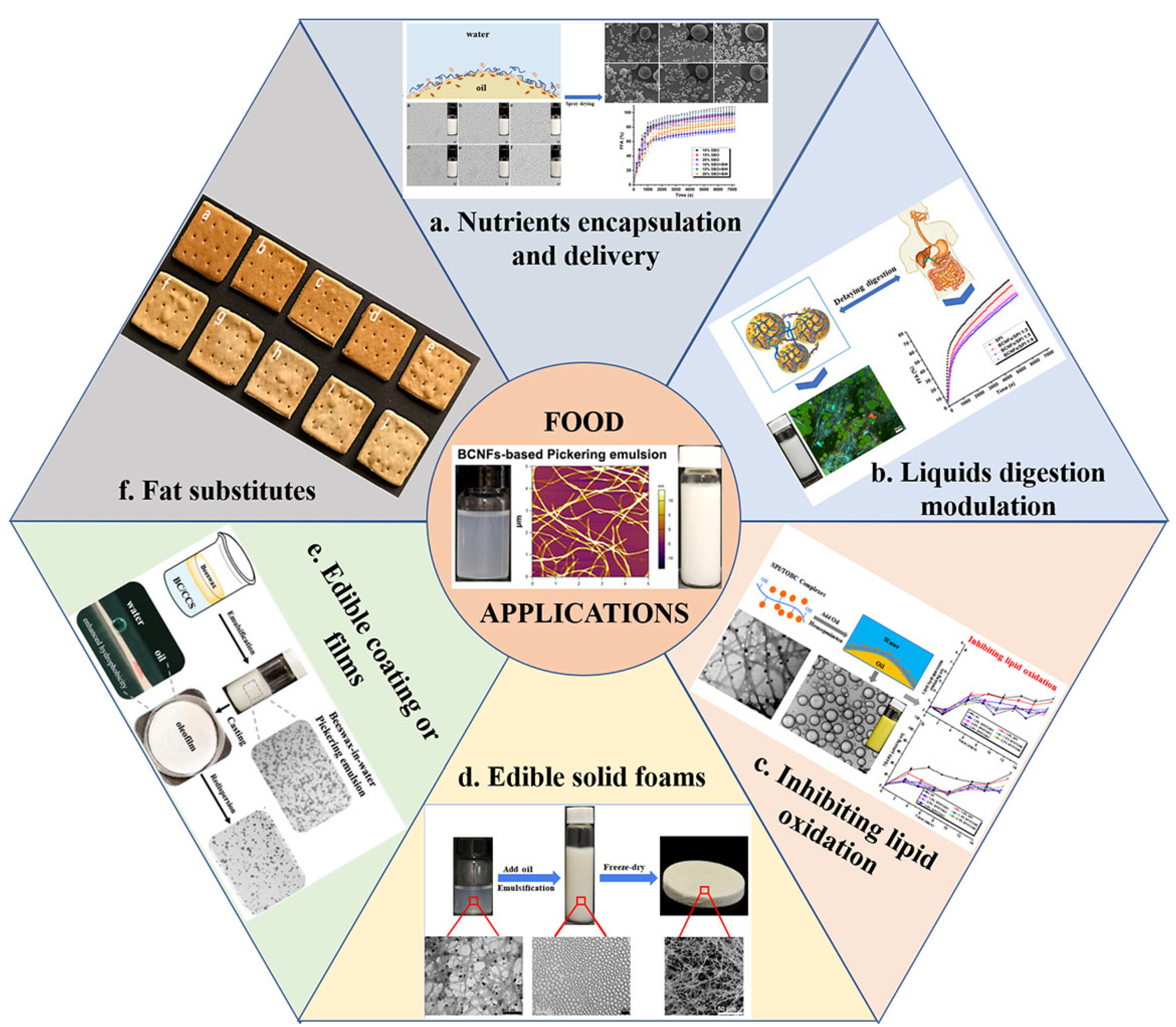
4. Applications of BCNF-Based Pickering Emulsions in the Food Industry
4.1. Nutrient Encapsulation and Delivery
4.2. Lipid Digestion Modulation
4.3. Inhibiting Lipid Oxidation
4.4. Edible Solid Foams
4.5. Edible Coatings or Films
4.6. Fat Substitutes
5. Challenges and Future Perspectives
- (1)
- There is an urgent requirement to deeply explore the toxicity and allergenicity of BCNF-based particles and their stabilized Pickering emulsions to ensure their safety in food applications. Consequently, the safety issues of BCNFs and their complex particles, as well as the influences of BCNF-based Pickering emulsions on gastrointestinal function and intestinal microflora can be further investigated by in vitro and in vivo studies;
- (2)
- Facile and low-cost preparation methods of BCNF-based Pickering stabilizers need to be provided and must be convenient for extensive commercial production and applications. Meanwhile, cost-effective modification methods of BCNF-based particles should be explored to improve their emulsification and functionality;
- (3)
- More potential applications of BCNF-based Pickering emulsions can be studied in the food industry, such as 3D-printed food, Pickering interfacial catalysis for enzymes, and probiotic encapsulation.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hong, X.; Zhao, Q.; Liu, Y.; Li, J. Recent advances on food-grade water-in-oil emulsions: Instability mechanism, fabrication, characterization, application, and research trends. Crit. Rev. Food Sci. Nutr. 2021, 61, 1–31. [Google Scholar] [CrossRef]
- Kiokias, S.; Varzakas, T. Innovative applications of food-related emulsions. Crit. Rev. Food Sci. Nutr. 2017, 57, 3165–3172. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; McClements, D.J. Application of advanced emulsion technology in the food industry: A review and critical evaluation. Foods 2021, 10, 812. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Yu, C.; Wei, Q.; Liu, D.; Qiu, J. Pickering Emulsion Catalysis: Interfacial Chemistry, Catalyst Design, Challenges, and Perspectives. Angew. Chem. Int. Ed. 2022, 61, e202115885. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Xue, C.; Wei, Z. Physicochemical characteristics, applications and research trends of edible Pickering emulsions. Trends Food Sci. Technol. 2021, 107, 1–15. [Google Scholar] [CrossRef]
- Wu, J.; Ma, G.H. Recent studies of Pickering emulsions: Particles make the difference. Small 2016, 12, 4633–4648. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.M.; Doost, A.S.; Nasrabadi, M.N.; Boostani, S.; Van der Meeren, P. Phytoparticles for the stabilization of Pickering emulsions in the formulation of novel food colloidal dispersions. Trends Food Sci. Technol. 2020, 98, 117–128. [Google Scholar] [CrossRef]
- Mu, R.; Hong, X.; Ni, Y.; Li, Y.; Pang, J.; Wang, Q.; Xiao, J.; Zheng, Y. Recent trends and applications of cellulose nanocrystals in food industry. Trends Food Sci. Technol. 2019, 93, 136–144. [Google Scholar] [CrossRef]
- Salas, C.; Nypelö, T.; Rodriguez-Abreu, C.; Carrillo, C.; Rojas, O.J. Nanocellulose properties and applications in colloids and interfaces. Curr. Opin. Colloid Interface Sci. 2014, 19, 383–396. [Google Scholar] [CrossRef]
- Sarkar, A.; Dickinson, E. Sustainable food-grade Pickering emulsions stabilized by plant-based particles. Curr. Opin. Colloid Interface Sci. 2020, 49, 69–81. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, Y.; Phillips, G.O.; Yang, G. Utilization of bacterial cellulose in food. Food Hydrocoll. 2014, 35, 539–545. [Google Scholar] [CrossRef]
- da Silva, C.J.G.; de Medeiros, A.D.; de Amorim, J.D.P.; do Nascimento, H.A.; Converti, A.; Costa, A.F.S.; Sarubbo, L.A. Bacterial cellulose biotextiles for the future of sustainable fashion: A review. Environ. Chem. Lett. 2021, 19, 2967–2980. [Google Scholar] [CrossRef]
- Lin, S.P.; Loira Calvar, I.; Catchmark, J.M.; Liu, J.R.; Demirci, A.; Cheng, K.C. Biosynthesis, production and applications of bacterial cellulose. Cellulose 2013, 20, 2191–2219. [Google Scholar] [CrossRef]
- Medronho, B.; Romano, A.; Miguel, M.G.; Stigsson, L.; Lindman, B. Rationalizing cellulose (in) solubility: Reviewing basic physicochemical aspects and role of hydrophobic interactions. Cellulose 2012, 19, 581–587. [Google Scholar] [CrossRef]
- Yan, H.; Chen, X.; Song, H.; Li, J.; Feng, Y.; Shi, Z.; Wang, X.; Lin, Q. Synthesis of bacterial cellulose and bacterial cellulose nanocrystals for their applications in the stabilization of olive oil pickering emulsion. Food Hydrocoll. 2017, 72, 127–135. [Google Scholar] [CrossRef]
- Zhai, X.; Lin, D.; Liu, D.; Yang, X. Emulsions stabilized by nanofibers from bacterial cellulose: New potential food-grade Pickering emulsions. Food Res. Int. 2018, 103, 12–20. [Google Scholar] [CrossRef] [Green Version]
- Kalashnikova, I.; Bizot, H.; Cathala, B.; Capron, I. New Pickering emulsions stabilized by bacterial cellulose nanocrystals. Langmuir 2011, 27, 7471–7479. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Wang, Y.; Luo, X.; Li, Y.; Li, B.; Wang, J.; Liu, S. Surface modification of cellulose nanofibrils with protein nanoparticles for enhancing the stabilization of O/W pickering emulsions. Food Hydrocoll. 2019, 97, 105180. [Google Scholar] [CrossRef]
- Rol, F.; Belgacem, M.N.; Gandini, A.; Bras, J. Recent advances in surface-modified cellulose nanofibrils. Prog. Polym. Sci. 2019, 88, 241–264. [Google Scholar] [CrossRef]
- Ortiz, D.G.; Pochat-Bohatier, C.; Cambedouzou, J.; Bechelany, M.; Miele, P. Current trends in Pickering emulsions: Particle morphology and applications. Engineering 2020, 6, 468–482. [Google Scholar] [CrossRef]
- Berton-Carabin, C.C.; Schroën, K. Pickering emulsions for food applications: Background, trends, and challenges. Annu. Rev. Food Sci. Technol. 2015, 6, 263–297. [Google Scholar] [CrossRef] [PubMed]
- Low, L.E.; Siva, S.P.; Ho, Y.K.; Chan, E.S.; Tey, B.T. Recent advances of characterization techniques for the formation, physical properties and stability of Pickering emulsion. Adv. Colloid. Interface Sci. 2020, 277, 102117. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Zhao, S.; Guan, X.; McClements, D.J.; Liu, X.; Liu, F.; Ngai, T. Polysaccharide-based Pickering emulsions: Formation, stabilization and applications. Food Hydrocoll. 2021, 119, 106812. [Google Scholar] [CrossRef]
- Hossain, H.; Wu, J.; Zhang, H.; Chen, Y.; Ma, L.; Huang, H.; Huang, Y.; Zhang, Y. Recent advances on cellulose nanocrystals for Pickering emulsions: Development and challenge. Trends Food Sci. Technol. 2020, 102, 16–29. [Google Scholar] [CrossRef]
- Kalashnikova, I.; Bizot, H.; Cathala, B.; Capron, I. Modulation of cellulose nanocrystals amphiphilic properties to stabilize oil/water interface. Biomacromolecules 2012, 13, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Kalashnikova, I.; Bizot, H.; Bertoncini, P.; Cathala, B.; Capron, I. Cellulosic nanorods of various aspect ratios for oil in water Pickering emulsions. Soft Matter 2013, 9, 952–959. [Google Scholar] [CrossRef]
- Paximada, P.; Tsouko, E.; Kopsahelis, N.; Koutinas, A.A.; Mandala, I. Bacterial cellulose as stabilizer of o/w emulsions. Food Hydrocoll. 2016, 53, 225–232. [Google Scholar] [CrossRef]
- Bai, L.; Huan, S.; Xiang, W.; Rojas, O.J. Pickering emulsions by combining cellulose nanofibrils and nanocrystals: Phase behavior and depletion stabilization. Green Chem. 2018, 20, 1571–1582. [Google Scholar] [CrossRef]
- Yan, X.; Ma, C.; Cui, F.; McClements, D.J.; Liu, X.; Liu, F. Protein-stabilized Pickering emulsions: Formation, stability, properties, and applications in foods. Trends Food Sci. Technol. 2020, 103, 293–303. [Google Scholar] [CrossRef]
- Liu, W.; Du, H.; Zhang, M.; Liu, K.; Liu, H.; Xie, H.; Zhang, X.; Si, C. Bacterial cellulose-based composite scaffolds for biomedical applications: A review. ACS Sustain. Chem. Eng. 2020, 8, 7536–7562. [Google Scholar] [CrossRef]
- Reiniati, I.; Hrymak, A.N.; Margaritis, A. Recent developments in the production and applications of bacterial cellulose fibers and nanocrystals. Crit. Rev. Biotechnol. 2017, 37, 510–524. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sanz, M.; Lopez-Rubio, A.; Lagaron, J.M. Optimization of the nanofabrication by acid hydrolysis of bacterial cellulose nanowhiskers. Carbohydr. Polym. 2011, 85, 228–236. [Google Scholar] [CrossRef]
- Winter, H.T.; Cerclier, C.; Delorme, N.; Bizot, H.; Quemener, B.; Cathala, B. Improved colloidal stability of bacterial cellulose nanocrystal suspensions for the elaboration of spin-coated cellulose-based model surfaces. Biomacromolecules 2010, 11, 3144–3151. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, Y.; Wu, Y.; He, K.; Li, Y.; Luo, X.; Li, B.; Wang, C.; Liu, S. Flexible cellulose nanofibrils as novel pickering stabilizers: The emulsifying property and packing behavior. Food Hydrocoll. 2019, 88, 180–189. [Google Scholar] [CrossRef]
- Jia, Y.; Zhai, X.; Fu, W.; Liu, Y.; Li, F.; Zhong, C. Surfactant-free emulsions stabilized by tempo-oxidized bacterial cellulose. Carbohydr. Polym. 2016, 151, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Apelgren, P.; Karabulut, E.; Amoroso, M.; Mantas, A.; Martínez Ávila, H.; Kölby, L.; Kondo, T.; Toriz, G.; Gatenholm, P. In vivo human cartilage formation in three-dimensional bioprinted constructs with a novel bacterial nanocellulose bioink. ACS Biomater. Sci. Eng. 2019, 5, 2482–2490. [Google Scholar] [CrossRef]
- Andrade, F.K.; Morais, J.P.S.; Muniz, C.R.; Nascimento, J.H.O.; Vieira, R.S.; Gama, F.M.P.; Rosa, M.F. Stable microfluidized bacterial cellulose suspension. Cellulose 2019, 26, 5851–5864. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Wu, Y.; Shabbir, M.; Pei, Y.; Liang, H.; Li, J.; Chen, Y.; Li, Y.; Li, B.; Luo, X.; et al. Coalescence behavior of eco-friendly Pickering-MIPES and HIPEs stabilized by using bacterial cellulose nanofibrils. Food Chem. 2021, 349, 129163. [Google Scholar] [CrossRef]
- Jia, Y.; Zheng, M.; Xu, Q.; Zhong, C. Rheological behaviors of Pickering emulsions stabilized by TEMPO-oxidized bacterial cellulose. Carbohydr. Polym. 2019, 215, 263–271. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.; Qiu, D.; Pei, Y.; Li, Y.; Li, B.; Liu, S. Effect of surface charge density of bacterial cellulose nanofibrils on the rheology property of O/W Pickering emulsions. Food Hydrocoll. 2021, 120, 106944. [Google Scholar] [CrossRef]
- Liu, Z.; Lin, D.; Shen, R.; Yang, X. Bacterial cellulose nanofibers improved the emulsifying capacity of soy protein isolate as a stabilizer for pickering high internal-phase emulsions. Food Hydrocoll. 2021, 112, 106279. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Y.; Li, Y.; Li, B.; Pei, Y.; Liu, S. Effects of the interaction between bacterial cellulose and soy protein isolate on the oil-water interface on the digestion of the Pickering emulsions. Food Hydrocoll. 2022, 126, 107480. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, J.; Chen, J.; Li, B.; Li, Y.; Liu, S. Edible foam based on pickering effect of bacterial cellulose nanofibrils and soy protein isolates featuring interfacial network stabilization. Food Hydrocoll. 2020, 100, 105440. [Google Scholar] [CrossRef]
- Wu, Y.; Lei, C.; Li, J.; Chen, Y.; Liang, H.; Li, Y.; Li, B.; Luo, X.; Pei, Y.; Liu, S. Improvement of O/W emulsion performance by adjusting the interaction between gelatin and bacterial cellulose nanofibrils. Carbohydr. Polym. 2022, 276, 118806. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Y.; Luo, X.; Lu, A.; Li, Y.; Li, B.; Liu, S. O/W Pickering emulsion templated organo-hydrogels with enhanced mechanical strength and energy storage capacity. ACS Appl. Bio Mater. 2018, 2, 480–487. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, S.; Tang, H.; Wan, S.; Qin, W.; Zeng, Q.; Huang, J.; Yu, G.; Feng, Y.; Li, J. Depletion stabilization of emulsions based on bacterial cellulose/carboxymethyl chitosan complexes. Carbohydr. Polym. 2022, 297, 119904. [Google Scholar] [CrossRef]
- Martins, D.; Estevinho, B.; Rocha, F.; Dourado, F.; Gama, M. A dry and fully dispersible bacterial cellulose formulation as a stabilizer for oil-in-water emulsions. Carbohydr. Polym. 2020, 230, 115657. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.S.; Lee, S.H.; Seo, H.M.; Shin, K.; Kang, M.H.; Lee, M.; Park, J.; Kim, J.W. Structuring Pickering emulsion interfaces with bilayered coacervates of cellulose nanofibers and hectorite nanoplatelets. Langmuir 2021, 37, 3828–3835. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Y.; Liu, Z.; Liu, S.; Huang, F.; Zheng, M. Novel bacterial cellulose-TiO2 stabilized Pickering emulsion for photocatalytic degradation. Cellulose 2022, 29, 5223–5234. [Google Scholar] [CrossRef]
- Seo, H.M.; Seo, M.; Shin, K.; Choi, S.; Kim, J.W. Bacterial cellulose nanofibrils-armored Pickering emulsions with limited influx of metal ions. Carbohydr. Polym. 2021, 258, 117730. [Google Scholar] [CrossRef]
- Li, Q.; Chen, P.; Li, Y.; Li, B.; Liu, S. Construction of cellulose-based Pickering stabilizer as a novel interfacial antioxidant: A bioinspired oxygen protection strategy. Carbohydr. Polym. 2020, 229, 115395. [Google Scholar] [CrossRef] [PubMed]
- Hondo, H.; Saito, T.; Isogai, A. Preparation of oxidized celluloses in a TEMPO/NaBr system using different chlorine reagents in water. Cellulose 2019, 26, 3021–3030. [Google Scholar] [CrossRef]
- Kedzior, S.A.; Gabriel, V.A.; Dubé, M.A.; Cranston, E.D. Nanocellulose in emulsions and heterogeneous water-based polymer systems: A review. Adv. Mater. 2021, 33, 2002404. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Luo, Y.; Huang, Y.; Ma, L.; Chen, H.; Fu, Y.; Yu, Y.; Zhu, H.; Wang, H.; Zhang, Y. Recent advances in protein-based emulsions: The key role of cellulose. Food Hydrocoll. 2022, 136, 108260. [Google Scholar] [CrossRef]
- Hossain, K.M.Z.; Deeming, L.; Edler, K.J. Recent progress in Pickering emulsions stabilised by bioderived particles. RSC Adv. 2021, 11, 39027–39044. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, Y.; Luo, X.; Wang, Y.; Li, Y.; Li, B.; Liu, S. Impact of pH on the interaction between soybean protein isolate and oxidized bacterial cellulose at oil-water interface: Dilatational rheological and emulsifying properties. Food Hydrocoll. 2021, 115, 106609. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Li, J.; Liang, H.; Chen, Y.; Li, B.; Luo, X.; Pei, Y.; Liu, S. Edible oil powders based on spray-dried Pickering emulsion stabilized by soy protein/cellulose nanofibrils. LWT 2022, 154, 112605. [Google Scholar] [CrossRef]
- Li, Q.; Ma, Q.; Wu, Y.; Li, Y.; Li, B.; Luo, X.; Liu, S. Oleogel films through the Pickering effect of bacterial cellulose nanofibrils featuring interfacial network stabilization. J. Agric. Food Chem. 2020, 68, 9150–9157. [Google Scholar] [CrossRef]
- Xie, Y.; Lei, Y.; Rong, J.; Zhang, X.; Li, J.; Chen, Y.; Liang, H.; Li, Y.; Li, B.; Fang, Z.; et al. Physico-chemical properties of reduced-fat biscuits prepared using O/W cellulose-based Pickering emulsion. LWT 2021, 148, 111745. [Google Scholar] [CrossRef]
- Bao, C.; Jiang, P.; Chai, J.; Jiang, Y.; Li, D.; Bao, W.; Liu, B.; Norde, W.; Li, Y. The delivery of sensitive food bioactive ingredients: Absorption mechanisms, influencing factors, encapsulation techniques and evaluation models. Food Res. Int. 2019, 120, 130–140. [Google Scholar] [CrossRef]
- Razavi, M.S.; Golmohammadi, A.; Nematollahzadeh, A.; Fiori, F.; Rovera, C.; Farris, S. Preparation of cinnamon essential oil emulsion by bacterial cellulose nanocrystals and fish gelatin. Food Hydrocoll. 2020, 109, 106111. [Google Scholar] [CrossRef]
- McClements, D.J.; Li, Y. Structured emulsion-based delivery systems: Controlling the digestion and release of lipophilic food components. Adv. Colloid Interface Sci. 2010, 159, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Lin, D.; Liu, Z.; Zhai, H.; Yang, X. Fabrication of bacterial cellulose nanofibers/soy protein isolate colloidal particles for the stabilization of high internal phase pickering emulsions by anti-solvent precipitation and their application in the delivery of curcumin. Front. Nutr. 2021, 8, 734620. [Google Scholar] [CrossRef]
- Madadlou, A.; Rakhshi, E.; Abbaspourrad, A. Engineered emulsions for obesity treatment. Trends Food Sci. Technol. 2016, 52, 90–97. [Google Scholar] [CrossRef]
- Golding, M.; Wooster, T.J. The influence of emulsion structure and stability on lipid digestion. Curr. Opin. Colloid Interface Sci. 2010, 15, 90–101. [Google Scholar] [CrossRef]
- Acevedo-Fani, A.; Singh, H. Biophysical insights into modulating lipid digestion in food emulsions. Prog. Lipid Res. 2022, 85, 101129. [Google Scholar] [CrossRef]
- Sarkar, A.; Zhang, S.; Holmes, M.; Ettelaie, R. Colloidal aspects of digestion of Pickering emulsions: Experiments and theoretical models of lipid digestion kinetics. Adv. Colloid. Interface Sci. 2019, 263, 195–211. [Google Scholar] [CrossRef] [Green Version]
- Waraho, T.; McClements, D.J.; Decker, E.A. Mechanisms of lipid oxidation in food dispersions. Trends Food Sci. Technol. 2011, 22, 3–13. [Google Scholar] [CrossRef]
- Villeneuve, P.; Bourlieu-Lacanal, C.; Durand, E.; Lecomte, J.; McClements, D.J.; Decker, E.A. Lipid oxidation in emulsions and bulk oils: A review of the importance of micelles. Crit. Rev. Food Sci. Nutr. 2021, volume 61, 1–41. [Google Scholar] [CrossRef]
- Laguerre, M.; Bily, A.; Roller, M.; Birtić, S. Mass transport phenomena in lipid oxidation and antioxidation. Annu. Rev. Food Sci. Technol. 2017, 8, 391–411. [Google Scholar] [CrossRef]
- McClements, D.J.; Decker, E. Interfacial antioxidants: A review of natural and synthetic emulsifiers and coemulsifiers that can inhibit lipid oxidation. J. Agric. Food Chem. 2018, 66, 20–35. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E. Biopolymer-based particles as stabilizing agents for emulsions and foams. Food Hydrocoll. 2017, 68, 219–231. [Google Scholar] [CrossRef]
- Van der Sman, R.G.M. Filler functionality in edible solid foams. Adv. Colloid Interface Sci. 2016, 231, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Falco, C.Y.; Geng, X.; Cárdenas, M.; Risbo, J. Edible foam based on Pickering effect of probiotic bacteria and milk proteins. Food Hydrocoll. 2017, 70, 211–218. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Food applications of emulsion-based edible films and coatings. Trends Food Sci. Technol. 2015, 45, 273–283. [Google Scholar] [CrossRef]
- Díaz-Montes, E.; Castro-Muñoz, R. Edible films and coatings as food-quality preservers: An overview. Foods 2021, 10, 249. [Google Scholar] [CrossRef]
- Xie, B.; Zhang, X.; Luo, X.; Wang, Y.; Li, Y.; Li, B.; Liu, S. Edible coating based on beeswax-in-water Pickering emulsion stabilized by cellulose nanofibrils and carboxymethyl chitosan. Food Chem. 2020, 331, 127108. [Google Scholar] [CrossRef]
- Bai, L.; Huan, S.; Zhu, Y.; Chu, G.; McClements, D.J.; Rojas, O.J. Recent advances in food emulsions and engineering foodstuffs using plant-based nanocelluloses. Annu. Rev. Food Sci. Technol. 2021, 12, 383–406. [Google Scholar] [CrossRef]
- Oliveira, A.A.N.; Mesquita, E.D.F.M.D.; Furtado, A.A.L. Use of bacterial cellulose as a fat replacer in emulsified meat products. Food Sci. Technol. 2021, 42, e42621. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, X.; Hao, W.; Xie, Y.; Chen, L.; Li, Z.; Zhu, B.; Feng, X. Nano-bacterial cellulose/soy protein isolate complex gel as fat substitutes in ice cream model. Carbohydr. Polym. 2018, 198, 620–630. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Anankanbil, S.; Guo, Z. Applications of nanocellulosic products in food: Manufacturing processes, structural features and multifaceted functionalities. Trends Food Sci. Technol. 2021, 113, 277–300. [Google Scholar] [CrossRef]
- Zhang, X.; Luo, X.; Wang, Y.; Li, Y.; Li, B.; Liu, S. Concentrated O/W Pickering emulsions stabilized by soy protein/cellulose nanofibrils: Influence of pH on the emulsification performance. Food Hydrocoll. 2020, 108, 106025. [Google Scholar] [CrossRef]

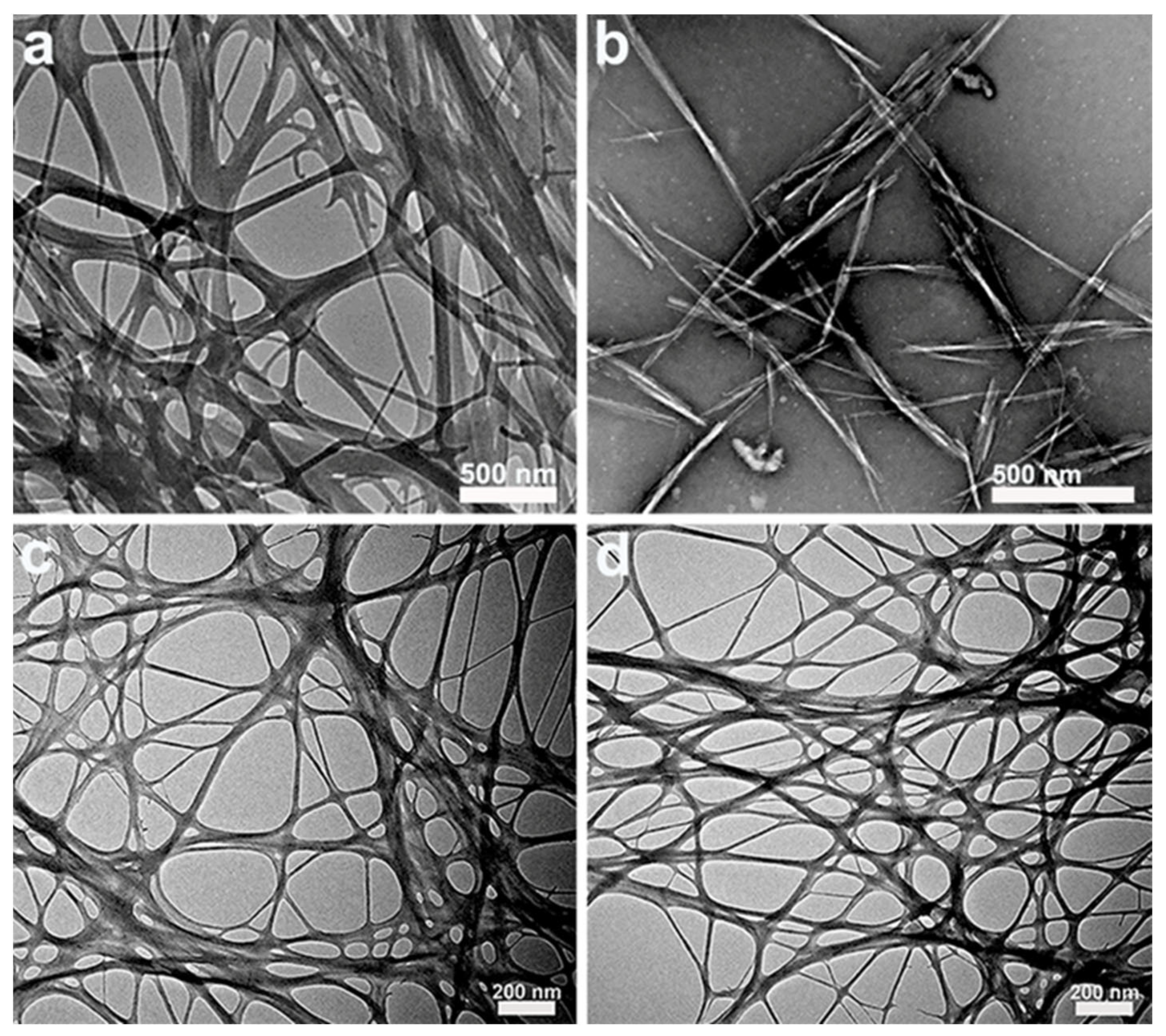
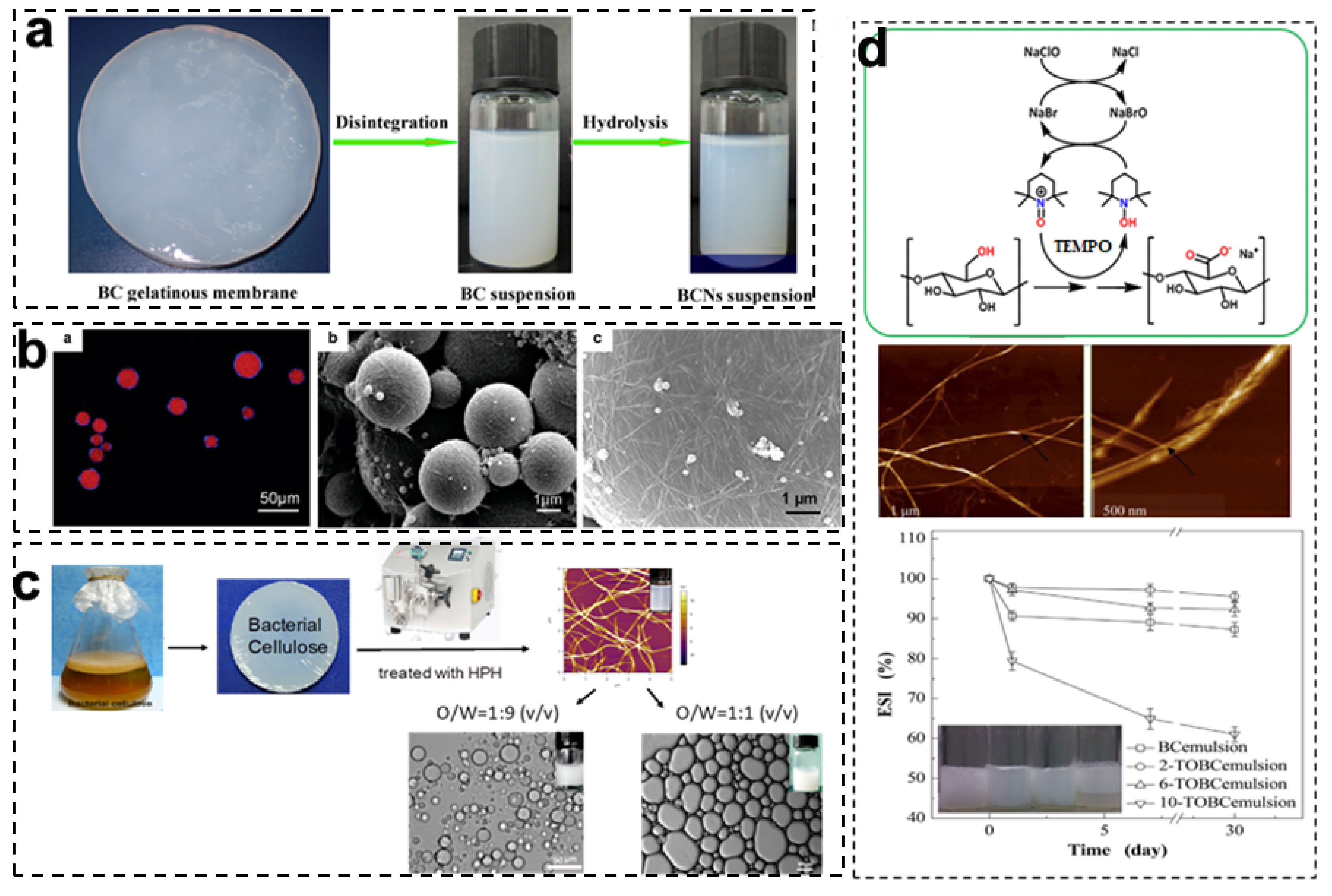
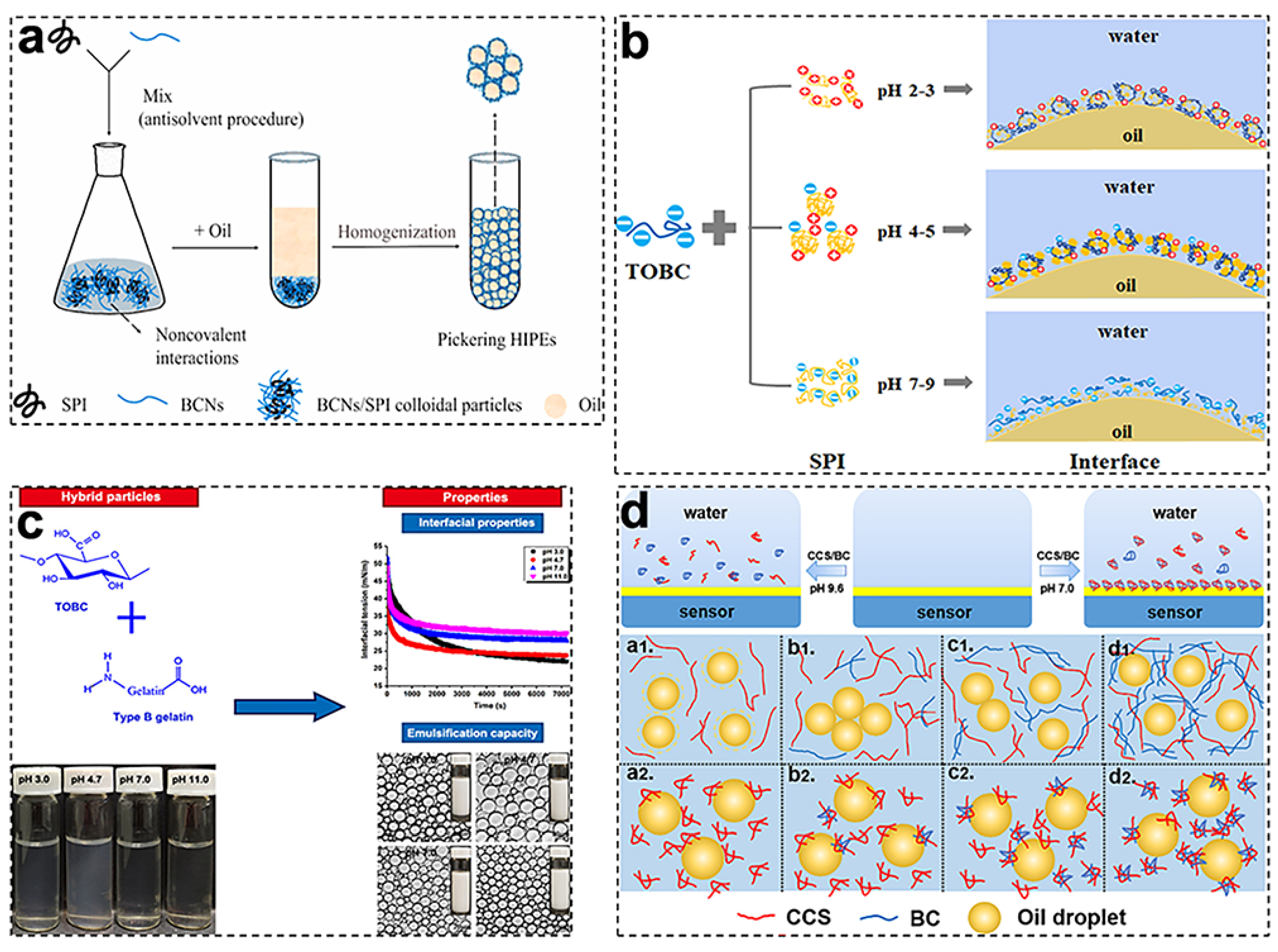
| Particle Type | Preparation Method | Size (d = Diameter, h = Height, w = Width, I = Length) | Particle Content | Emulsification Method | Emulsion Type | Oil-Phase Type and Content | References | |
|---|---|---|---|---|---|---|---|---|
| BCNFs | High-pressure homogenization | 6 < h < 13 nm 97 < w < 127 nm I < 10 μm | 0.1–0.5 wt% | High-speed shear, 10,000 rpm, 2 min | O/W | Dodecane (10–50 v%) | [34] | |
| High-pressure homogenization | 3.8 < h < 8.9 nm 66.8 < w < 128.6 nm | 0.1–0.5 wt% | High-speed shear, 12,000 rpm, 2 min | O/W | Dodecane (50–75 v%) | [38] | ||
| Hydrochloric acid hydrolysis | 30 < d < 80 nm | 0.05% (w/v) | High-speed shear, 15,000 rpm, 1 min, and high-pressure homogenization, 600 bar, 1 min | O/W | Peanut oil (5–30 v%) | [16] | ||
| TEMPO oxidation | 5 < w < 50 nm I < 5 μm | 0.18–0.70 wt% | Ultrasound emulsification, 40 W, 5 min, 50% ultrasonic pulse | O/W | Liquid paraffin (50 v%) | [35,39] | ||
| High-pressure homogenization and TEMPO oxidation | 5.7 < h < 10.8 nm 70.3 < w < 101.6 nm | 0.3 wt% | Ultrasound emulsification, 20 kHz, 95% amplitude, 2 min | O/W | Dodecane (10 v%) | [40] | ||
| BCNF-based complexes | ||||||||
| BCNF-protein | BCNF-soy protein isolate (SPI) | Antisolvent precipitation approach | 927.0 < d < 1510.3 nm | 2.0 wt% | High-speed shear, 20,000 rpm, 3 min | O/W | Sunflower seed oil (75 v%) | [41] |
| BCNF-SPI | Electrostatic interaction | 10.3 < h < 19.1 nm 130.9 < w < 160.7 nm I < 10 μm | 1.0 wt% | High-speed shear, 16,000 rpm, 2 min | O/W | Medium-chain triglyceride (MCT) oil | [42] | |
| TEMPO-oxidized BCNF (TOBC)-SPI | Electrostatic interaction | 8.7 < h < 14.9 nm 102.4 < w < 121.6 nm I < 10 μm | 1.08-3.24 wt% | High-speed shear, 16,000 rpm, 2 min | O/W | Dodecane (50–74.05 v%) | [18,43] | |
| BCNF-gelatin (GLT) | Electrostatic interaction | 1.0 wt% | High-speed shear, 10,000 rpm, 2 min | O/W | Dodecane (75 v%) | [44] | ||
| BCNF-polysaccharide | BCNF-sodium alginate (SA) | High-pressure homogenization and mixing | 6.8 < h < 7.6 nm 55.0 < w < 60.2 nm | 0.1–0.5 wt% | Ultrasound emulsification, 20 kHz, 3 min | O/W | Dodecane (10–30 v%) | [45] |
| BCNF-carboxymethyl chitosan (CCS) | Electrostatic interaction and depletion effect | 0.01-0.20 wt% | High-speed shear, 22,000 rpm, 4 min | O/W | Soybean oil (50 v%) | [46] | ||
| BCNF-carboxymethyl cellulose (CMC) | Mixing and spray drying | 581.3 < d < 620.7 nm | 0.1–0.5 wt% | High-speed shear, 20,000 rpm, 4 min | O/W | Isohexadecane (10 v%) | [47] | |
| BCNF-attractive hectorite nanoplatelets (AHNPs) | High-pressure homogenization and TEMPO oxidation | I ≈ 3 μm | 0.65 wt% | High-speed shear, 15,000 rpm for 10 min | O/W & W/O | silicone oil (10–90 v%) | [48] | |
| BCNF-titanium oxide (TiO2) | High-pressure homogenization and ultrasonic treatment | 0.2–0.6 wt% | High-speed shear, 20,000 rpm, 2 min | O/W | Dodecane (10–80 v%) | [49] | ||
| BCNF-octadecylamine (C18) | TEMPO oxidation and grafting | 5 < w < 10 nm | 0.05–0.1 wt% | High-speed shear, 15,000 rpm, 10 min | O/W | N-decane; olive oil; fomblin oil; silicone oil (10–40 v%) | [50] | |
| BCNF-tea polyphenols (TPs) | High-pressure homogenization and physical adsorption | 0.1–0.5 wt% | High-speed shear, 10,000 rpm, 2 min | O/W | Dodecane; camellia seed oil (10 v%) | [51] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Wang, D.; Liu, S.; Tang, J. Bacterial Cellulose Nanofibril-Based Pickering Emulsions: Recent Trends and Applications in the Food Industry. Foods 2022, 11, 4064. https://doi.org/10.3390/foods11244064
Zhang X, Wang D, Liu S, Tang J. Bacterial Cellulose Nanofibril-Based Pickering Emulsions: Recent Trends and Applications in the Food Industry. Foods. 2022; 11(24):4064. https://doi.org/10.3390/foods11244064
Chicago/Turabian StyleZhang, Xingzhong, Dan Wang, Shilin Liu, and Jie Tang. 2022. "Bacterial Cellulose Nanofibril-Based Pickering Emulsions: Recent Trends and Applications in the Food Industry" Foods 11, no. 24: 4064. https://doi.org/10.3390/foods11244064




