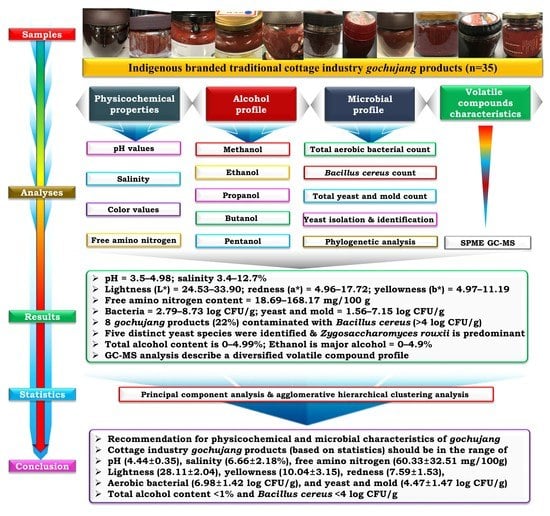
Physicochemical, Microbial, and Volatile Compound Characteristics of Gochujang, Fermented Red Pepper Paste, Produced by Traditional Cottage Industries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
Instruments and Apparatus
2.2. Sample Collection
2.3. Physicochemical Characteristics
2.3.1. Determination of pH, Salinity, Color Values, and Free Amino Nitrogen
2.3.2. Determination of Total Alcohol Content
2.4. Determination of Volatile Compounds
2.5. Microbial Profile
2.6. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Analysis of Gochujang Products
3.1.1. pH
3.1.2. Salinity
3.1.3. Free Amino Nitrogen Content
3.1.4. Color Values
3.1.5. Alcohol Content
3.2. Volatile Compounds
3.3. Microbial Profile Analysis
3.3.1. Aerobic Mesophilic Bacteria and Yeast/Mold
3.3.2. Detection of B. cereus in Gochujang Products
3.4. Principal Component Analysis and Hierarchical Clustering of Gochujang Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramalingam, S.; Bahuguna, A.; Lim, S.; Joe, A.R.; Lee, J.S.; Kim, S.Y.; Kim, M. Quantification of biogenic amines in 35 Korean cottage industry traditional gochujang (fermented red pepper paste) products. Foods 2021, 10, 2370. [Google Scholar] [CrossRef]
- Information Statistics System, 2018, Food Market Newsletter Gochujang-2018. Available online: https://www.atfis.or.kr/article/M001010000/view.do?articleId=3034 (accessed on 19 February 2021).
- CODEX Alimentarius. Regional Standard for Gochujang (Asia): CODEX STAN 294R-2009. 2009. Available online: http://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B294R-2009%252FCXS_294Re.pdf (accessed on 31 December 2018).
- Kim, H.E.; Han, S.Y.; Kim, Y.S. Quality characteristics of gochujang meju prepared with different fermentation tools and inoculation time of Aspergillus oryzae. Food Sci. Biotechnol. 2010, 19, 1579–1585. [Google Scholar] [CrossRef]
- Shin, D.; Jeong, D. Korean traditional fermented soybean products: Jang. J. Ethn. Foods 2015, 2, 2–7. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.H.; Choi, H.J. Physicochemical properties of kochujang prepared by Bacillus sp. koji. Korean J. Food Sci. Technol. 2003, 35, 1174–1181. [Google Scholar]
- Jang, S.J.; Kim, Y.J.; Park, J.M.; Park, Y.S. Analysis of microflora in gochujang, Korean traditional fermented food. Food Sci. Biotechnol. 2011, 20, 1435–1440. [Google Scholar] [CrossRef]
- Ryu, J.A.; Kim, E.; Kim, M.-J.; Lee, S.; Yoon, S.R.; Ryu, J.G.; Kim, H.Y. Physicochemical Characteristics and Microbial Communities in Gochujang, a Traditional Korean Fermented Hot Pepper Paste. Front. Microbiol. 2021, 11, 620478. [Google Scholar] [CrossRef]
- Burges, P. Modification of a traditional Korean food product (gochujang) to enhance its consumer acceptability as an ethnic food. J. Ethn. Foods 2014, 1, 13–18. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.B.; Kim, C.W.; Cho, S.H.; No, W.S.; Kim, W.J. Proposal of statistical sampling plans for in Korean fermented soybean pastes. Food Sci. Biotechnol. 2015, 24, 765–770. [Google Scholar] [CrossRef]
- Park, Y.K.; Lee, J.H.; Mah, J.H. Occurrence and reduction of biogenic amines in kimchi and Korean fermented seafood products. Foods 2019, 8, 547. [Google Scholar] [CrossRef] [Green Version]
- Ramalingam, S.; Dhatchanamoorthi, I.; Arumugam, A.; Bahuguna, A.; Krishnamoorthy, M.; Lee, J.S.; Devarajan, N.; Kim, M. Functional, nutritional, antinutritional, and microbial assessment of novel fermented sugar syrup fortified with pre-mature fruits of Totapuri mango and star gooseberry. LWT-Food Sci. Technol. 2021, 136, 110276. [Google Scholar] [CrossRef]
- Korea Food and Drug Administration (KFDA). 2019. Available online: http://www.foodsafetykorea.go.kr/foodcode/01_03.jsp?idx=308 (accessed on 27 August 2020).
- Lee, J.S.; Ramalingam, S.; Jo, I.G.; Kwon, Y.S.; Bahuguna, A.; Oh, Y.S.; Kwon, O.-J.; Kim, M. Comparative study of the physicochemical, nutritional, and antioxidant properties of some commercial refined and non-centrifugal sugars. Food Res. Inter. 2018, 109, 614–625. [Google Scholar] [CrossRef] [PubMed]
- Korea Food and Drug Administration (KFDA). 2014. Available online: http://www.foodsafetykorea.go.kr/foodcode/01_03.jsp?idx=11009 (accessed on 26 August 2020).
- Cho, K.M.; Kang, J.R.; Kim, G.M.; Kang, M.J.; Hwang, C.E.; Jeong, Y.S.; Kim, J.H.; Shin, J.H. Quality characteristics of low salted garlic Doenjang during fermentation. Korean J. Food Preserv. 2014, 21, 627–635. [Google Scholar] [CrossRef]
- Lee, J.S.; Choi, Y.J.; Kwon, S.J.; Yoo, J.Y.; Chung, D.H. Screening and characterization of osmotolerant and gas-producing yeasts from traditional Doenjang and Kochujang. Food Sci. Biotechnol. 1996, 5, 54–58. [Google Scholar]
- Gil, N.Y.; Song, J.; Eom, J.S.; Park, S.Y.; Choi, H.S. Changes of physicochemical properties of Cheonggukjang prepared with various soybean cultivars and Bacillus subtilis HJ18-9. Korean J. Food Preserv. 2016, 23, 811–818. [Google Scholar] [CrossRef] [Green Version]
- National Institute of Standards and Technology (NIST). Standard Reference Database. 2014. Available online: https://www.nist.gov/system/files/documents/srd/NIST1aVer22Man.pdf (accessed on 27 August 2020).
- Association of Official Analytical Chemists (AOAC). Official methods of analysis. In Official Methods of Analysis, 16th ed.; AOAC: Washington, DC, USA, 1999; Available online: https://www.aoac.org/AOAC_Prod_Imis/AOAC_Member/Default.aspx?WebsiteKey=2e25ab5a-1f6d-4d78-a498-19b9763d11b4&hkey=8fc2171a-6051-4e64-a928-5c47dfa25797 (accessed on 19 February 2020).
- Bird, P.; Flannery, J.; Crowley, E.; Agin, J.; Goins, D.; Jechorek, R. Evaluation of the 3M™ Petrifilm™ rapid yeast and mold count plate for the enumeration of yeast and mold in food: Collaborative study, first action 2014.05. J. AOAC Inter. 2015, 98, 767–783. [Google Scholar] [CrossRef]
- United States Food and Drug Administration (USFDA). Code of Federal Regulations, revised on 2021. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=114&showFR=1 (accessed on 2 November 2020).
- Lee, S.; Yoo, S.M.; Park, B.R.; Han, H.M.; Kim, H.Y. Analysis of quality state for gochujang produced by regional rural families. J. Korean Soc. Food Sci. Nutr. 2014, 43, 1088–1094. [Google Scholar] [CrossRef]
- Ryu, M.H.; Zhang, J.; Toth, T.; Khokhani, D.; Geddes, B.A.; Mus, F.; Amaya, G.C.; Peters, J.W.; Poole, J.M.; Ane, C.A. Control of nitrogen fixation in bacteria that associate with cereals. Nat. Microbiol. 2020, 5, 314–330. [Google Scholar] [CrossRef]
- Oh, H.I.; Shon, S.H.; Kim, J.M. Changes in microflora and enzyme activities of kochujang prepared with Aspergillus oryzae, Bacillus licheniformis and Saccharomyces rouxii during fermentation. Korean J. Food Sci. Technol. 2000, 32, 410–416. [Google Scholar]
- Kim, G.T.; Hwang, Y.I.; Lim, S.I.; Lee, D.S. Carbon dioxide production and quality changes in korean fermented soybean paste and hot pepper-soybean paste. J. Korean Soc. Food Sci. Nutr. 2000, 29, 807–813. [Google Scholar]
- Baek, S.Y.; Gil1, N.Y.; Han, M.H.; Kang, H.Y.; Lee, H.Y.; Yoon, Y.S.; Lee, J.; Song, Y.E.; Lee, S.K.; Ryu, J.A.; et al. Study on relationship between quality characteristics and exterior environment of the Korean traditional gochujang produced in 2018 by 8 regions of Korea. Korean J. Food Preserv. 2019, 26, 2287–7428. [Google Scholar] [CrossRef]
- Yang, H.J.; Lee, Y.S.; Choi, I.S. Comparison of physicochemical properties and antioxidant activities of fermented soybean-based red pepper paste, gochujang, prepared with five different red pepper (Capsicum annuum L.) varieties. J. Food Sci. Technol. 2018, 55, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.E.; Stewart, G.G. Free amino nitrogen in brewing. Fermentation 2019, 5, 22. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Li, C.; Zhao, M.; Feng, Y.; Sun, L.; Wang, Y. Assessment on the improvement of soy sauce fermentation by Aspergillus oryzae HG76. Biocatal. Agric. Biotechnol. 2013, 2, 344–351. [Google Scholar] [CrossRef]
- Jang, Y.K.; Shin, G.R.; Jung, E.S.; Lee, S.; Lee, S.; Singh, D.; Jang, E.S.; Shin, D.J.; Kim, H.J.; Shin, H.W.; et al. Process specific differential metabolomes for industrial gochujang types (pepper paste) manufactured using white rice, brown rice, and wheat. Food Chem. 2017, 234, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Devanthi, P.V.P.; Gkatzionis, K. Soy sauce fermentation: Microorganisms, aroma formation, and process modification. Food Res. Inter. 2019, 120, 364–374. [Google Scholar] [CrossRef]
- Alzeer, J.; Hadeed, K.A. Ethanol and its Halal status in food industries. Trends Food Sci. Technol. 2016, 58, 14–20. [Google Scholar] [CrossRef]
- Buratti, S.; Benedetti, S. Alcoholic fermentation using electronic nose and electronic tongue. In Electronic Noses and Tongues in Food Science; Academic Press: Cambridge, MA, USA, 2016; pp. 291–299. [Google Scholar]
- Park, E.S.; Heo, J.H.; Ju, J.; Park, K.Y. Changes in quality characteristics of gochujang prepared with different ingredients and meju starters. J. Korean Soc. Food Sci. Nutr. 2016, 45, 880–888. [Google Scholar] [CrossRef]
- Choi, J.Y.; Lee, T.S.; Noh, B.S. Characteristics of volatile flavor compounds in improved kochujang prepared with glutinous rice koji during fermentation. Korean J. Food Sci. Technol. 1999, 31, 1221–1226. [Google Scholar]
- Kang, K.M.; Baek, H.H. Aroma quality assessment of Korean fermented red pepper paste (gochujang) by aroma extract dilution analysis and headspace solid-phase micro extraction–gas chromatography–olfactometry. Food Chem. 2014, 145, 488–495. [Google Scholar] [CrossRef]
- Choi, J.Y.; Lee, T.S.; Noh, B.S. Characteristics of volatile flavor compounds in kochujangs with meju and soybean koji during fermentation. Korean J. Food Sci. Technol. 2000, 32, 1035–1042. [Google Scholar]
- Choi, J.Y.; Lee, T.S. Characteristics of volatile flavor compounds in kochujang prepared with commercial enzyme during fermentation. J. Korean Soc. Agric. Chem. Biotechnol. 2003, 46, 207–213. [Google Scholar]
- Park, H.K.; Kim, J.G. Character impact compounds of kochujang. Korea Soybean Digest. 2003, 20, 1–11. [Google Scholar]
- Oh, J.Y.; Kim, Y.S.; Kim, Y.H.; Shin, D.H. Volatile flavor components of kochujang prepared with different kojis. Food Sci. Biotechnol. 2001, 10, 45–53. [Google Scholar]
- Shon, S.; Kim, J.; Oh, H.; Ha, J. Volatile flavor components of kochujang prepared with Aspergillus oryzae, Bacillus licheniformis and Saccharomyces rouxii. Food Sci. Biotechnol. 2003, 12, 18–22. [Google Scholar]
- Byun, H.O.; Park, M.J.; Park, Y.S.; Bai, H.S. Analysis of microflora and volatile flavor components in traditional gochujang with different concentrations of salt during fermentation. Food Eng. Prog. 2014, 18, 282–292. [Google Scholar] [CrossRef]
- Choi, J.Y.; Lee, T.S.; Park, S.O. Characteristics of volatile flavor compounds in improved kochujang prepared with soybean koji during fermentation. Korean J. Food Sci. Technol. 1997, 29, 1144–1150. [Google Scholar]
- Choi, J.Y.; Lee, T.S.; Park, S.O.; Noh, B.S. Changes of volatile flavor compounds in traditional kochujang during fermentation. Korean J. Food Sci. Technol. 1997, 29, 745–751. [Google Scholar]
- Kim, Y.S.; Oh, H.I. Volatile flavor components of traditional and commercial kochujang. Korean J. Food Sci. Technol. 1993, 25, 494–501. [Google Scholar]
- Zhang, L.; Che, Z.; Xu, W.; Yue, P.; Li, R.; Li, Y.; Pei, X.; Zeng, P. Dynamics of physicochemical factors and microbial communities during ripening fermentation of Pixian Doubanjiang, a typical condiment in Chinese cuisine. Food Microbiol. 2020, 86, 103342. [Google Scholar] [CrossRef]
- Cho, S.H.; Park, H.S.; Jo, S.W.; Yim, E.J.; Yang, H.Y.; Ha, G.S.; Jeong, D.Y. Comparison of microbial community profiling on traditional fermented soybean products (Deonjang, Gochujang) produced in Jeonbuk, Jeonnam, and Jeju province area. Korean J. Microbiol. 2017, 53, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Nam, Y.D.; Park, S.I.; Lim, S.I. Microbial composition of the Korean traditional food “kochujang” analyzed by a massive sequencing technique. J. Food Sci. 2012, 77, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Schifferdecker, A.J.; Gamero, A.; Compagno, C.; Boekhout, T.; Piškur, J.; Knecht, W. Kazachstania gamospora and Wickerhamomyces subpelliculosus: Two alternative baker’s yeasts in the modern bakery. Inter. J. Food Microbiol. 2017, 250, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Oh, N.S.; Shin, D.B.; In, M.J.; Chang, Y.I.; Han, M. Effects of capsaicin on the growth and ethanol production of Zygosaccharomyces rouxii KFY80 isolated from gochujang (fermented hot pepper paste). Food Sci. Biotechnol. 2004, 13, 749–753. [Google Scholar]
- Hong, Y.J.; Son, S.H.; Kim, H.Y.; Hwang, I.G.; Yoo, S.S. Volatile components of traditional gochujang produced from small farms according to each cultivation region. J. East Asian Soc. Diet. Life 2013, 23, 451–460. [Google Scholar]
- Korea Food and Drug Administration (KFDA). Food Borne Pathogen Test Methods, Seoul, Korea. 2010. Available online: http://www.kfda.go.kr (accessed on 5 April 2020).
- Yim, J.H.; Kim, K.Y.; Chon, J.W.; Kim, D.H.; Kim, H.S.; Choi, D.S.; Choi, I.S.; Seo, K.H. Incidence, antibiotic susceptibility, and toxin profiles of Bacillus cereus sensulato isolated from Korean fermented soybean products. J. Food Sci. 2015, 80, M1266–M1270. [Google Scholar] [CrossRef]


| Product Code | pH # | Salinity (%) # | Color Values # | Free Amino Nitrogen (mg/100 g) # | ||
|---|---|---|---|---|---|---|
| Lightness (L*) | Redness (a*) | Yellowness (b*) | ||||
| Go-1 | 4.96 ± 0.01 b | 5.01 ± 0.30 pq | 29.70 ± 0.58 bcdefg | 13.36 ± 0.63 d | 8.74 ± 0.13 cdef | 28.03 ± 8.09 kl |
| Go-2 | 4.49 ± 0.01 n | 10.59 ± 0.32 c | 28.26 ± 0.19 bcdefgh | 12.24 ± 0.10 e | 8.92 ± 0.07 cd | 65.40 ± 8.09 defgh |
| Go-3 | 4.78 ± 0.01 d | 7.74 ± 0.00 fg | 26.98 ± 0.59 bcdefgh | 10.16 ± 0.16 hi | 7.09 ± 0.13 jk | 74.74 ± 14.01 cdefg |
| Go-4 | 4.65 ± 0.01 i | 8.16 ± 0.00 ef | 27.25 ± 0.29 bcdefgh | 10.12 ± 0.13 hi | 7.95 ± 0.08 gh | 46.71 ± 14.01 hijk |
| Go-5 | 4.74 ± 0.01 ef | 4.81 ± 0.27 qr | 28.22 ± 0.31 bcdefgh | 12.03 ± 0.34 ef | 8.93 ± 0.11 cd | 74.74 ± 14.01 cdefg |
| Go-6 | 4.74 ± 0.01 f | 5.22 ± 0.26 opq | 29.21 ± 0.87 bcdefg | 11.35 ± 0.69 g | 8.86 ± 0.30 cde | 51.38 ± 8.09 ghijk |
| Go-7 | 4.12 ± 0.02 u | 6.64 ± 0.33 jk | 26.91 ± 0.51 cdefgh | 9.33 ± 0.59 jk | 7.24 ± 0.44 jk | 65.40 ± 16.18 defgh |
| Go-8 | 4.62 ± 0.01 j | 4.72 ± 0.00 qr | 26.06 ± 1.16 efghi | 5.76 ± 0.39 n | 5.47 ± 0.30 opq | 130.80 ± 0.00 b |
| Go-9 | 4.20 ± 0.01 t | 7.03 ± 0.00 hij | 24.53 ± 0.34 hi | 6.88 ± 0.10 m | 5.89 ± 0.01 no | 51.38 ± 16.18 ghjik |
| Go-10 | 4.30 ± 0.03 p | 6.0 ± 0.00 klmn | 25.95 ± 0.68 fghi | 7.26 ± 0.07 m | 6.47 ± 0.06 lm | 93.43 ± 8.09 c |
| Go-11 | 3.99 ± 0.00 v | 4.98 ± 0.00 pq | 26.01 ± 1.03 efghi | 5.58 ± 0.78 no | 5.80 ± 0.29 no | 37.37 ± 8.09 ijkl |
| Go-12 | 4.26 ± 0.01 q | 5.67 ± 0.67 mnop | 28.05 ± 0.26 bcdefgh | 10.64 ± 0.16 h | 8.47 ± 0.04 def | 60.73 ± 0.00 efghi |
| Go-13 | 4.40 ± 0.01 o | 4.81 ± 0.25 qr | 25.88 ± 1.21 fghi | 6.03 ± 0.50 n | 5.74 ± 0.15 nop | 32.70 ± 0.00 jkl |
| Go-14 | 4.58 ± 0.02 k | 7.35 ± 0.31 ghi | 26.33 ± 0.34 defgh | 8.91 ± 0.10 jkl | 7.07 ± 0.01 jk | 42.04 ± 8.09 hijkl |
| Go-15 | 3.84 ± 0.01 x | 5.81 ± 0.53 lmno | 26.72 ± 0.74 cdefgh | 7.47 ± 0.55 m | 7.03 ± 0.16 jk | 37.37 ± 8.09 ijkl |
| Go-16 | 4.62 ± 0.01 j | 5.95 ± 0.78 klmn | 26.76 ± 0.34 cdefgh | 9.50 ± 0.12 ij | 7.36 ± 0.06 ij | 46.71 ± 0.00 hijk |
| Go-17 | 4.84 ± 0.01 c | 5.14 ± 0.89 opq | 27.30 ± 0.68 bcdefgh | 10.52 ± 0.09 h | 8.10 ± 0.02 fgh | 51.38 ± 8.09 ghijk |
| Go-18 | 4.26 ± 0.01 qr | 6.46 ± 0.50 jkl | 27.28 ± 0.43 cdefghi | 12.46 ± 0.21 e | 9.00 ± 0.03 cde | 37.37 ± 8.09 ijkl |
| Go-19 | 4.69 ± 0.01 g | 7.54 ± 0.30 fgh | 28.75 ± 0.62 bcdefgh | 11.45 ± 0.23 fg | 7.88 ± 0.01 gh | 56.06 ± 8.09 fghij |
| Go-20 | 4.67 ± 0.01 h | 4.20 ± 0.32 r | 26.09 ± 0.08 efghi | 4.96 ± 0.12 o | 4.97 ± 0.10 q | 42.04 ± 8.09 hijkl |
| Go-21 | 4.98 ± 0.01 a | 4.93 ± 0.30 q | 27.97 ± 0.16 bcdefgh | 9.22 ± 0.24 jk | 6.77 ± 0.09 kl | 42.04 ± 8.09 hijkl |
| Go-22 | 4.29 ± 0.02 p | 8.52 ± 0.50 de | 31.21 ± 0.57 ab | 16.63 ± 0.22 b | 11.19 ± 0.14 a | 168.17 ± 16.18 a |
| Go-23 | 4.23 ± 0.01 s | 5.01 ± 0.31 pq | 30.28 ± 0.14 abcde | 12.63 ± 0.12 e | 8.38 ± 0.07 efg | 65.4 ± 16.18 defgh |
| Go-24 | 3.94 ± 0.01 w | 6.06 ± 0.33 klm | 30.41 ± 0.17 abcd | 8.77 ± 0.21 kl | 6.05 ± 0.24 mn | 126.13 ± 16.18 b |
| Go-25 | 4.79 ± 0.02 d | 6.95 ± 0.00 hij | 25.67 ± 0.10 ghi | 6.95 ± 0.31 m | 5.42 ± 0.10 opq | 28.03 ± 8.09 kl |
| Go-26 | 4.55 ± 0.01 l | 12.68 ± 0.33 a | 30.90 ± 0.07 abc | 14.76 ± 0.04 c | 10.08 ± 0.02 b | 79.41 ± 8.09 cdef |
| Go-27 | 4.56 ± 0.01 l | 11.36 ± 0.57 b | 28.39 ± 0.03 bcdefgh | 8.33 ± 0.04 l | 7.06 ± 0.02 jk | 46.71 ± 14.01 hijk |
| Go-28 | 4.78 ± 0.00 d | 6.63 ± 0.34 jk | 29.14 ± 0.22 i | 10.40 ± 0.15 ij | 7.98 ± 0.09 cde | 18.69 ± 0.00 l |
| Go-29 | 3.99 ± 0.01 v | 5.68 ± 0.00 mnop | 30.09 ± 0.04 abcdef | 13.93 ± 0.07 d | 9.05 ± 0.01 c | 18.69 ± 0.00 l |
| Go-30 | 3.57 ± 0.01 z | 6.82 ± 0.57 ij | 30.57 ± 0.35 abcd | 17.72 ± 0.13 a | 10.52 ± 0.03 b | 79.41 ± 21.41 cdef |
| Go-31 | 4.52 ± 0.01 m | 5.30 ± 0.33 nopq | 30.85 ± 0.17 abc | 13.43 ± 0.17 d | 9.13 ± 0.03 c | 37.37 ± 8.09 ijkl |
| Go-32 | 3.76 ± 0.01 y | 5.41 ± 0.33 mnopq | 33.90 ± 0.23 a | 6.82 ± 0.14 m | 7.04 ± 0.07 jk | 84.08 ± 16.18 cde |
| Go-33 | 4.76 ± 0.01 e | 8.94 ± 0.28 d | 28.24 ± 0.49 bcdefgh | 9.20 ± 0.02 jk | 7.07 ± 0.02 jk | 65.40 ± 21.41 defgh |
| Go-34 | 4.24 ± 0.00 rs | 3.44 ± 0.00 s | 28.11 ± 0.08 bcdefgh | 10.49 ± 0.23 h | 7.76 ± 0.11 hi | 32.70 ± 14.01 jkl |
| Go-35 | 4.53 ± 0.01 m | 11.58 ± 0.00 b | 25.77 ± 0.04 fghi | 5.93 ± 0.06 n | 5.24 ± 0.03 pq | 93.43 ± 21.41 c |
| Mean ± SD | 4.44 ± 0.35 | 6.66 ± 2.18 | 28.11 ± 2.04 | 10.04 ± 3.15 | 7.59 ± 1.53 | 60.33 ± 32.51 |
| Product Code | Aerobic Bacteria (log CFU/g) * | Yeast and Mold (log CFU/g) * | Isolated and Identified Yeast | GenBank Accession Number |
|---|---|---|---|---|
| Go-1 | 6.64 ± 0.16 o | 3.89 ± 0.04 hi | Zygosaccharomyces rouxii | OL679471 |
| Go-2 | 7.20 ± 0.17 kl | 3.71 ± 0.02 i | Zygosaccharomyces rouxii | OL679472 |
| Go-3 | 8.29 ± 0.07 b | 2.67 ± 0.05 kl | Zygosaccharomyces rouxii | OL679473 |
| Go-4 | 7.97 ± 0.23 d | 4.92 ± 0.03 ef | Zygosaccharomyces rouxii | OL679474 |
| Go-5 | 7.75 ± 0.16 fg | 3.74 ± 0.10 hi | Zygosaccharomyces rouxii | OL679475 |
| Go-6 | 7.82 ± 0.08 ef | 3.10 ± 0.08 jk | Zygosaccharomyces rouxii | OL679476 |
| Go-7 | 6.04 ± 0.12 q | 5.16 ± 0.06 def | Zygosaccharomyces rouxii | OL679477 |
| Go-8 | 7.31 ± 0.15 h | 3.66 ± 0.09 i | Zygosaccharomyces rouxii | OL679478 |
| Go-9 | 7.19 ± 0.11 i | 2.69 ± 0.01 m | Zygosaccharomyces rouxii | OL679479 |
| Go-10 | 7.10 ± 0.12 lm | 6.22 ± 0.03 b | Zygosaccharomyces rouxii | OL679480 |
| Go-11 | 6.17 ± 0.52 s | 3.15 ± 0.04 j | Zygosaccharomyces rouxii | OL679481 |
| Go-12 | 7.94 ± 0.17 efg | 6.10 ± 0.04 b | Zygosaccharomyces rouxii | OL679482 |
| Go-13 | 7.86 ± 0.11 fg | 5.96 ± 0.04 bc | Starmerella lactis-condensi | OL679483 |
| Go-14 | 7.92 ± 0.22 g | 4.94 ± 0.12 ef | Starmerella lactis-condensi | OL679484 |
| Go-15 | 7.92 ± 0.17 efg | 5.84 ± 0.10 bc | Zygosaccharomyces rouxii | OL679485 |
| Go-16 | 7.93 ± 0.00 e | 4.68 ± 0.03 fg | Zygosaccharomyces rouxii | OL679486 |
| Go-17 | 8.42 ± 0.04 a | 5.30 ± 0.15 de | Zygosaccharomyces rouxii | OL679487 |
| Go-18 | 6.35 ± 0.54 s | 2.37 ± 0.05 l | Starmerella lactis-condensi | OL679488 |
| Go-19 | 8.12 ± 0.09 c | 4.04 ± 0.03 hi | Zygosaccharomyces rouxii | OL679489 |
| Go-20 | 7.01 ± 0.06 kl | 4.02 ± 0.10 hi | Zygosaccharomyces rouxii | OL679490 |
| Go-21 | 6.10 ± 0.17 r | 4.23 ± 0.02 gh | Zygosaccharomyces rouxii | OL679491 |
| Go-22 | 7.33 ± 0.20 jk | 4.23 ± 0.04 gh | Zygosaccharomyces rouxii | OL679492 |
| Go-23 | 6.39 ± 0.22 p | 5.50 ± 0.04 cd | Zygosaccharomyces rouxii | OL679493 |
| Go-24 | 8.73 ± 0.30 a | 6.13 ± 0.03 b | Zygosaccharomyces rouxii | OL679494 |
| Go-25 | 8.06 ± 0.17 d | 2.29 ± 0.02 l | Zygosaccharomyces rouxii | OL679495 |
| Go-26 | 7.86 ± 0.09 efg | 6.12 ± 0.03 b | Zygosaccharomyces rouxii | OL679496 |
| Go-27 | 4.65 ± 0.14 n | 5.31 ± 0.01 de | Zygosaccharomyces rouxii | OL679497 |
| Go-28 | 7.84 ± 0.09 efg | 5.06 ± 0.07 def | Zygosaccharomyces rouxii | OL679498 |
| Go-29 | 3.48 ± 0.12 s | 7.15 ± 0.02 a | Zygosaccharomyces rouxii | OL679499 |
| Go-30 | 7.23 ± 0.19 j | 4.82 ± 0.02 ef | Zygosaccharomyces rouxii | OL679500 |
| Go-31 | 7.28 ± 0.24 kl | 6.90 ± 0.02 a | Wikerhamomyces subpelliculosus | OL679501 |
| Go-32 | 3.55 ± 0.43 u | 1.56 ± 0.06 m | Cladosporium welwitschiicola | OL679502 |
| Go-33 | 7.53 ± 0.13 h | 2.50 ± 0.02 l | Zygosaccharomyces rouxii | OL679503 |
| Go-34 | 2.79 ± 0.10 t | 6.14 ± 0.03 b | Pichia membranifaciens | OL679504 |
| Go-35 | 4.97 ± 0.50 m | 2.37 ± 0.04 l | Wikerhamomyces subpelliculosus | OL679505 |
| Mean ± SD | 6.98 ± 1.42 | 4.47 ± 1.47 |
| Product Code | Bacillus cereus (Log CFU/g) * |
|---|---|
| Go-13 | 4.26 |
| Go-16 | 5.30 |
| Go-17 | 4.60 |
| Go-19 | 4.60 |
| Go-22 | 5.90 |
| Go-24 | 6.26 |
| Go-26 | 6.94 |
| Go-31 | 5.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramalingam, S.; Bahuguna, A.; Lim, S.; Joe, A.-R.; Lee, J.-S.; Kim, S.-Y.; Kim, M. Physicochemical, Microbial, and Volatile Compound Characteristics of Gochujang, Fermented Red Pepper Paste, Produced by Traditional Cottage Industries. Foods 2022, 11, 375. https://doi.org/10.3390/foods11030375
Ramalingam S, Bahuguna A, Lim S, Joe A-R, Lee J-S, Kim S-Y, Kim M. Physicochemical, Microbial, and Volatile Compound Characteristics of Gochujang, Fermented Red Pepper Paste, Produced by Traditional Cottage Industries. Foods. 2022; 11(3):375. https://doi.org/10.3390/foods11030375
Chicago/Turabian StyleRamalingam, Srinivasan, Ashutosh Bahuguna, SeMi Lim, Ah-Ryeong Joe, Jong-Suk Lee, So-Young Kim, and Myunghee Kim. 2022. "Physicochemical, Microbial, and Volatile Compound Characteristics of Gochujang, Fermented Red Pepper Paste, Produced by Traditional Cottage Industries" Foods 11, no. 3: 375. https://doi.org/10.3390/foods11030375