Bioactivity and Bioaccessibility of Bioactive Compounds in Gastrointestinal Digestion of Tomato Bagasse Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Samples
2.3. In Vitro GID
2.4. Recovery and Bioaccessibility Indexes of Polyphenolic and Carotenoids Compounds throughout In Vitro GID Digestion
2.5. Analysis of Gastrointestinal Fractions
2.5.1. Total Phenolic Content (TPC)
2.5.2. Total Carotenoids Content (TCC)
2.5.3. HPLC-Analysis (Phenols and Carotenoids)
2.5.4. UPLC-qTOF MS Analysis (Phenolic Compounds)
2.6. Bioactivities
2.6.1. Antioxidant Activity
2.6.2. Prebiotic Effect
2.6.3. ACE-Inhibitory Activity Assay (iACE)
2.6.4. Citocines Inhibition—Anti-Inflammatory Activity
2.7. Statistical Analysis
3. Results and Discussion
3.1. Extract Phenolic and Carotenoids Characterization
3.1.1. Phenolic Characterization
3.1.2. Carotenoids
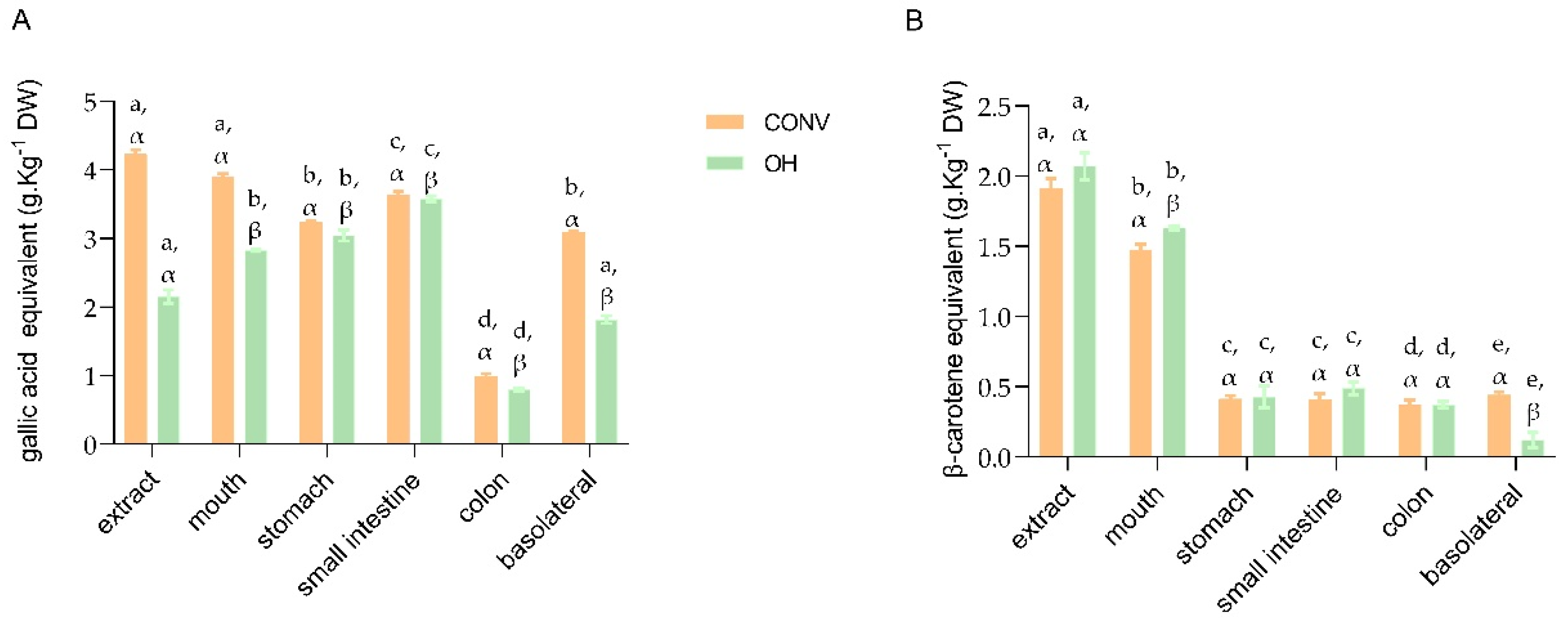
3.2. Recovery and Bioaccessibility Indexes of Polyphenolic and Carotenoids Compounds throughout In Vitro GID
3.2.1. Phenolic Compounds
3.2.2. Carotenoids
3.3. Bioactive Properties of Liquid Extracts after Simulated Enzymatic GID
3.3.1. Antioxidant Activities of Liquid Digest Extracts throughout In Vitro GID
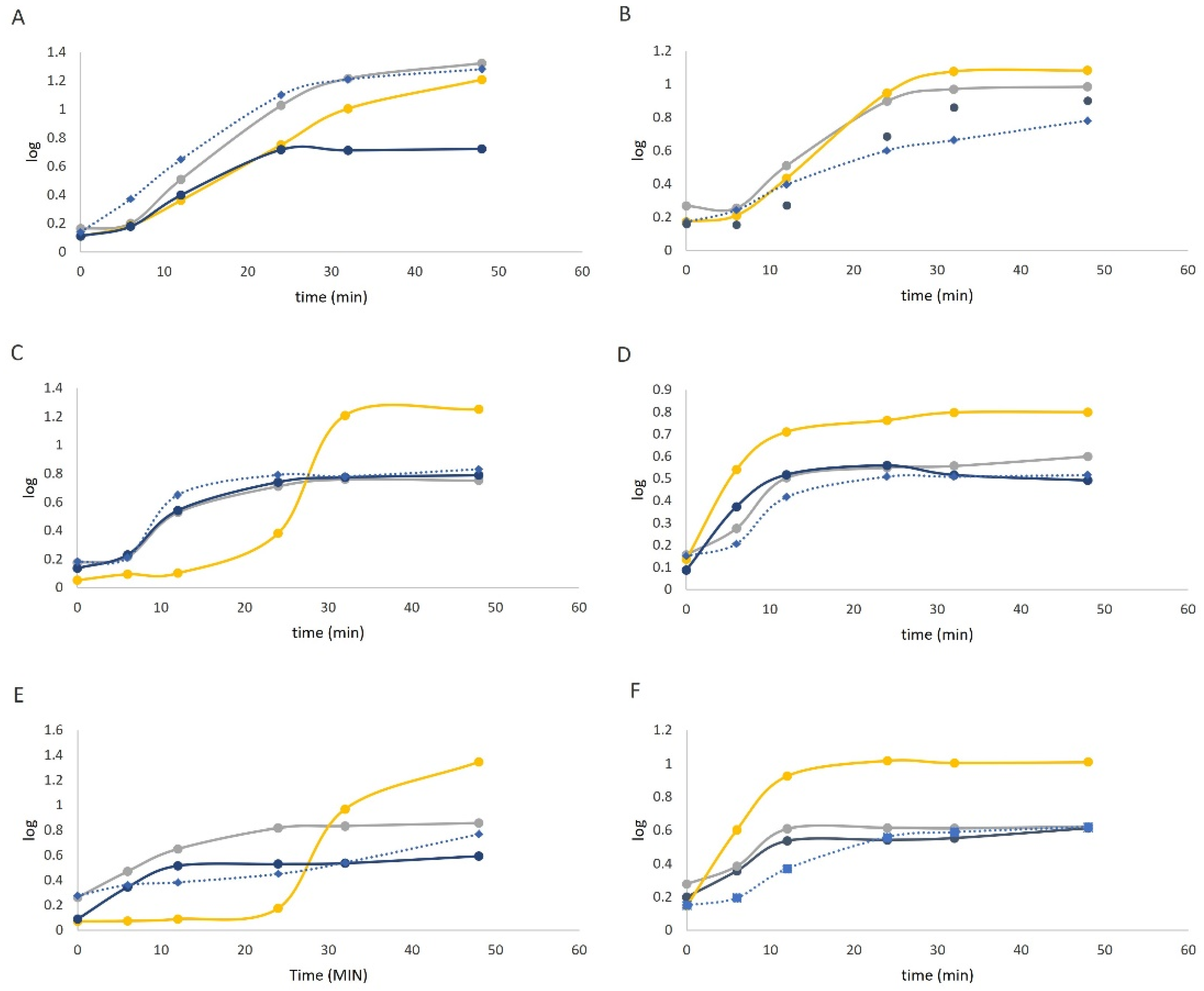
3.3.2. Prebiotic Effect of Digested OH and CONV Extracts
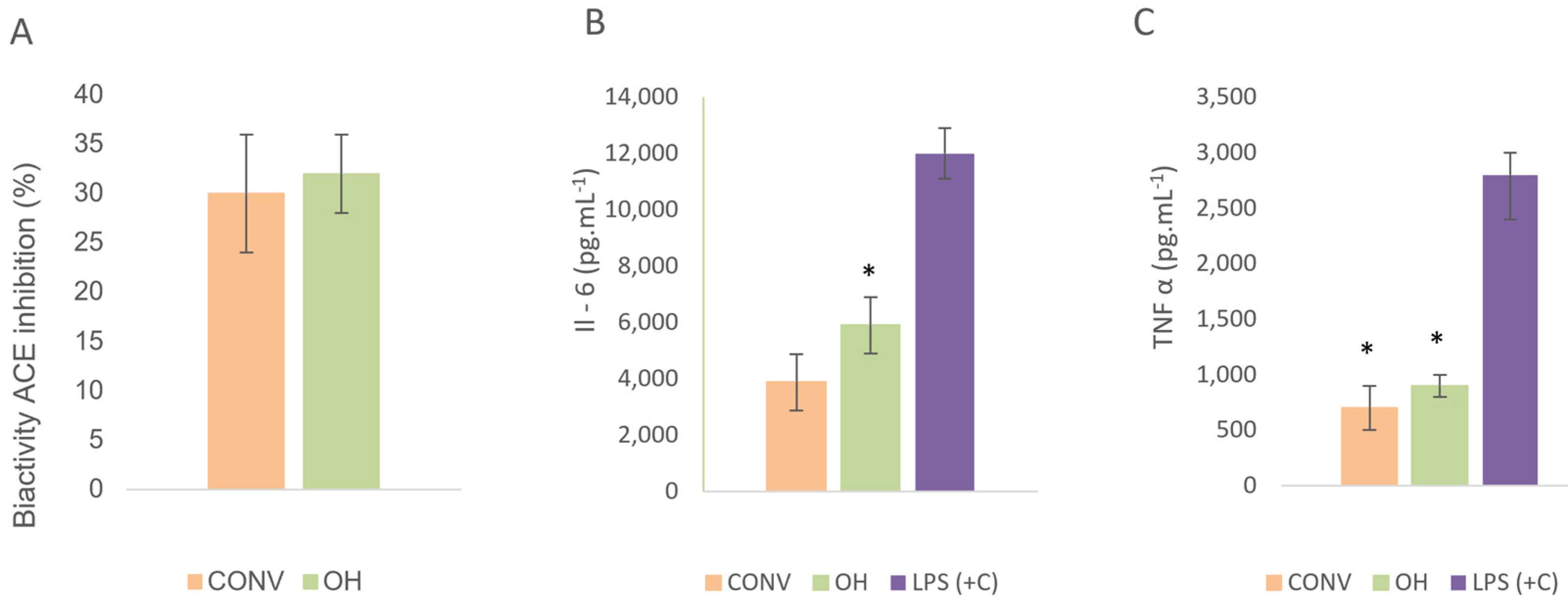
3.3.3. Inhibition of Angiotensin-Converting Enzyme Activity (iACE)
3.3.4. Anti-Inflammatory Inhibition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galanakis, C.M. The Food Systems in the Era of the Coronavirus (COVID-19) Pandemic Crisis. Foods 2020, 9, 523. [Google Scholar] [CrossRef]
- Ribeiro, T.B.; Oliveira, A.; Campos, D.; Nunes, J.; Vicente, A.A.; Pintado, M. Simulated Digestion of an Olive Pomace Water-Soluble Ingredient: Relationship between the Bioaccessibility of Compounds and Their Potential Health Benefits. Food Funct. 2020, 11, 2238–2254. [Google Scholar] [CrossRef]
- Coelho, M.C.; Pereira, R.N.; Rodrigues, A.S.; Teixeira, J.A.; Pintado, M.E. The Use of Emergent Technologies to Extract Added Value Compounds from Grape By-Products. Trends Food Sci. Technol. 2020, 106, 182–197. [Google Scholar] [CrossRef]
- Knirsch, M.C.; Alves dos Santos, C.; Martins de Oliveira Soares Vicente, A.A.; Vessoni Penna, T.C. Ohmic Heating—A Review. Trends Food Sci. Technol. 2010, 21, 436–441. [Google Scholar] [CrossRef]
- Coelho, M.; Pereira, R.; Rodrigues, A.S.; Teixeira, J.A.; Pintado, M.E. Extraction of Tomato By-Products’ Bioactive Compounds Using Ohmic Technology. Food Bioprod. Process. 2019, 117, 329–339. [Google Scholar] [CrossRef] [Green Version]
- Coelho, M.; Silva, S.; Costa, E.; Pereira, R.N.; Rodrigues, A.S.; Teixeira, J.A.; Pintado, M. Anthocyanin Recovery from Grape By-Products by Combining Ohmic Heating with Food-Grade Solvents: Phenolic Composition, Antioxidant, and Antimicrobial Properties. Molecules 2021, 26, 3838. [Google Scholar] [CrossRef]
- Barba, F.J.; Brianceau, S.; Turk, M.; Boussetta, N.; Vorobiev, E. Effect of Alternative Physical Treatments (Ultrasounds, Pulsed Electric Fields, and High-Voltage Electrical Discharges) on Selective Recovery of Bio-Compounds from Fermented Grape Pomace. Food Bioprocess Technol. 2015, 8, 1139–1148. [Google Scholar] [CrossRef]
- Coman, V.; Teleky, B.E.; Mitrea, L.; Martău, G.A.; Szabo, K.; Călinoiu, L.F.; Vodnar, D.C. Bioactive Potential of Fruit and Vegetable Wastes. Adv. Food Nutr. Res. 2020, 91, 157–225. [Google Scholar] [CrossRef]
- Coelho, M.C.; Ribeiro, T.B.; Oliveira, C.; Batista, P.; Castro, P.; Monforte, A.R.; Rodrigues, A.S.; Teixeira, J.; Pintado, M. In Vitro Gastrointestinal Digestion Impact on the Bioaccessibility and Antioxidant Capacity of Bioactive Compounds from Tomato Flours Obtained after Conventional and Ohmic Heating Extraction. Foods 2021, 10, 554. [Google Scholar] [CrossRef]
- Abbasi-Parizad, P.; de Nisi, P.; Pepè Sciarria, T.; Scarafoni, A.; Squillace, P.; Adani, F.; Scaglia, B. Polyphenol Bioactivity Evolution during the Spontaneous Fermentation of Vegetal By-Products. Food Chem. 2022, 374, 131791. [Google Scholar] [CrossRef]
- Lazzarini, C.; Casadei, E.; Valli, E.; Tura, M.; Ragni, L.; Bendini, A.; Gallina Toschi, T. Sustainable Drying and Green Deep Eutectic Extraction of Carotenoids from Tomato Pomace. Foods 2022, 11, 405. [Google Scholar] [CrossRef]
- Bot, F.; Verkerk, R.; Mastwijk, H.; Anese, M.; Fogliano, V.; Capuano, E. The Effect of Pulsed Electric Fields on Carotenoids Bioaccessibility: The Role of Tomato Matrix. Food Chem. 2018, 240, 415–421. [Google Scholar] [CrossRef]
- Oliveira, A.; Alexandre, E.; Coelho, M.; Barros, R.M.; Almeida, D.P.F.; Pintado, M. Peach Polyphenol and Carotenoid Content as Affected by Frozen Storage and Pasteurization. LWT—Food Sci. Technol. 2016, 66, 361–368. [Google Scholar] [CrossRef]
- Kimura, M.; Rodriguez-Amaya, D.B.; Godoy, H.T. Assessment of the Saponification Step in the Quantitative Determination of Carotenoids and Provitamins A. Food Chem. 1990, 35, 187–195. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Barros, A.S.; Silva Ferreira, A.C.; Silva, A.M.S. Influence of the Temperature and Oxygen Exposure in Red Port Wine: A Kinetic Approach. Food Res. Int. 2015, 75, 337–347. [Google Scholar] [CrossRef]
- Dávalos, A.; Gómez-Cordovés, C.; Bartolomé, B. Extending Applicability of the Oxygen Radical Absorbance Capacity (ORAC-Fluorescein) Assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef]
- Coscueta, E.R.; Brassesco, M.E.; Pintado, M. Collagen-Based Bioactive Bromelain Hydrolysate from Salt-Cured Cod Skin. Appl. Sci. 2021, 11, 8538. [Google Scholar] [CrossRef]
- Fernandes, J.C.; Spindola, H.; de Sousa, V.; Santos-Silva, A.; Pintado, M.E.; Malcata, F.X.; Carvalho, J.E. Anti-Inflammatory Activity of Chitooligosaccharides in Vivo. Mar. Drugs 2010, 8, 1763–1768. [Google Scholar] [CrossRef]
- Peschel, W.; Sánchez-Rabaneda, F.; Diekmann, W.; Plescher, A.; Gartzía, I.; Jiménez, D.; Lamuela-Raventós, R.; Buxaderas, S.; Codina, C. An Industrial Approach in the Search of Natural Antioxidants from Vegetable and Fruit Wastes. Food Chem. 2006, 97, 137–150. [Google Scholar] [CrossRef]
- Kim, H.S.; Chin, K.B. Evaluation of Different Drying Temperatures on Physico-Chemical and Antioxidant Properties of Water-Soluble Tomato Powders and on Their Use in Pork Patties. J. Sci. Food Agric. 2016, 96, 742–750. [Google Scholar] [CrossRef]
- Preedy, V.R. Tomatoes and Tomato Products: Nutritional, Medicinal and Therapeutic Properties. Nutr. Food Sci. 2009, 39, 702. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Jáuregui, O.; Medina-Remón, A.; Andrés-Lacueva, C.; Lamuela-Raventós, R.M. Improved Characterization of Tomato Polyphenols Using Liquid Chromatography/Electrospray Ionization Linear Ion Trap Quadrupole Orbitrap Mass Spectrometry and Liquid Chromatography/Electrospray Ionization Tandem Mass Spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 2986–2992. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.Y. Biochemical Basis of Anti-Cancer-Effects of Phloretin—a Natural Dihydrochalcone. Molecules 2019, 24, 278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zidorn, C. Isoetin and Its Derivatives: Analytics, Chemosystematics, and Bioactivities. Biochem. Syst. Ecol. 2015, 61, 402–412. [Google Scholar] [CrossRef]
- Nguyen, M.; Francis, D.; Schwartz, S. Thermal Isomerisation Susceptibility of Carotenoids in Different Tomato Varieties. J. Sci. Food Agric. 2001, 81, 910–917. [Google Scholar] [CrossRef]
- Sabio, E.; Lozano, M.; Montero De Espinosa, V.; Mendes, R.L.; Pereira, A.P.; Palavra, A.F.; Coelho, J.A. Lycopene and β-Carotene Extraction from Tomato Processing Waste Using Supercritical CO2. Ind. Eng. Chem. Res. 2003, 42, 6641–6646. [Google Scholar] [CrossRef]
- Caseiro, M.; Ascenso, A.; Costa, A.; Creagh-Flynn, J.; Johnson, M.; Simões, S. Lycopene in Human Health. LWT 2020, 127, 109323. [Google Scholar] [CrossRef]
- D’Evoli, L.; Lombardi-Boccia, G.; Lucarini, M. Influence of Heat Treatments on Carotenoid Content of Cherry Tomatoes. Foods 2013, 2, 352–363. [Google Scholar] [CrossRef] [Green Version]
- Lucas-González, R.; Viuda-Martos, M.; Pérez Álvarez, J.A.; Fernández-López, J. Changes in Bioaccessibility, Polyphenol Profile and Antioxidant Potential of Flours Obtained from Persimmon Fruit (Diospyros Kaki) Co-Products during in Vitro Gastrointestinal Digestion. Food Chem. 2018, 256, 252–258. [Google Scholar] [CrossRef]
- David, L.; Danciu, V.; Moldovan, B.; Filip, A. Effects of in Vitro Gastrointestinal Digestion on the Antioxidant Capacity and Anthocyanin Content of Cornelian Cherry Fruit Extract. Antioxidants 2019, 8, 114. [Google Scholar] [CrossRef] [Green Version]
- Pavan, V.Ô.; Sancho, R.A.S.; Pastore, G.M. The Effect of In vitro Digestion on the Antioxidant Activity of Fruit Extracts (Carica Papaya, Artocarpus Heterophillus and Annona Marcgravii). LWT—Food Sci. Technol. 2014, 59, 1247–1251. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Solana, R.; Coelho, N.; Santos-Rufo, A.; Gonçalves, S.; Pérez-Santín, E.; Romano, A. The Influence of in Vitro Gastrointestinal Digestion on the Chemical Composition and Antioxidant and Enzyme Inhibitory Capacities of Carob Liqueurs Obtained with Different Elaboration Techniques. Antioxidants 2019, 8, 563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gullón, B.; Gullón, P.; Tavaria, F.; Alonso, J.L.; Pintado, M. In Vitro Assessment of the Prebiotic Potential of Aloe Vera Mucilage and Its Impact on the Human Microbiota. Food Funct. 2015, 6, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Barba, F.J.; Mariutti, L.R.B.; Bragagnolo, N.; Mercadante, A.Z.; Barbosa-Cánovas, G.v.; Orlien, V. Bioaccessibility of Bioactive Compounds from Fruits and Vegetables after Thermal and Nonthermal Processing. Trends Food Sci. Technol. 2017, 67, 195–206. [Google Scholar] [CrossRef]
- Martínez-Huélamo, M.; Vallverdú-Queralt, A.; di Lecce, G.; Valderas-Martínez, P.; Tulipani, S.; Jáuregui, O.; Escribano-Ferrer, E.; Estruch, R.; Illan, M.; Lamuela-Raventós, R.M. Bioavailability of Tomato Polyphenols Is Enhanced by Processing and Fat Addition: Evidence from a Randomized Feeding Trial. Mol. Nutr. Food Res. 2016, 60, 1578–1589. [Google Scholar] [CrossRef]
- del Pino-García, R.; González-SanJosé, M.L.; Rivero-Pérez, M.D.; García-Lomillo, J.; Muñiz, P. Total Antioxidant Capacity of New Natural Powdered Seasonings after Gastrointestinal and Colonic Digestion. Food Chem. 2016, 211, 707–714. [Google Scholar] [CrossRef] [Green Version]
- Courraud, J.; Berger, J.; Cristol, J.P.; Avallone, S. Stability and Bioaccessibility of Different Forms of Carotenoids and Vitamin A during in Vitro Digestion. Food Chem. 2013, 136, 871–877. [Google Scholar] [CrossRef]
- Scrob, T.; Hosu, A.; Cimpoiu, C. The Influence of in Vitro Gastrointestinal Digestion of Brassica Oleracea Florets on the Antioxidant Activity and Chlorophyll, Carotenoid and Phenolic Content. Antioxidants 2019, 8, 212. [Google Scholar] [CrossRef] [Green Version]
- Reboul, E. Mechanisms of Carotenoid Intestinal Absorption: Where Do We Stand? Nutrients 2019, 11, 838. [Google Scholar] [CrossRef] [Green Version]
- Salter-Venzon, D.; Kazlova, V.; Izzy Ford, S.; Intra, J.; Klosner, A.E.; Gellenbeck, K.W. Evidence for Decreased Interaction and Improved Carotenoid Bioavailability by Sequential Delivery of a Supplement. Food Sci. Nutr. 2017, 5, 424–433. [Google Scholar] [CrossRef]
- Domínguez-Avila, J.A.; Wall-Medrano, A.; Velderrain-Rodríguez, G.R.; Chen, C.Y.O.; Salazar-López, N.J.; Robles-Sánchez, M.; González-Aguilar, G.A. Gastrointestinal Interactions, Absorption, Splanchnic Metabolism and Pharmacokinetics of Orally Ingested Phenolic Compounds. Food Funct. 2017, 8, 15–38. [Google Scholar] [CrossRef] [PubMed]
- Taveira, M.; Silva, L.R.; Vale-Silva, L.A.; Pinto, E.; Valentão, P.; Ferreres, F.; Guedes De Pinho, P.; Andrade, P.B. Lycopersicon Esculentum Seeds: An Industrial Byproduct as an Antimicrobial Agent. J. Agric. Food Chem. 2010, 58, 9529–9536. [Google Scholar] [CrossRef] [PubMed]
- Palafox-Carlos, H.; Ayala-Zavala, J.F.; González-Aguilar, G.A. The Role of Dietary Fiber in the Bioaccessibility and Bioavailability of Fruit and Vegetable Antioxidants. J. Food Sci. 2011, 76, R6–R15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Jiménez, J.; Serrano, J.; Tabernero, M.; Arranz, S.; Díaz-Rubio, M.E.; García-Diz, L.; Goñi, I.; Saura-Calixto, F. Bioavailability of Phenolic Antioxidants Associated with Dietary Fiber: Plasma Antioxidant Capacity after Acute and Long-Term Intake in Humans. Plant Foods Hum. Nutr. 2009, 64, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Tagliazucchi, D.; Verzelloni, E.; Bertolini, D.; Conte, A. In Vitro Bio-Accessibility and Antioxidant Activity of Grape Polyphenols. Food Chem. 2010, 120, 599–606. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic Acids: Natural Versatile Molecules with Promising Therapeutic Applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Coscueta, E.R.; Campos, D.A.; Osório, H.; Nerli, B.B.; Pintado, M. Enzymatic Soy Protein Hydrolysis: A Tool for Biofunctional Food Ingredient Production. Food Chem. X 2019, 1, 100006. [Google Scholar] [CrossRef]
- de Carvalho, N.M.; Teixeira, F.; Silva, S.; Madureira, A.R.; Pintado, M.E. Potential Prebiotic Activity of Tenebrio Molitor Insect Flour Using an Optimized in Vitro Gut Microbiota Model. Food Funct. 2019, 10, 3909–3922. [Google Scholar] [CrossRef]
- Costa, J.R.; Amorim, M.; Vilas-Boas, A.; Tonon, R.v.; Cabral, L.M.C.; Pastrana, L.; Pintado, M. Impact of: In Vitro Gastrointestinal Digestion on the Chemical Composition, Bioactive Properties, and Cytotoxicity of Vitis vinifera L. Cv. Syrah Grape Pomace Extract. Food Funct. 2019, 10, 1856–1869. [Google Scholar] [CrossRef]
- Moayedi, A.; Mora, L.; Aristoy, M.C.; Hashemi, M.; Safari, M.; Toldrá, F. ACE-Inhibitory and Antioxidant Activities of Peptide Fragments Obtained from Tomato Processing By-Products Fermented Using Bacillus Subtilis: Effect of Amino Acid Composition and Peptides Molecular Mass Distribution. Appl. Biochem. Biotechnol. 2017, 181, 48–64. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef] [Green Version]
- Guerrero, L.; Castillo, J.; Quiñones, M.; Garcia-Vallvé, S.; Arola, L.; Pujadas, G.; Muguerza, B. Inhibition of Angiotensin-Converting Enzyme Activity by Flavonoids: Structure-Activity Relationship Studies. PLoS ONE 2012, 7, e49493. [Google Scholar] [CrossRef] [Green Version]
- Ghavipour, M.; Saedisomeolia, A.; Djalali, M.; Sotoudeh, G.; Eshraghyan, M.R.; Moghadam, A.M.; Wood, L.G. Tomato Juice Consumption Reduces Systemic Inflammation in Overweight and Obese Females. Br. J. Nutr. 2013, 109, 2031–2035. [Google Scholar] [CrossRef] [PubMed]
- Riso, P.; Visioli, F.; Grande, S.; Guarnieri, S.; Gardana, C.; Simonetti, P.; Porrini, M. Effect of a Tomato-Based Drink on Markers of Inflammation, Immunomodulation, and Oxidative Stress. J. Agric. Food Chem. 2006, 54, 2563–2566. [Google Scholar] [CrossRef]
- Mohri, S.; Takahashi, H.; Sakai, M.; Takahashi, S.; Waki, N.; Aizawa, K.; Suganuma, H.; Ara, T.; Matsumura, Y.; Shibata, D.; et al. Wide-Range Screening of Anti-Inflammatory Compounds in Tomato Using LC-MS and Elucidating the Mechanism of Their Functions. PLoS ONE 2018, 13, e0191203. [Google Scholar] [CrossRef] [PubMed] [Green Version]



 ), 1% extract obtained with CONV process (
), 1% extract obtained with CONV process (  ), positive control—FOS (
), positive control—FOS (  ) and negative control without sugar source (
) and negative control without sugar source (  ).
).
 ), 1% extract obtained with CONV process (
), 1% extract obtained with CONV process (  ), positive control—FOS (
), positive control—FOS (  ) and negative control without sugar source (
) and negative control without sugar source (  ).
).
| Name | OH Samples | CONV Samples | tr (min) | DAD | Formula | m/z Experimental | m/z Calculated | MSMS Fragments | Err [mDa] | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No identified | ✓ | ✓ | 7.7 | 295 | C15H2ON2O4 | 291.1355 | 291.1350 | 291.1 (22) | 171.1 (51) | 145.0 (51) | 119.1 (100) | 145.0 (19) | 0.5 |
| Caffeoyl-glucose | ✓ | ✓ | 8.3 | 370 | C15H18O9 | 341.0897 | 341.0878 | 341.1 (6) | 179.0 (100) | 1.9 | |||
| Quinic acid | ✓ | ✓ | 8.5 | 330 | C7H12O6 | 191.6634 | 192.0634 | 191.0 (100) | 146.9 (33) | 119.0 (54) | 102.9 (75) | 0.4 | |
| Kaempferol 3-sophorotrioside | ✓ | ✓ | 8.7 | 266/360 | C33H40O21 | 771.2004 | 771.1989 | 771.2 (100) | 609.1 (25) | 463.1 (6) | 301.0 (4) | 1.4 | |
| (2-phenylethyl) alpha-L-gulo-hexopyranosyl-(1->6)-alpha-L-gulo-hexopyranoside | ✓ | ✓ | 9.8 | 285 | C20H30O11 | 445.1709 | 445.1715 | 445.1 (12) | 267.1 (31) | 221.1 (28) | 179.0 (100) | 0.6 | |
| Quercetin-3-O-sophoroside | ✓ | ✓ | 10.5 | 266/366 | C27H30O17 | 625.1421 | 625.1410 | 300.0 (74) | 179.0 (6) | 741.2 (100) | 1.1 | ||
| Quercetin 3-(2G-xylosylrutinoside) | ✓ | ✓ | 10.9 | 266/356 | C32H38O20 | 741.0956 | 741.19564 | 714.2 (0.01) | 300.0 (22) | 1.1 | |||
| Malvidin 3-(6-malonylglucoside) 5-glucoside | ✓ | ✕ | 11.1 | 510 | C32H38O20 | 741.1895 | 741.884 | 741.14 (100) | 300.0 (16) | 0.7 | |||
| Quercetin-3-O-neohesperidoside | ✓ | ✓ | 11.3 | 262 | C27H30O16 | 609.1474 | 609.1461 | 609.1 (100) | 429.1 (3) | 284.0 (53) | 179.0 (4) | 1.3 | |
| p-coumaric acid | ✓ | ✓ | 11.3 | 314 | C9H8O3 | 163.04 | 163.0401 | 163.0 (14) | 119.0 (100) | 0.1 | |||
| Rutin | ✓ | ✓ | 11.8 | 262/356 | C27H30O16 | 609.1442 | 609.1461 | 609.1 (100) | 301.0 (34) | 300.0 (38) | 179.0 (2) | 151.0 (1) | 1.9 |
| Phloridzinyl glucoside | ✓ | ✓ | 12.2 | 290 | C27H34O15 | 597.1825 | 597.1824 | 597.2 (100) | 477.1 (33) | 417.1 (24) | 387.1 (76) | 357.1 (100) | 0.1 |
| N-acetyl-D-tryptophan | ✓ | ✓ | 12.4 | 281 | C13H14N2O3 | 245.0936 | 245.0932 | 245.1 (53) | 203.1 (100) | 159.1 (5) | 116.0 (29) | 98.0 (7) | 0.5 |
| Naringenin-7-O-glucoside | ✓ | ✓ | 13.7 | 290 | C21H22O10 | 433.1 | 433.114 | 433.1 (2) | 271.1 (100) | 151.0 (8) | 0.5 | ||
| cis-2-Coumarate | ✓ | ✕ | 16.2 | C9H8O4 | 163.0396 | 163.0396 | 163 (100) | 0.5 | |||||
| Flavimycin A | ✓ | ✕ | 17.3 | C18H18O9 | 377.0862 | 377.0862 | 377.1 (56) | 341 (100) | 215 (33) | 179 (56) | 161 (11) | 1.6 | |
| Naringenin | ✓ | ✓ | 18 | 290 | C15H12O5 | 271.0608 | 271.0612 | 271.1 (84) | 177.01 (13) | 151.0 (100) | 119.0 (32) | 0.4 | |
| 4-Coumarate | ✕ | ✓ | 11.5 | C9H8O3 | 163.0401 | 163.0406 | 0.5 | ||||||
| No identified | ✕ | ✓ | 16.2 | C54H67O10 | 875.4739 | 875.4723 | 874.5 (33.0) | 97.0 (100) | 1.6 | ||||
| (2S)-2-[[2-(diethylamino)-5-[ethyl(piperidine-1-carbonyl)amino]pyrimidin-4-yl]amino]-3-[4-(pyrrolidine-1-carbonyloxy)phenyl]propanoic acid | ✕ | ✓ | 20.7 | C30H42N7O5 | 580.3253 | 580.32474 | 580.0 (1) | 514.3 (100) | 0.6 | ||||
| Compounds | Recovery Index (%) | Bioaccesibility Index (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mouth | Stomach | Small Intestine | Colon | Basolateral | ||||||||
| CONV | OH | CONV | OH | CONV | OH | CONV | OH | CONV | OH | CONV | OH | |
| Total Phenolic compounds | 92.32 ± 1.78 a | 131.05 ± 1.25 a | 76.53 ± 2.23 b | 141.19 ± 7.25 b | 86.12 ± 2.14 a | 165.98 ± 3.51 c | 23.44 ± 1.25 d | 36.87 ± 1.78 d | 73.10 ± 2.62 b | 84.25 ± 1.89 e | 75.72 ± 1.45 α | 69.56 ± 1.53 β |
| 4-Hydroxybenzoic acid | 10.69 ± 1.25 a | 94.46 ± 1.84 a | 1.35 ± 0.09 b | 105.94 ± 3.28 b | 2.50 ± 0.56 b | 44.00 ± 1.98 c | 3.95 ± 1.25 c | 9.54 ± 1.33 d | 8.18 ± 1.44 d | 4.94 ± 0.78 e | 67.41 ± 2.73 α | 34.13 ± 1.23 β |
| trans-cinnamic acid | 92.19 ± 4.13 a | 122.70 ± 5.67 a | n.d. | 296.89 ± 8.23 b | n.d. | 197.20 ± 3.63 b | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| vanillin | n.d. | 65.51 ± 2.59 a | n.d. | 175.37 ± 4.17 b | n.d. | 59.83 ± 1.88 a | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| caffeic acid | 96.54 ± 3.32 a | 138.16 ± 1.61 a | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 47.73 ± 9.85 b | n.d. | 10.00 ± 1.23 α |
| luteolin | n.d. | 167.40 ± 4.21 a | n.d. | 143.45 ± 3.78 b | n.d. | 57.91 ± 3.21 c | n.d. | 101.99 ± 4.45 d | n.d. | n.d. | n.d. | n.d. |
| rutin | 59.71 ± 2.24 a | 145.44 ± 2.13 a | 3.40 ± 0.78 b | 236.33 ± 5.45 b | 41.90 ± 2.32 c | 131.40 ± 2.41 a | 35.72 ± 1.69 d | 32.99 ± 2.44 c | 33.01 ± 1.12 d | 13.72 ± 2.56 d | 48.03 ± 3.24 β | 29.37 ± 4.71 α |
| naringenin | n.d. | 107.49 ± 3.27 a | n.d. | 149.67 ± 2.98 b | n.d. | 224.15 ±7.42 c | 62.22 ± 4.44 a | 67.60 ± 1.94 d | 52.50 ± 2.41 a | 12.95 ± 1.76 e | 45.76 ± 1.85 β | 16.08 ± 1.89 α |
| Total carotenoids | 77.03 ± 4.67 a/α | 78.75 ± 2.25 a/α | 21.58 ± 2.85 a/β | 20.65 ± 2.43 a/β | 21.27 ± 4.25 a/β | 23.67 ± 2.81 a/β | 19.38 ± 0.97 a/β | 17.92 ± 1.71 a/β | 22.967 ± 2.43 a/β | 5.76 ± 3.12 a/ỿ | 93.62 ± 1.41 α | 25.86 ± 1.12 β |
| Total antioxidant activity | 92.75 ± 2.01 | 104.08 ± 12.99 | 76.23 ± 4.67 | 115.51 ± 12.01 | 86.38 ± 3.21 | 106.53 ± 11.84 | 23.19 ± 1.24 | 41.80 ± 2.78 | 73.04 ± 5.87 | 95.51 ± 3.32 | 84.89 ± 2.98 α | 89.66 ± 2.44 β |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho, M.; Oliveira, C.; Coscueta, E.R.; Fernandes, J.; Pereira, R.N.; Teixeira, J.A.; Rodrigues, A.S.; Pintado, M.E. Bioactivity and Bioaccessibility of Bioactive Compounds in Gastrointestinal Digestion of Tomato Bagasse Extracts. Foods 2022, 11, 1064. https://doi.org/10.3390/foods11071064
Coelho M, Oliveira C, Coscueta ER, Fernandes J, Pereira RN, Teixeira JA, Rodrigues AS, Pintado ME. Bioactivity and Bioaccessibility of Bioactive Compounds in Gastrointestinal Digestion of Tomato Bagasse Extracts. Foods. 2022; 11(7):1064. https://doi.org/10.3390/foods11071064
Chicago/Turabian StyleCoelho, Marta, Carla Oliveira, Ezequiel R. Coscueta, João Fernandes, Ricardo N. Pereira, José A. Teixeira, António Sebastião Rodrigues, and Manuela E. Pintado. 2022. "Bioactivity and Bioaccessibility of Bioactive Compounds in Gastrointestinal Digestion of Tomato Bagasse Extracts" Foods 11, no. 7: 1064. https://doi.org/10.3390/foods11071064