Evaluating the Use of a Similarity Index (SI) Combined with near Infrared (NIR) Spectroscopy as Method in Meat Species Authenticity
Abstract
:1. Introduction
2. Materials and Methods
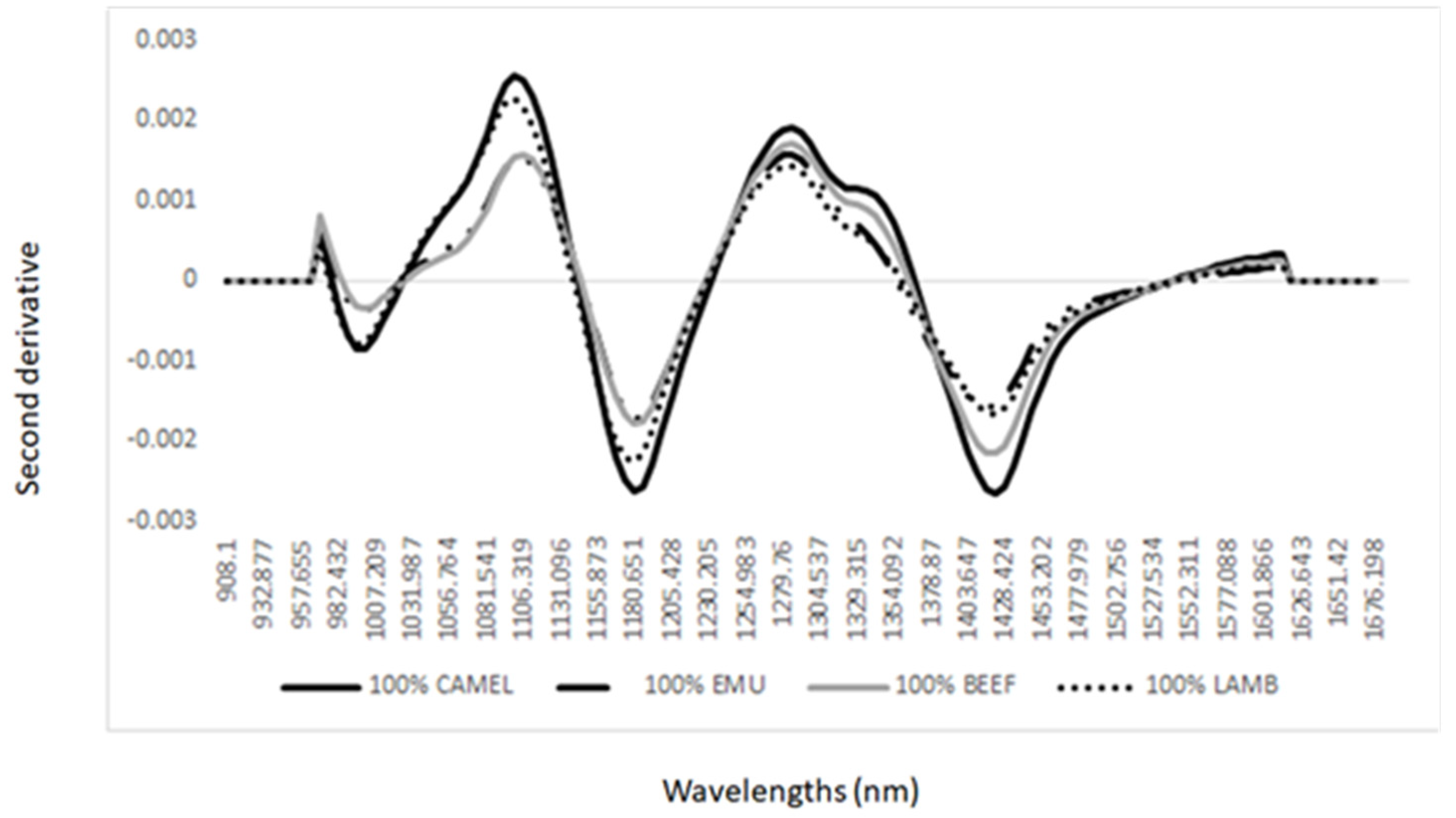
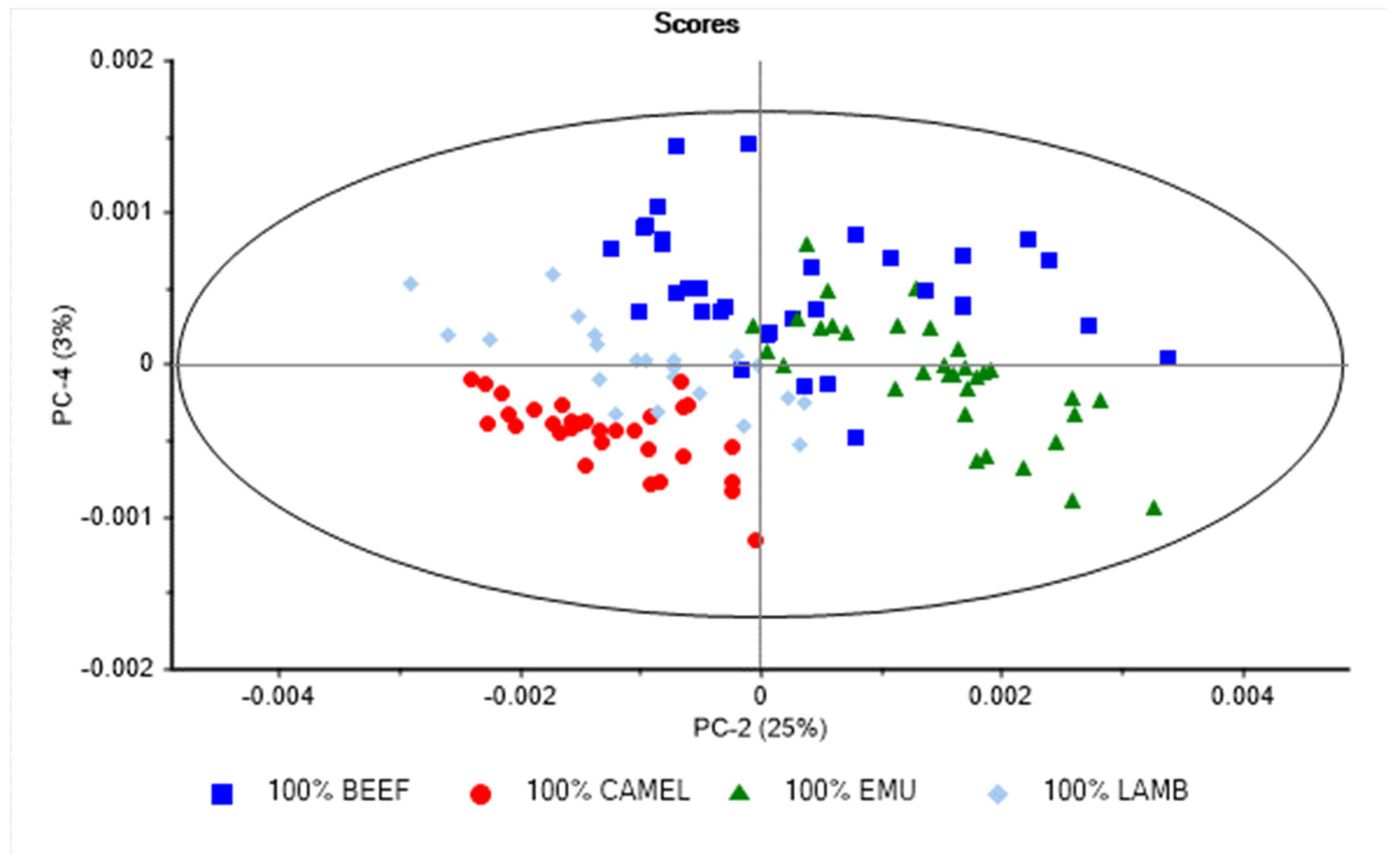
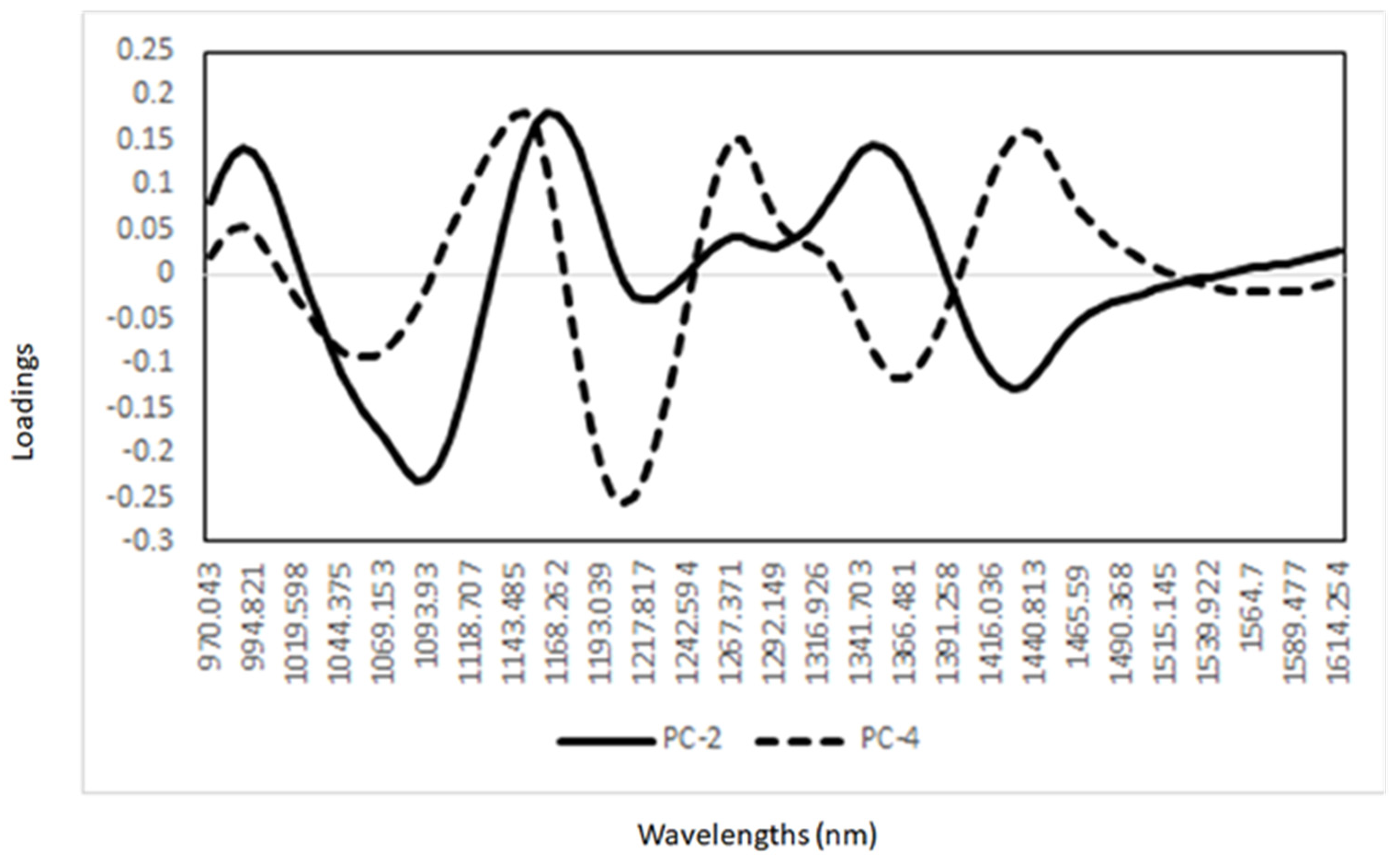
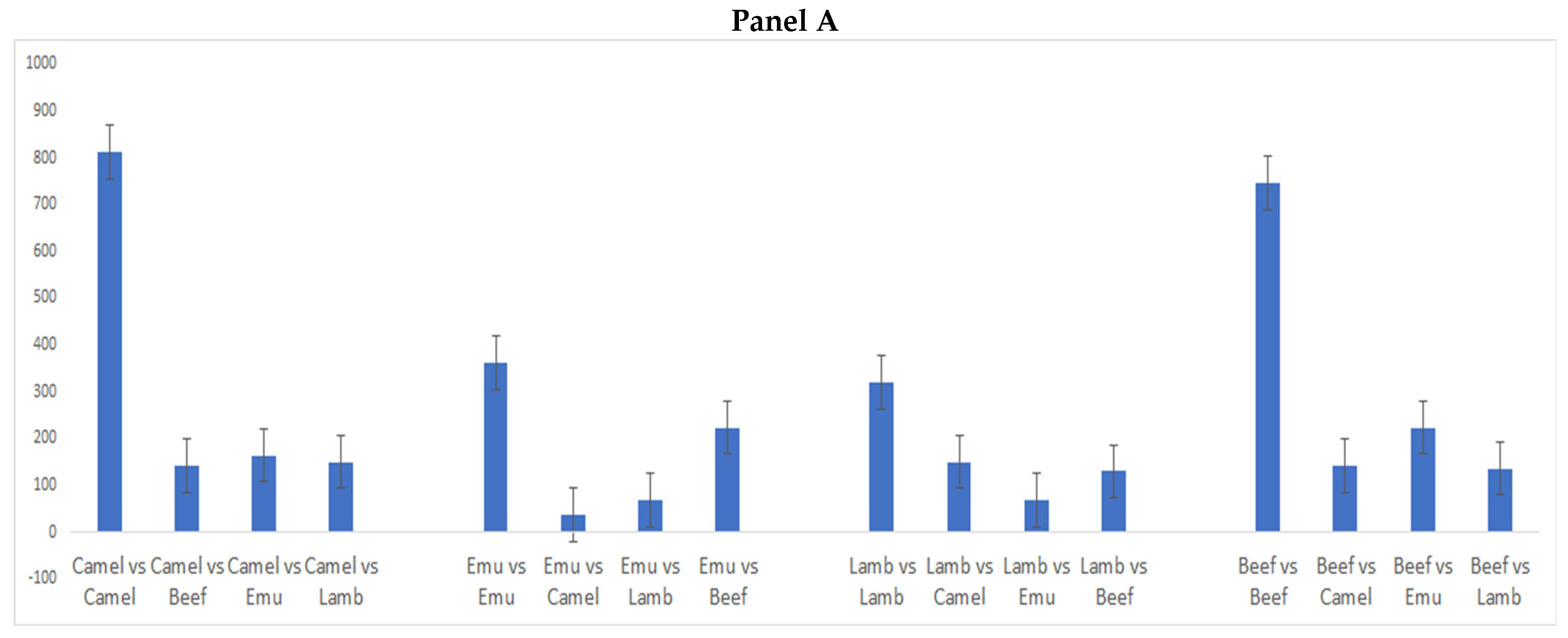
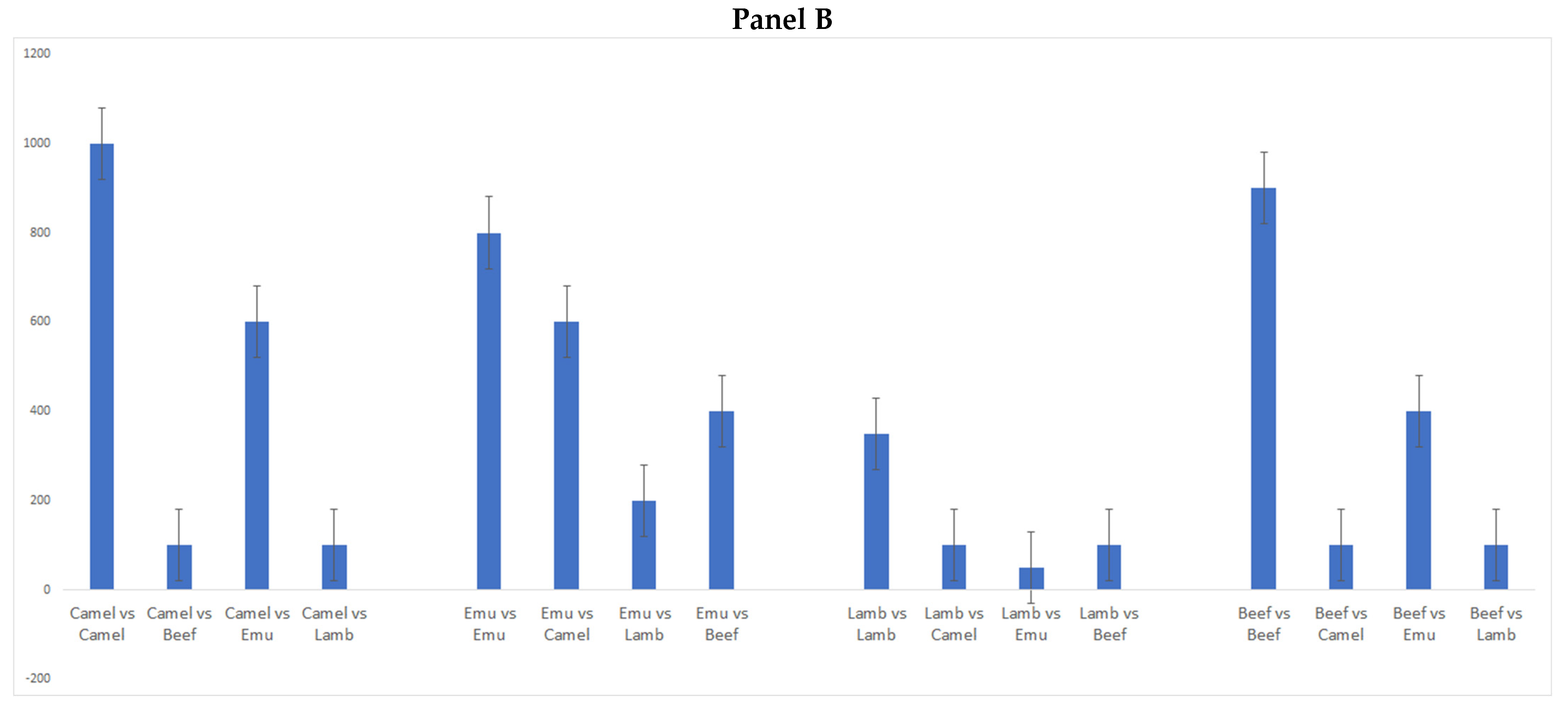
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prieto, N.; Roehe, R.; Lavin, P.; Batten, G.; Andres, S. Application of near infrared reflectance spectroscopy to predict meat and meat products quality: A review. Meat Sci. 2009, 83, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Prieto, N.; Pawluczyk, O.; Edward, M.; Dugan, R.; Aalhus, J.L. A Review of the Principles and Applications of Near-Infrared Spectroscopy to Characterize Meat, Fat, and Meat Products. Appl. Spectrocopy 2017, 7, 1406–1426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pieszczek, L.; Czarnik-Matusewicz, H.; Daszykowski, M. Identification of ground meat species using near-infrared spectroscopy and class modeling techniques—Aspects of optimization and validation using a one-class classification model. Meat Sci. 2018, 139, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.; Elbourne, A.; Truong, V.K.; Cozzolino, D. Shining light into meat—A review on the recent advances in in vivo and carcass applications of near infrared spectroscopy. Int. J. Food Sci. Technol. 2020, 55, 935–941. [Google Scholar] [CrossRef]
- Bai, Y.; Liu, H.; Zhang, B.; Zhang, J.; Wu, H.; Zhao, S.; Qie, M.; Guo, J.; Wang, Q.; Zhao, Y. Research Progress on Traceability and Authenticity of Beef. Food Rev. Int. 2021, 1–21. [Google Scholar] [CrossRef]
- Mamani-Linares, L.W.; Gallo, C.; Alomar, D. Identification of cattle, llama and horse meat by near infrared reflectance or transflectance spectroscopy. Meat Sci. 2012, 90, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Dumalisile, P.; Manley, M.; Hoffman, L.; Williams, P.J. Discriminating muscle type of selected game species using near infrared (NIR) spectroscopy. Food Control. 2020, 110, 106981. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, M.; Zhu, R.; Ma, R. Detection of adulteration in mutton using digital images in time domain combined with deep learning algorithm. Meat Sci. 2022, 192, 108850. [Google Scholar] [CrossRef]
- Cozzolino, D.; Murray, I. Identification of animal meat muscles by visible and near infrared reflectance spectroscopy. Lebensm.-Wiss. Und-Technol. 2004, 37, 447–452. [Google Scholar] [CrossRef]
- Cozzolino, D. Near Infrared Spectroscopy and Food Authenticity. In Advances in Food Traceability Techniques and Technologies; Elsevier: Amsterdam, The Netherlands, 2016; pp. 119–136. [Google Scholar] [CrossRef]
- López-Maestresalas, A.; Insausti, K.; Jarén, C.; Pérez-Roncal, C.; Urrutia, O.; Beriain, M.J.; Arazuri, S. Detection of minced lamb and beef fraud using NIR spectroscopy. Food Control 2019, 98, 465–473. [Google Scholar] [CrossRef]
- Boyaci, I.H.; Temiz, H.T.; Uysal, R.S.; Velioglu, H.M.; Yadegari, R.J.; Rishkan, M.M. A novel method for discrimination of beef and horsemeat using Raman spectroscopy. Food Chem. 2014, 148, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Kamruzzaman, M.; Makino, Y.; Oshita, S.; Liu, S. Assessment of Visible Near-Infrared Hyperspectral Imaging as a Tool for Detection of Horsemeat Adulteration in Minced Beef. Food Bioprocess Technol. 2015, 8, 1054–1062. [Google Scholar] [CrossRef]
- Qu, J.-H.; Liu, D.; Cheng, J.-H.; Sun, D.-W.; Ma, J.; Pu, H.; Zeng, X.-A. Applications of Near-infrared Spectroscopy in Food Safety Evaluation and Control: A Review of Recent Research Advances. Crit. Rev. Food Sci. Nutr. 2015, 55, 1939–1954. [Google Scholar] [CrossRef] [PubMed]
- Ropodi, A.I.; Pavlidis, D.E.; Mohareb, F.; Panagou, E.Z.; Nychas, G.-J.E. Multispectral image analysis approach to detect adulteration of beef and pork in raw meats. Food Res. Int. 2015, 67, 12–18. [Google Scholar] [CrossRef]
- Rady, A.; Adedeji, A. Assessing different processed meats for adulterants using visible-near-infrared spectroscopy. Meat Sci. 2018, 136, 59–67. [Google Scholar] [CrossRef]
- Cawthorn, D.-M.; Steinman, H.A.; Hoffman, L.C. A high incidence of species substitution and mislabelling detected in meat products sold in South Africa. Food Control 2013, 32, 440–449. [Google Scholar] [CrossRef] [Green Version]
- Edwards, K.; Manley, M.; Hoffman, L.C.; Williams, P.J. Non-Destructive Spectroscopic and Imaging Techniques for the Detection of Processed Meat Fraud. Foods 2021, 10, 448. [Google Scholar] [CrossRef]
- Ballin, R. Lametsch Analytical methods for authentication of fresh vs. thawed meat-A review. Meat Sci. 2008, 80, 151–158. [Google Scholar] [CrossRef]
- Coene, M.P.D.; Grinter, R.; Davies, A.M.C. The use of quadratic regression in qualitative near infrared and visible spectroscopic analysis. J. Near Infrared Spectrosc. 1996, 4, 153–161. [Google Scholar] [CrossRef]
- Bevin, C.; Fergusson, A.; Perry, W.; Janik, L.J.; Cozzolino, D. Development of a Rapid “Fingerprinting” System for Wine Authenticity by Mid-infrared Spectroscopy. J. Agric. Food Chem. 2006, 54, 9713–9718. [Google Scholar] [CrossRef]
- Bi, Y.; Li, S.; Zhang, L.; Li, Y.; He, W.; Tie, J.; Li, F.; Hao, X.; Tian, X.; Tang, L.; et al. Quality evaluation of flue-cured tobacco by near infrared spectroscopy and spectral similarity method. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 215, 398–404. [Google Scholar] [CrossRef]
- Saviztky, A.; Golay, M.J.E. Smoothing and differentiation of data by simplified least squares procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Bureau, S.; Cozzolino, D.; Clark, C.J. Contributions of Fourier-transform mid infrared (FT-MIR) spectroscopy to the study of fruit and vegetables: A review. Postharvest Biol. Technol. 2019, 148, 1–14. [Google Scholar] [CrossRef]
- Næs, T.; Isaksson, T.; Fearn, T.; Davies, T. A User-Friendly Guide to Multivariate Calibration and Classification, 2nd ed; NIR Chichester: Chichester, UK, 2002; Volume 6. [Google Scholar] [CrossRef]
- Williams, P.; Dardenne, P.; Flinn, P. Tutorial: Items to be include in a report on a near infrared spectroscopy project. J. Near Infrared Spectrosc. 2017, 25, 85–90. [Google Scholar] [CrossRef]
- Workman, J.; Weyer, L. Practical Guide to Interpretive Near-Infrared Spectroscopy; CRC Press Taylor and Francis Group: Boca Raton, FL, USA, 2008. [Google Scholar]
| Camel | Emu | Beef | Lamb | |
|---|---|---|---|---|
| Camel | 34 (92%) | 0 | 0 | 3 |
| Emu | 0 | 33 (89%) | 3 | 0 |
| Beef | 4 | 0 | 32 (86%) | 1 |
| Lamb | 2 | 0 | 3 | 31 (84%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cozzolino, D.; Bureš, D.; Hoffman, L.C. Evaluating the Use of a Similarity Index (SI) Combined with near Infrared (NIR) Spectroscopy as Method in Meat Species Authenticity. Foods 2023, 12, 182. https://doi.org/10.3390/foods12010182
Cozzolino D, Bureš D, Hoffman LC. Evaluating the Use of a Similarity Index (SI) Combined with near Infrared (NIR) Spectroscopy as Method in Meat Species Authenticity. Foods. 2023; 12(1):182. https://doi.org/10.3390/foods12010182
Chicago/Turabian StyleCozzolino, Daniel, Daniel Bureš, and Louwrens C. Hoffman. 2023. "Evaluating the Use of a Similarity Index (SI) Combined with near Infrared (NIR) Spectroscopy as Method in Meat Species Authenticity" Foods 12, no. 1: 182. https://doi.org/10.3390/foods12010182





