In Vitro Assessment of the Prebiotic Potential of Xylooligosaccharides from Barley Straw
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material and Pretreatment
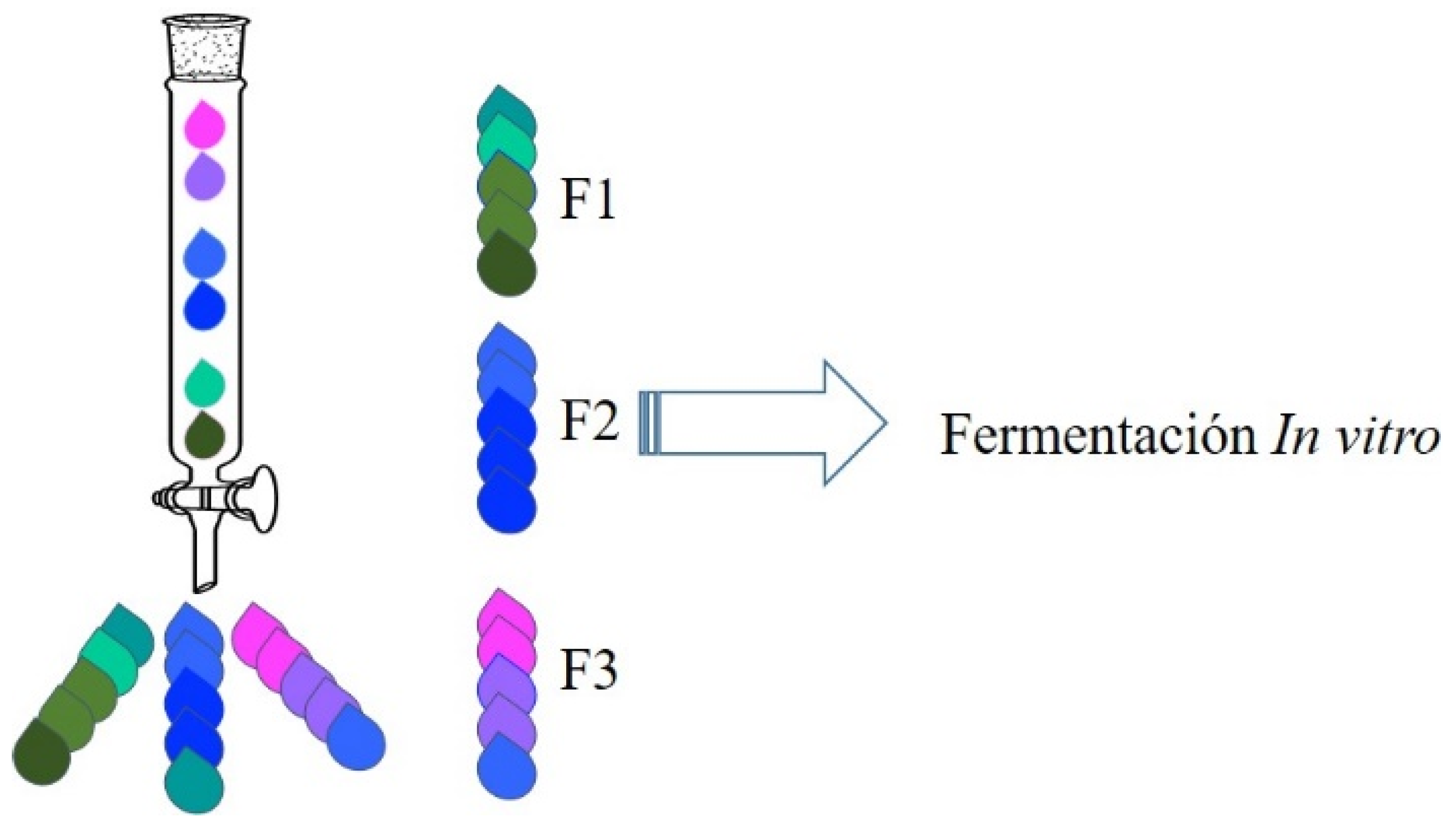
2.2. Fractionation and Purification of the Liquid Fraction
2.3. Fermentation of XOS
2.4. Analytical Methods
2.4.1. Sugars, Phenolic Compounds and Degradation Products
2.4.2. Determination of Short Chain Fatty Acids (SCFAs)
2.4.3. Structural Characterisation of Xylooligosaccharides
High-Performance Size Exclusion Chromatography (HPSEC) and High-Performance Anion Exchange Chromatography with Pulsed Amperometric Detection (HPAEC-PAD)
Matrix-Assisted Laser Desorption/Ionization-Time of Flight-Mass Spectrometry (MALDI-TOF-MS)
Attenuated Total Reflection-Fourier Transformed Infrared Radiation (FTIR) Spectroscopy
2.5. Statistical Analysis
3. Results and Discussion
3.1. Liquid Fraction from Steam-Exploded Barley Straw
3.2. Fractionation Step
3.3. Structural Characterisation of Xylooligosaccharides in F2
3.3.1. MALDI-TOF-MS
3.3.2. FTIR
3.4. Analysis of Prebiotic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Nie, Y.; Lu, X.; Zhang, X.; He, H.; Fengjiao, P.; Le, Z.; Liu, X.; Ji, X.; Zhang, S. Cascade Utilization of Lignocellulosic Biomass to High-Value Products. Green Chem. 2019, 21, 3499–3535. [Google Scholar] [CrossRef]
- Palaniappan, A.; Antony, U.; Emmambux, M.N. Current Status of Xylooligosaccharides: Production, Characterization, Health Benefits and Food Application. Trends Food Sci. Technol. 2021, 5000, 506–519. [Google Scholar] [CrossRef]
- Álvarez, C.; González, A.; Negro, M.J.; Ballesteros, I.; Oliva, J.M.; Sáez, F. Optimized Use of Hemicellulose within a Biorefinery for Processing High Value-Added Xylooligosaccharides. Ind. Crops Prod. 2017, 99, 41–48. [Google Scholar] [CrossRef]
- Álvarez, C.; González, A.; Ballesteros, I.; Negro, M.J. Production of Xylooligosaccharides, Bioethanol, and Lignin from Structural Components of Barley Straw Pretreated with a Steam Explosion. Bioresour. Technol. 2021, 342, 125953. [Google Scholar] [CrossRef]
- Gullón, P.; González-Muñoz, M.J.; van Gool, M.P.; Schols, H.A.; Hirsch, J.; Ebringerová, A.; Parajó, J.C. Production, Refining, Structural Characterization and Fermentability of Rice Husk Xylooligosaccharides. J. Agric. Food Chem. 2010, 58, 3632–3641. [Google Scholar] [CrossRef]
- Ávila, P.F.; Martins, M.; de Almeida Costa, F.A.; Goldbeck, R. Xylooligosaccharides Production by Commercial Enzyme Mixture from Agricultural Wastes and Their Prebiotic and Antioxidant Potential. Bioact. Carbohydr. Diet. Fibre 2020, 24, 100234. [Google Scholar] [CrossRef]
- Singh, R.D.; Nadar, C.G.; Muir, J.; Arora, A. Green and Clean Process to Obtain Low Degree of Polymerisation Xylooligosaccharides from Almond Shell. J. Clean. Prod. 2019, 241, 118237. [Google Scholar] [CrossRef]
- Pham, T.; Thomas Teoh, K.; Savary, B.J.; Chen, M.H.; McClung, A.; Lee, S.O. In Vitro Fermentation Patterns of Rice Bran Components by Human Gut Microbiota. Nutrients 2017, 9, 1237. [Google Scholar] [CrossRef] [Green Version]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Bonif, T.; Catarino, M.D.; Vilas-boas, A.A.; Ribeiro, T.B.; Campos, D.A.; Teixeira, J.A.; Pintado, M. Impact of Circular Brewer’s Spent Grain Flour after In Vitro Gastrointestinal Digestion on Human Gut Microbiota. Foods 2022, 11, 2279. [Google Scholar]
- Chen, Y.; Xie, Y.; Ajuwou, K.M.; Zhong, R.; Li, T.; Chen, L.; Zhang, H.; Beckers, Y. Evaraet Xylo-Oligosaccharides, Preparation and Application to Human and Animal Health:A Review. Front. Nutr. 2021, 8, 731930. [Google Scholar] [CrossRef] [PubMed]
- Khat-udomkiri, N.; Toejing, P.; Sirilun, S.; Chaiyasut, C.; Lailerd, N. Antihyperglycemic Effect of Rice Husk Derived Xylooligosaccharides in High-Fat Diet and Low-Dose Streptozotocin-Induced Type 2 Diabetic Rat Model. Food Sci. Nutr. 2020, 8, 428–444. [Google Scholar] [CrossRef] [PubMed]
- Childs, C.E.; Röytiö, H.; Alhoniemi, E.; Fekete, A.A.; Forssten, S.D.; Hudjec, N.; Lim, Y.N.; Steger, C.J.; Yaqoob, P.; Tuohy, K.M.; et al. Xylo-Oligosaccharides Alone or in Synbiotic Combination with Bifidobacterium Animalis Subsp. Lactis Induce Bifidogenesis and Modulate Markers of Immune Function in Healthy Adults: A Double-Blind, Placebo-Controlled, Randomised, Factorial Cross-over Study. Br. J. Nutr. 2014, 111, 1945–1956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santibañez, L.; Herníquez, C.; Corro-Tejeda, R.; Bernal, S.; Armijo, B.; Salazar, O. Xylooligosaccharides from Lignocellulosic Biomass: A Comprehensive Review. Carbohydr. Polym. 2021, 251, 117118. [Google Scholar] [CrossRef]
- de Capetti, C.C.M.; Vacilotto, M.M.; Dabul, A.N.G.; Sepulchro, A.G.V.; Pellegrini, V.O.A.; Polikarpov, I. Recent Advances in the Enzymatic Production and Applications of Xylooligosaccharides. World J. Microbiol. Biotechnol. 2021, 37, 169. [Google Scholar] [CrossRef]
- Turck, D.; Bresson, J.L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of Xylo-Oligosaccharides (XOS) as a Novel Food Pursuant to Regulation (EU) 2015/2283. EFSA J. 2018, 16, 5361. [Google Scholar] [CrossRef] [Green Version]
- Moreno, A.D.; Tomás-Pejó, E.; Ballesteros, M.; Negro, M.J. Pretreatment Technologies for Lignocellulosic Biomass Deconstruction within a Biorefinery Perspective. In Biofuels: Alternative Feedstocks and Conversion Processes for the Production of Liquid and Gaseous Biofuels; Pandey, A., Larroche, C., Dussap, C.-G., Gnansounou, E., Khanal, S.K., Ricke, S.C., Eds.; Academic Press: Amsterdam, The Netherlands, 2019; pp. 379–399. [Google Scholar]
- Yue, P.; Hu, Y.; Tian, R.; Bian, J.; Peng, F. Hydrothermal Pretreatment for the Production of Oligosaccharides: A Review. Bioresour. Technol. 2022, 343, 126075. [Google Scholar] [CrossRef]
- Huang, C.; Wang, X.; Liang, C.; Jiang, X.; Yang, G.; Xu, J.; Yong, Q. A Sustainable Process for Procuring Biologically Active Fractions of High-Purity Xylooligosaccharides and Water-Soluble Lignin from Moso Bamboo Prehydrolyzate. Biotechnol. Biofuels 2019, 12, 189. [Google Scholar] [CrossRef] [Green Version]
- Sluiter, J.B.; Ruiz, R.O.; Scarlata, C.J.; Sluiter, A.D.; Templeton, D.W. Compositional Analysis of Lignocellulosic Feedstocks. 1. Review and Description of Methods. J. Agric. Food Chem. 2010, 58, 9043–9053. [Google Scholar] [CrossRef]
- Negro, M.J.; Álvarez, C.; Doménech, P.; Iglesias, R.; Ballesteros, I. Sugars Production from Municipal Forestry and Greening Wastes Pretreated by an Integrated Steam Explosion-Based Process. Energies 2020, 13, 4432. [Google Scholar] [CrossRef]
- Duque, A.; Manzanares, P.; Ballesteros, I.; Ballesteros, M. Steam Explosion as Lignocellulosic Biomass Pretreatment. In Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery; Mussatto, S.I., Ed.; Elservier: Amsterdam, The Netherlands, 2016; pp. 349–368. ISBN 9780128025611. [Google Scholar]
- Álvarez, C.; Sáez, F.; González, A.; Ballesteros, I.; Oliva, J.M.; Negro, M.J. Production of Xylooligosaccharides and Cellulosic Ethanol from Steam-Exploded Barley Straw. Holzforschung 2018, 73, 35–44. [Google Scholar] [CrossRef]
- Negro, M.J.; Álvarez, C.; Ballesteros, I.; Romero, I.; Ballesteros, M.; Castro, E.; Manzanares, P.; Moya, M.; Oliva, J.M. Ethanol Production from Glucose and Xylose Obtained from Steam Exploded Water-Extracted Olive Tree Pruning Using Phosphoric Acid as Catalyst. Bioresour. Technol. 2014, 153, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Basit, A.; Miao, T.; Liu, J.; Wen, J.; Song, L.; Zheng, F.; Lou, H.; Jiang, W. Highly Efficient Degradation of Xylan into Xylose by a Single Enzyme. ACS Sustain. Chem. Eng. 2019, 7, 11360–11368. [Google Scholar] [CrossRef]
- Gullón, B.; Gullón, P.; Tavaria, F.; Pintado, M.; Gomes, A.M.; Alonso, J.L.; Parajó, J.C. Structural Features and Assessment of Prebiotic Activity of Refined Arabinoxylooligosaccharides from Wheat Bran. J. Funct. Foods 2014, 6, 438–449. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, H.; Jiao, N.; Xu, G.; Xu, Y. Fractionation of Poplar Using Hydrothermal and Acid Hydrotropic Pretreatments for Co-Producing Xylooligosaccharides, Fermentable Sugars, and Lignin Nanoparticles. Ind. Crop. Prod. 2022, 181, 114853. [Google Scholar] [CrossRef]
- Bhatia, R.; Lad, J.B.; Bosch, M.; Bryant, D.N.; Leak, D.; Hallett, J.P.; Franco, T.T.; Gallagher, J.A. Production of Oligosaccharides and Biofuels from Miscanthus Using Combinatorial Steam Explosion and Ionic Liquid Pretreatment. Bioresour. Technol. 2021, 323, 124625. [Google Scholar] [CrossRef]
- Moure, A.; Gullón, P.; Domínguez, H.; Parajó, J.C. Advances in the Manufacture, Purification and Applications of Xylo-Oligosaccharides as Food Additives and Nutraceuticals. Process Biochem. 2006, 41, 1913–1923. [Google Scholar] [CrossRef]
- Xiao, X.; Wen, J.; Wang, Y.; Bian, J.; Li, M.; Peng, F.; Sun, R. NMR and ESI—MS Spectrometry Characterization of Autohydrolysis Xylo- Oligosaccharides Separated by Gel Permeation Chromatography. Carbohydr. Polym. 2018, 195, 303–310. [Google Scholar] [CrossRef]
- Cara, C.; Ruiz, E.; Carvalheiro, F.; Moura, P.; Ballesteros, I.; Castro, E.; Gírio, F. Production, Purification and Characterisation of Oligosaccharides from Olive Tree Pruning Autohydrolysis. Ind. Crops Prod. 2012, 40, 225–231. [Google Scholar] [CrossRef] [Green Version]
- Xiang, Z.; Tang, N.; Jin, X.; Gao, W. Fabrications and Applications of Hemicellulose-Based Bio-Adsorbents. Carbohydr. Polym. 2022, 278, 118945. [Google Scholar] [CrossRef]
- Rastall, R.A.; Gibson, G.R.; Gill, H.S.; Guarner, F.; Klaenhammer, T.R.; Pot, B.; Reid, G.; Rowland, I.R.; Sanders, M.E. Modulation of the Microbial Ecology of the Human Colon by Probiotics, Prebiotics and Synbiotics to Enhance Human Health: An Overview of Enabling Science and Potential Applications. FEMS Microbiol. Ecol. 2005, 52, 145–152. [Google Scholar] [CrossRef]
- Álvarez, C.; González, A.; Alonso, J.L.; Sáez, F.; Negro, M.J.; Gullón, B. Xylooligosaccharides from Steam-Exploded Barley Straw: Structural Features and Assessment of Bifidogenic Properties. Food Bioprod. Process. 2020, 124, 131–142. [Google Scholar] [CrossRef]
- Kabel, M.A.; Kortenoeven, L.; Schols, H.A.; Voragen, A.G.J. In Vitro Fermentability of Differently Substituted Xylo-Oligosaccharides. J. Agric. Food Chem. 2002, 50, 6205–6210. [Google Scholar] [CrossRef]
- Pastell, H.; Westermann, P.; Meyer, A.S.; Tuomainen, P.; Tenkanen, M. In Vitro Fermentation of Arabinoxylan-Derived Carbohydrates by Bifidobacteria and Mixed Fecal Microbiota. J. Agric. Food Chem. 2009, 57, 8598–8606. [Google Scholar] [CrossRef]
- Moniz, P.; Pereira, H.; Quilhó, T.; Carvalheiro, F. Characterisation and Hydrothermal Processing of Corn Straw towards the Selective Fractionation of Hemicelluloses. Ind. Crops Prod. 2013, 50, 145–153. [Google Scholar] [CrossRef]
- Sun, R.; Fang, J.M.; Goodwin, A.; Lawther, J.M.; Bolton, A.J. Isolation and Characterization of Polysaccharides from Abaca Fiber. J. Agric. Food Chem. 1998, 46, 2817–2822. [Google Scholar] [CrossRef]
- Sun, X.F.; Xu, F.; Sun, R.C.; Geng, Z.C.; Fowler, P.; Baird, M.S. Characteristics of Degraded Hemicellulosic Polymers Obtained from Steam Exploded Wheat Straw. Carbohydr. Polym. 2005, 60, 15–26. [Google Scholar] [CrossRef]
- Dávila, I.; Gullón, B.; Alonso, J.L.; Labidi, J.; Gullón, P. Vine Shoots as New Source for the Manufacture of Prebiotic Oligosaccharides. Carbohydr. Polym. 2019, 207, 34–43. [Google Scholar] [CrossRef]
- Zhang, P.; Dong, S.J.; Ma, H.H.; Zhang, B.X.; Wang, Y.F.; Hu, X.M. Fractionation of Corn Stover into Cellulose, Hemicellulose and Lignin Using a Series of Ionic Liquids. Ind. Crops Prod. 2015, 76, 688–696. [Google Scholar] [CrossRef]
- Chen, J.; Wang, L.; Su, X.; Wang, K.; Wu, X.; Chen, L.; Xiong, X.; Zhou, H.; Liu, Y. Structure, Morphology, Thermostability and Irradiation-Mediated Degradation Fractions of Hemicellulose Treated with Γ-Irradiation. Waste Biomass Valorization 2016, 7, 1415–1425. [Google Scholar] [CrossRef]
- Fang, J.M.; Sun, R.C.; Tomkinson, J. Isolation and Characterization of Hemicelluloses and Cellulose from Rye Straw by Alkaline Peroxide Extraction. Cellulose 2000, 7, 87–107. [Google Scholar] [CrossRef]
- Buruiana, C.T.; Gómez, B.; Vizireanu, C.; Garrote, G. Manufacture and Evaluation of Xylooligosaccharides from Corn Stover as Emerging Prebiotic Candidates for Human Health. LWT-Food Sci. Technol. 2017, 77, 449–459. [Google Scholar] [CrossRef]
- Míguez, B.; Gullón, P.; Cotos-Yáñez, T.; Massot-Cladera, M.; Pérez-Cano, F.J.; Vila, C.; Alonso, J.L. Manufacture and Prebiotic Potential of Xylooligosaccharides Derived From Eucalyptus nitens Wood. Front. Chem. Eng. 2021, 3, 670440. [Google Scholar] [CrossRef]
- Moniz, P.; Ho, A.L.; Duarte, L.C.; Kolida, S.; Rastall, R.A.; Pereira, H.; Carvalheiro, F. Assessment of the Bifidogenic Effect of Substituted Xylo-Oligosaccharides Obtained from Corn Straw. Carbohydr. Polym. 2016, 136, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Shukla, G. Metabiotics: One Step Ahead of Probiotics; an Insight into Mechanisms Involved in Anticancerous Effect in Colorectal Cancer. Front. Microbiol. 2016, 7, 1940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz, E.; Gullón, B.; Moura, P.; Carvalheiro, F.; Eibes, G.; Cara, C.; Castro, E. Bifidobacterial Growth Stimulation by Oligosaccharides Generated from Olive Tree Pruning Biomass. Carbohydr. Polym. 2017, 169, 149–156. [Google Scholar] [CrossRef]
- Zeng, H. Mechanisms Linking Dietary Fiber, Gut Microbiota and Colon Cancer Prevention. World J. Gastrointest. Oncol. 2014, 6, 41. [Google Scholar] [CrossRef]
- Moles, L.; Otaegui, D. The Impact of Diet on Microbiota Evolution and Human Health. Is Diet an Adequate Tool for Microbiota Modulation? Nutrients 2020, 12, 1654. [Google Scholar] [CrossRef]
- Hernández-Hernández, O.; Côté, G.L.; Kolida, S.; Rastall, R.A.; Sanz, M.L. In Vitro Fermentation of Alternansucrase Raffinose-Derived Oligosaccharides by Human Gut Bacteria. J. Agric. Food Chem. 2011, 59, 10901–10906. [Google Scholar] [CrossRef]
- den Besten, G.; Lange, K.; Havinga, R.; van Dijk, T.H.; Gerding, A.; van Eunen, K.; Müller, M.; Groen, A.K.; Hooiveld, G.J.; Bakker, B.M.; et al. Gut-Derived Short-Chain Fatty Acids Are Vividly Assimilated into Host Carbohydrates and Lipids. Am. J. Physiol.-Gastrointest. Liver Physiol. 2013, 305, 900–910. [Google Scholar] [CrossRef]
- Xu, J.; Wang, R.; Zhang, H.; Wu, J.; Zhu, L.; Zhan, X. In Vitro Assessment of Prebiotic Properties of Oligosaccharides Derived from Four Microbial Polysaccharides. Lebensm.-Wiss. Technol. 2021, 147, 111544. [Google Scholar] [CrossRef]



| Fraction | Oligosaccharides (g/L) | Monosaccharides (g/L) | ||||
|---|---|---|---|---|---|---|
| GOS | XOS | AOS | Glucose | Xylose | Arabinose | |
| F1 | 4.8 | 8.8 | 1.0 | 0 | 0 | 0 |
| F2 | 0.1 | 10.9 | 0.4 | 0.8 | 0.4 | 0.2 |
| F3 | 0 | 0.16 | 0.2 | 0.3 | 3.0 | 2.0 |
| m/z | Structure | m/z | Structure |
|---|---|---|---|
| 569 | [P4Na]+ | 833 | [P6Na]+ |
| 611 | [P4AcNa]+ | 875 | [P6AcNa]+ |
| 653 | [P4Ac2Na]+ | 917 | [P6Ac2Na]+ |
| 701 | [P5Na]+ | 965 | [P7Na]+ |
| 743 | [P5AcNa]+ | 1007 | [P7AcNa]+ |
| 785 | [P5Ac2Na]+ | 1097 | [P8Na]+ |
| Carbon Source | Time (h) | Lactic Acid (mM) | Acetic Acid (mM) | Formic Acid (mM) | Butyric Acid (mM) | Propionic Acid (mM) | SCFAs Total (mM) | pH |
|---|---|---|---|---|---|---|---|---|
| Control | 0 | 0.3 ± 0.5 | 2.0 ± 0.8 | 1.6 ± 0.7 | 0.4 ± 0.4 | 2.34 ± 0.14 | 4.7 ± 1.1 | 6.9 ± 0.04 |
| 4 | 0.04 ± 0.08 | 2.5 ± 0.6 | 0.5 ± 0.9 | 0.3 ± 0.2 | 2.5 ± 0.4 | 5.4 ± 1.3 | 6.9 ± 0.05 | |
| 7 | 0.0 ± 0.0 | 5.8 ± 2.5 | 0.2± 0.3 | 1.0 ± 0.7 | 3.5 ± 0.4 | 10.3 ± 3.5 | 7.1 ± 0.07 | |
| 10 | 0.0 ± 0.0 | 8.5 ± 3.3 | 0.0 ± 0.00 | 1.6 ± 1.0 | 4.3 ± 0.4 | 14.8 ± 3.9 | 7.2 ± 0.2 | |
| 24 | 0.0 ± 0.0 | 18.8 ± 1.2 | 0.0 ± 0.0 | 5.3 ± 0.6 | 6.6 ± 0.3 | 30.8 ± 1.3 | 7.1 ± 0.7 | |
| 30 | 0.0 ± 0.0 | 17.3 ± 1.8 | 0.17 ± 0.3 | 4.7 ± 0.4 | 5.5 ± 1.1 | 27.6 ± 3.2 | 6.7 ± 0.08 | |
| FOS | 0 | 0.44 ± 0.07 a | 3.4 ± 2.2 a | 1.8 ± 0.3 a | 1.3 ± 1.5 a | 2.5 ± 0.4 a | 7.2 ± 3.7 a | 6.9 ± 0.04 |
| 4 | 0.8 ± 0.4 a | 4.4 ± 2.0 a | 2.6 ± 2.1 a | 2.5 ± 2.7 a | 2.8 ± 0.7 a | 9.7 ± 0.75 a | 6.7 ± 0.1 | |
| 7 | 8.3 ± 6.5 a | 23.2 ± 12.0 a | 10.2 ± 5.2 a | 6.2 ± 4.1 a | 5.6 ± 2,7 a | 35.0 ± 17.0 a | 6.1 ± 1.0 | |
| 10 | 6.7 ± 7.3 a | 33.5 ± 5.5 a | 12.5 ± 4.6 a.b | 11.6 ± 3.1 a | 9.4 ± 2,4 a | 54.4 ± 7.7 a | 5.1 ± 0.8 | |
| 24 | 5.6 ± 9.4 a | 40.4 ± 3.0 a | 14.6 ± 2.3 a | 18.4 ± 3.3 a | 13.7 ± 5.6 ab | 72.5 ± 6.8 a | 5.0 ± 0.3 | |
| 30 | 4.4 ± 7.6 a | 38.3 ± 4.5 a | 9.5 ± 2.4 a | 15.0 ± 5.4 a.b | 9.7 ± 4.9 ab | 63.0 ± 13.3 a | 4.9 ± 0.2 | |
| F2 | 0 | 0.9 ± 1.5 | 3.6 ± 2.1 a | 4.0 ± 1.7 a | 0.17 ± 0.3 a | 2.1 ± 0.2 a | 5.9 ± 2.2 a | 7.4 ± 0.03 |
| 4 | 1.0 ± 0.29 | 6.1 ± 2.3 a | 2.1 ± 0.9 a | 0.6 ± 0.4 a | 2.9 ± 0.8 a | 9.6 ± 3.2 a | 7.2 ± 0.03 | |
| 7 | 3.1 ± 1.60 | 29.7 ± 12.9 a.b | 1.9 ± 0.95 b | 3.8 ± 2.6 a | 6.6 ± 2.6 a | 40.2 ± 17.3 a | 6.5 ± 0.2 | |
| 10 | 2.5 ± 2.6 | 51.5 ± 1.5 b | 4.1 ± 1.2 c | 8.2 ± 3.5 a | 10.6 ± 1.3 a | 70.3 ± 4.2 a | 5.8 ± 0.1 | |
| 24 | 0.0 ± 0.0 | 63.1 ± 3.8 c | 0.6 ± 0.5 c | 12.5 ± 2.3 b | 11.9 ± 3.0 a,b | 87.5 ± 3.2 ac | 6.0 ± 0.08 | |
| 30 | 0.0 ± 0.0 | 65.2 ± 5.2 b.c | 0 ± 0 b | 12.7 ± 3.2 a.b | 12.2 ± 2.5 a,b | 90.1 ± 2.3 b | 5.9 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez, C.; González, A.; Ballesteros, I.; Gullón, B.; Negro, M.J. In Vitro Assessment of the Prebiotic Potential of Xylooligosaccharides from Barley Straw. Foods 2023, 12, 83. https://doi.org/10.3390/foods12010083
Álvarez C, González A, Ballesteros I, Gullón B, Negro MJ. In Vitro Assessment of the Prebiotic Potential of Xylooligosaccharides from Barley Straw. Foods. 2023; 12(1):83. https://doi.org/10.3390/foods12010083
Chicago/Turabian StyleÁlvarez, Cristina, Alberto González, Ignacio Ballesteros, Beatriz Gullón, and María José Negro. 2023. "In Vitro Assessment of the Prebiotic Potential of Xylooligosaccharides from Barley Straw" Foods 12, no. 1: 83. https://doi.org/10.3390/foods12010083





