Glycomacropeptide Protects against Inflammation and Oxidative Stress, and Promotes Wound Healing in an Atopic Dermatitis Model of Human Keratinocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and AD Model of Keratinocyte
2.2. MTT Assay
2.3. Cell Apoptosis Assay
2.4. Nitric Oxide Determination
2.5. Measurement of Cellular Hydroperoxide Lipids
2.6. RNA Extraction, Reverse Transcription, and qPCR
2.7. Wound Healing Assay
2.8. Statistical Analysis
3. Results
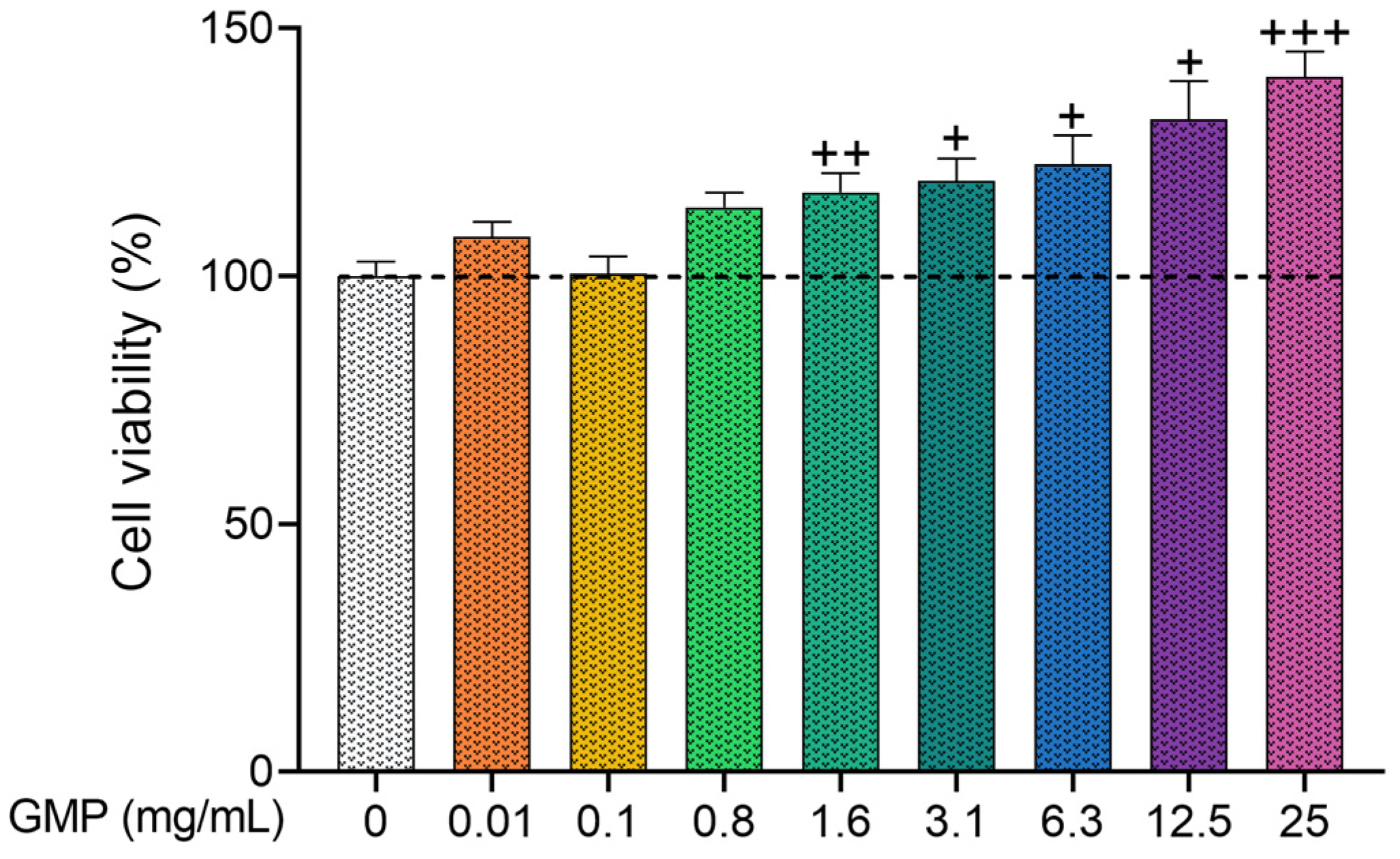
3.1. GMP Does Not Present Cytotoxic Activity on HaCaT Cells
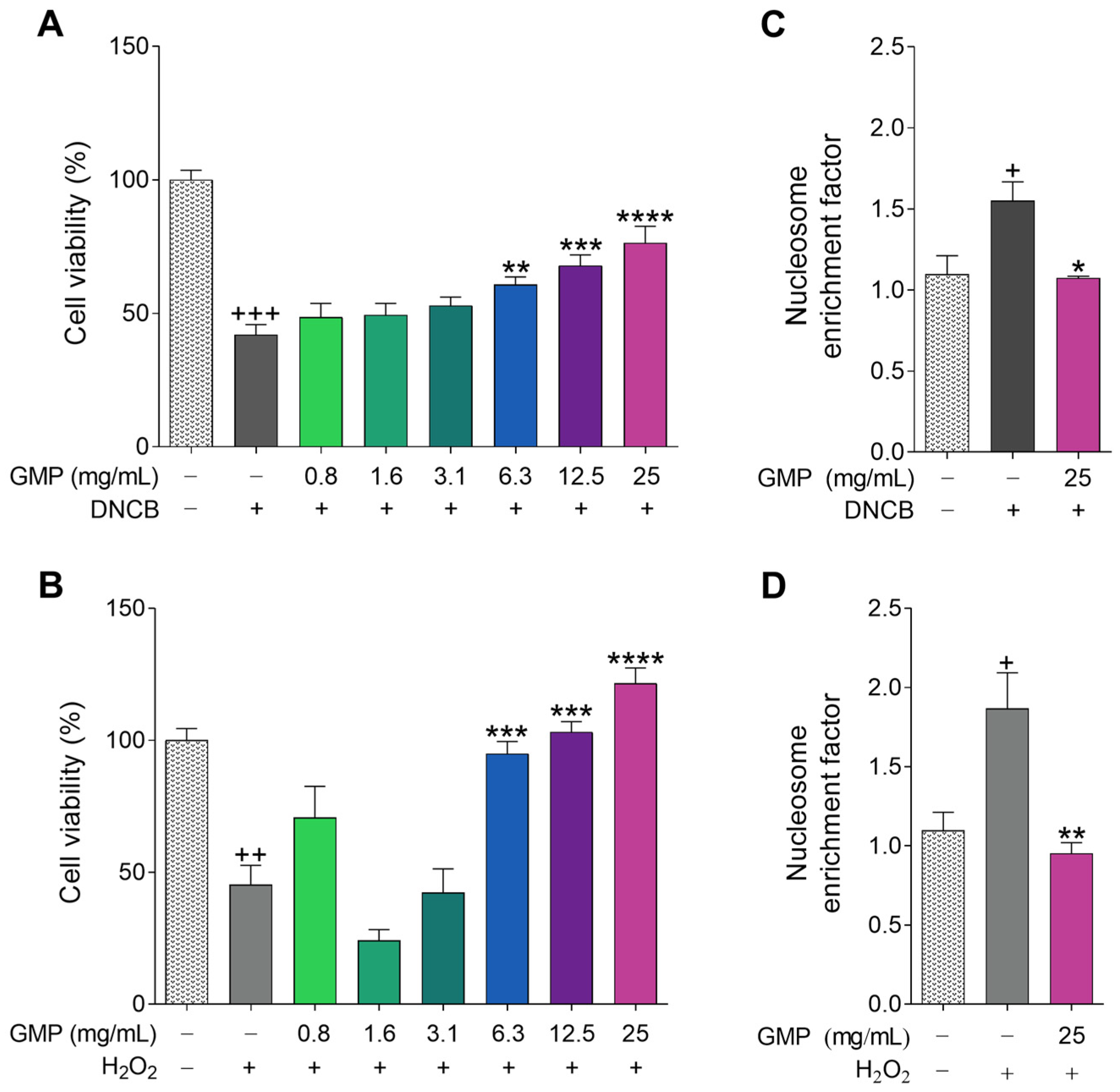
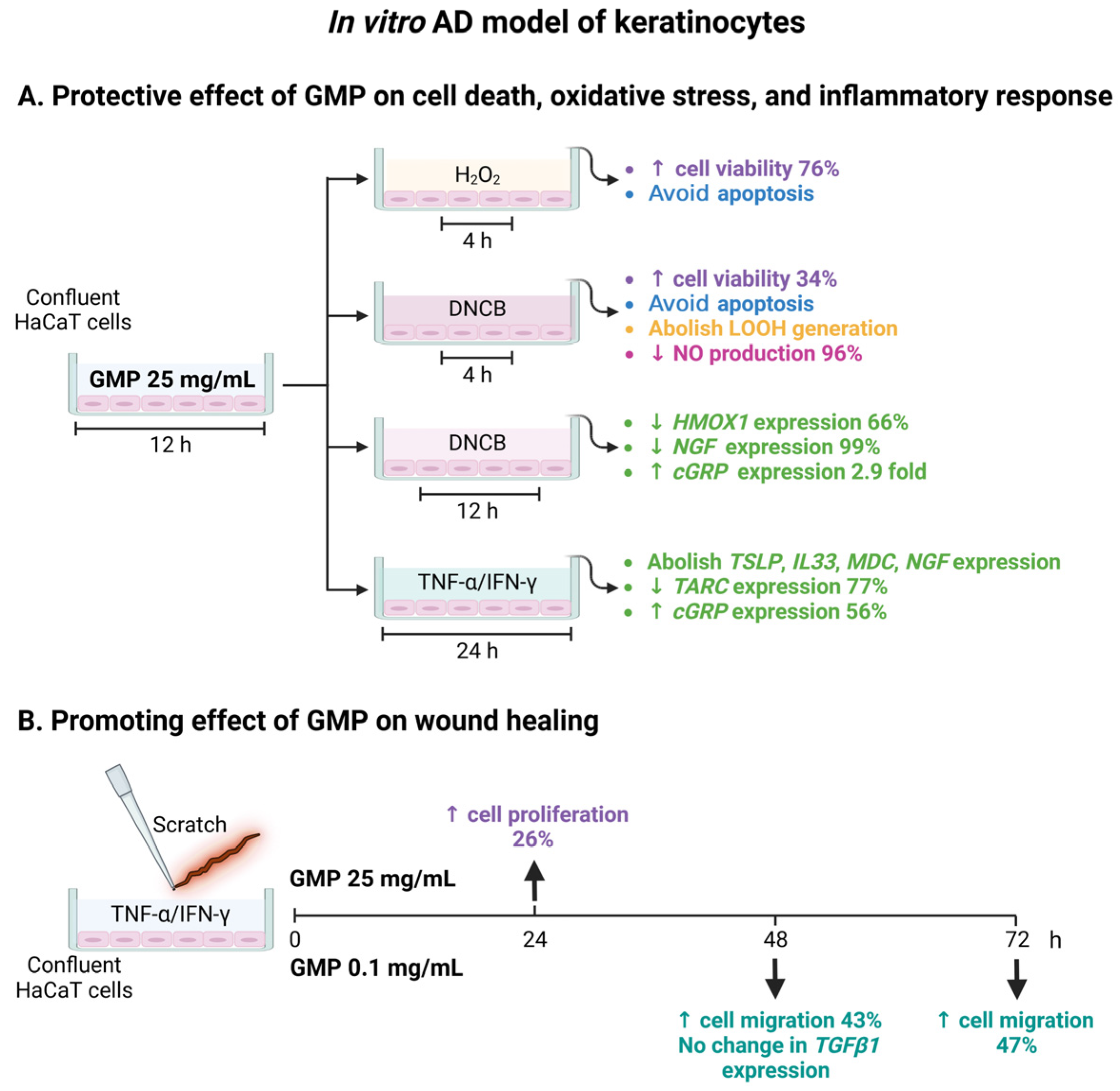
3.2. Protective Activity of GMP against Cell Death and Apoptosis
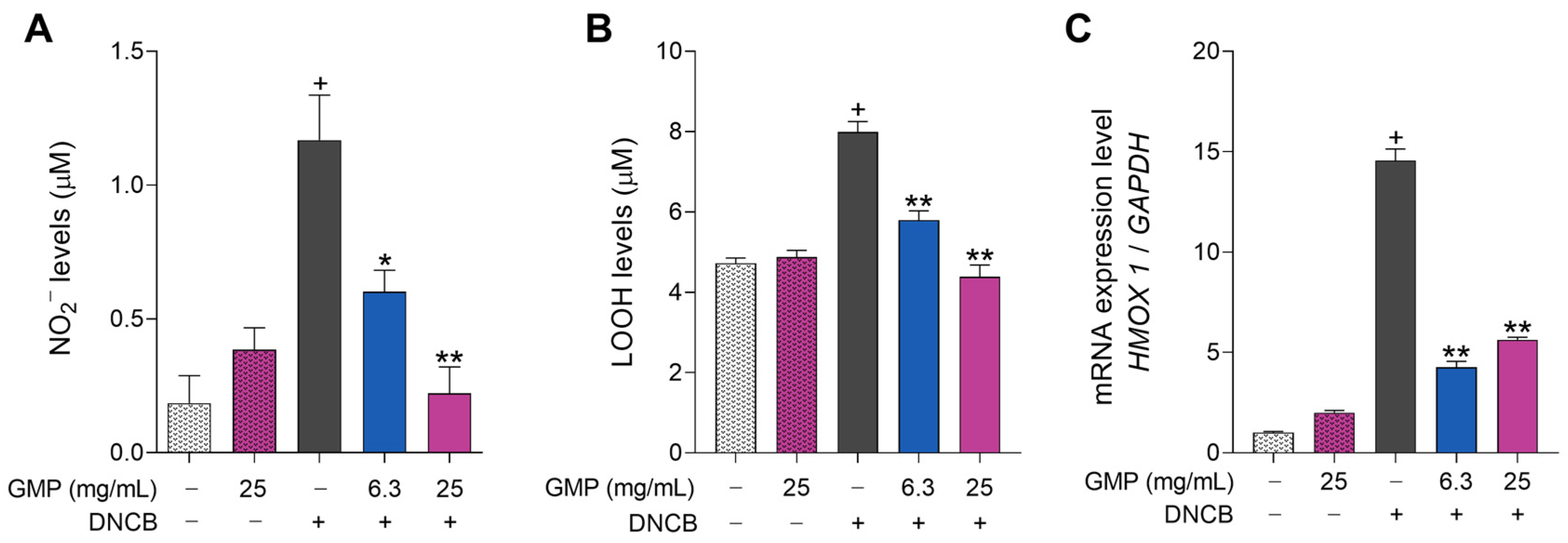
3.3. GMP Protects Keratinocytes from Oxidative Damage
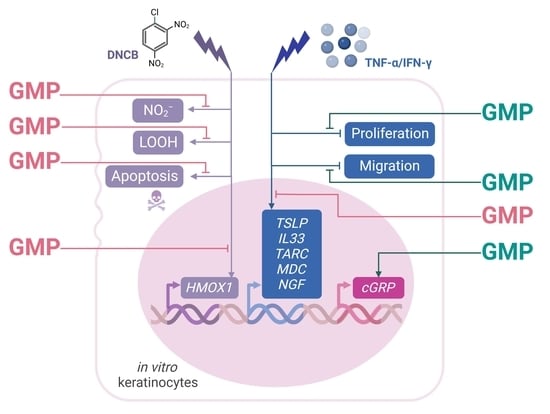
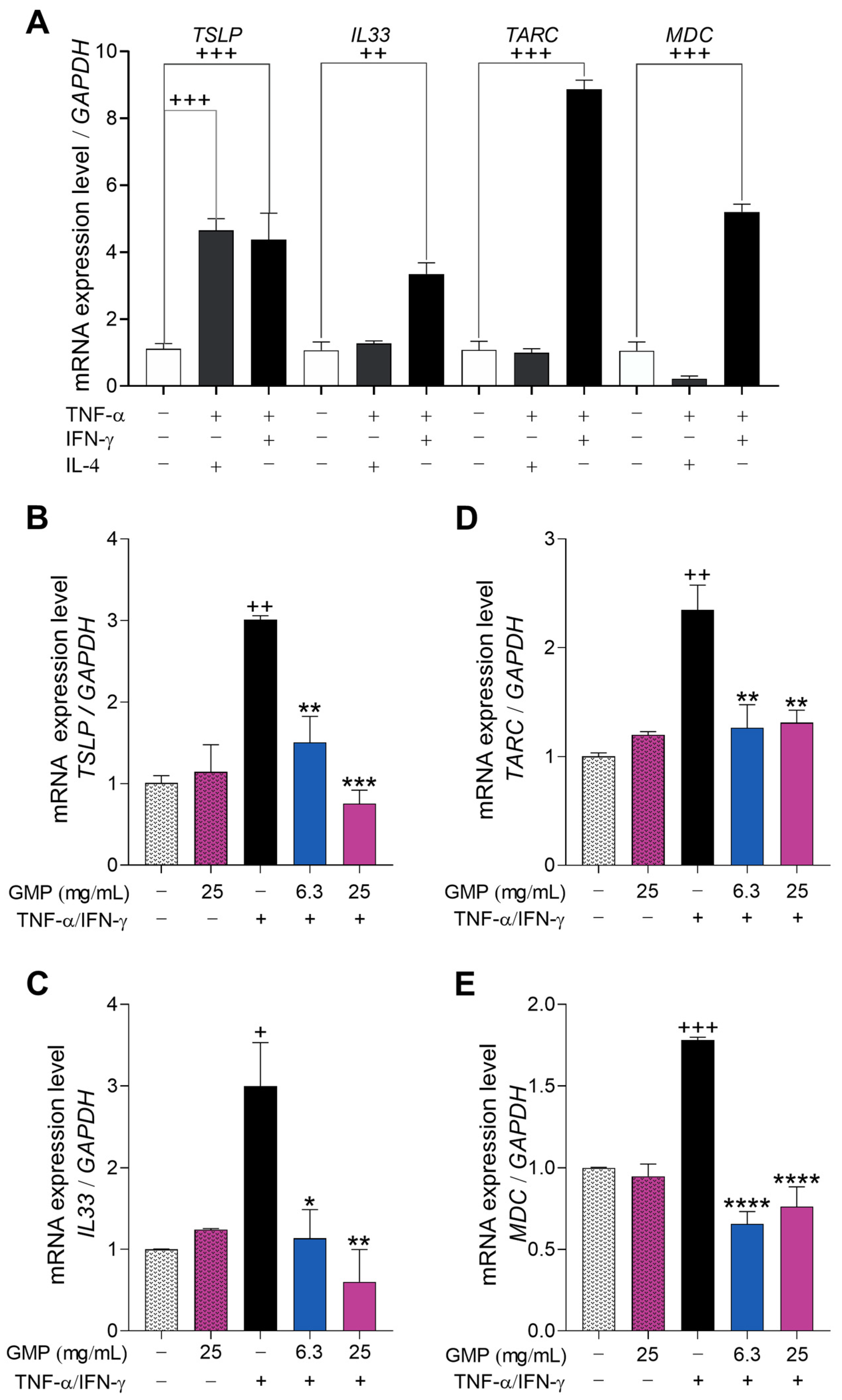
3.4. GMP Down-Regulates Gene Expression Associated with Type-2 Inflammatory Response in Keratinocytes
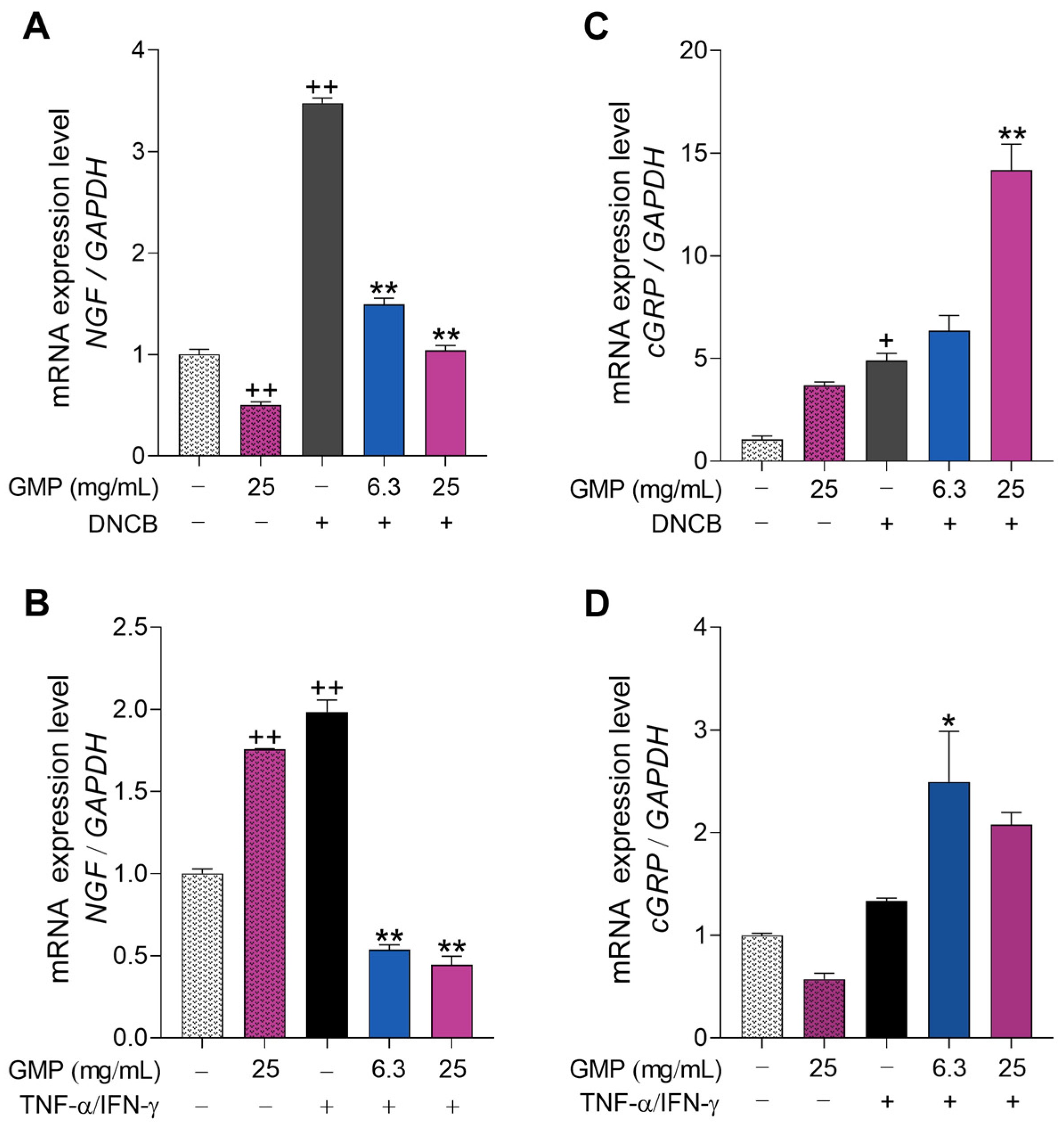
3.5. GMP Modifies Gene Expression Related to Itch and Neurogenic Inflammation
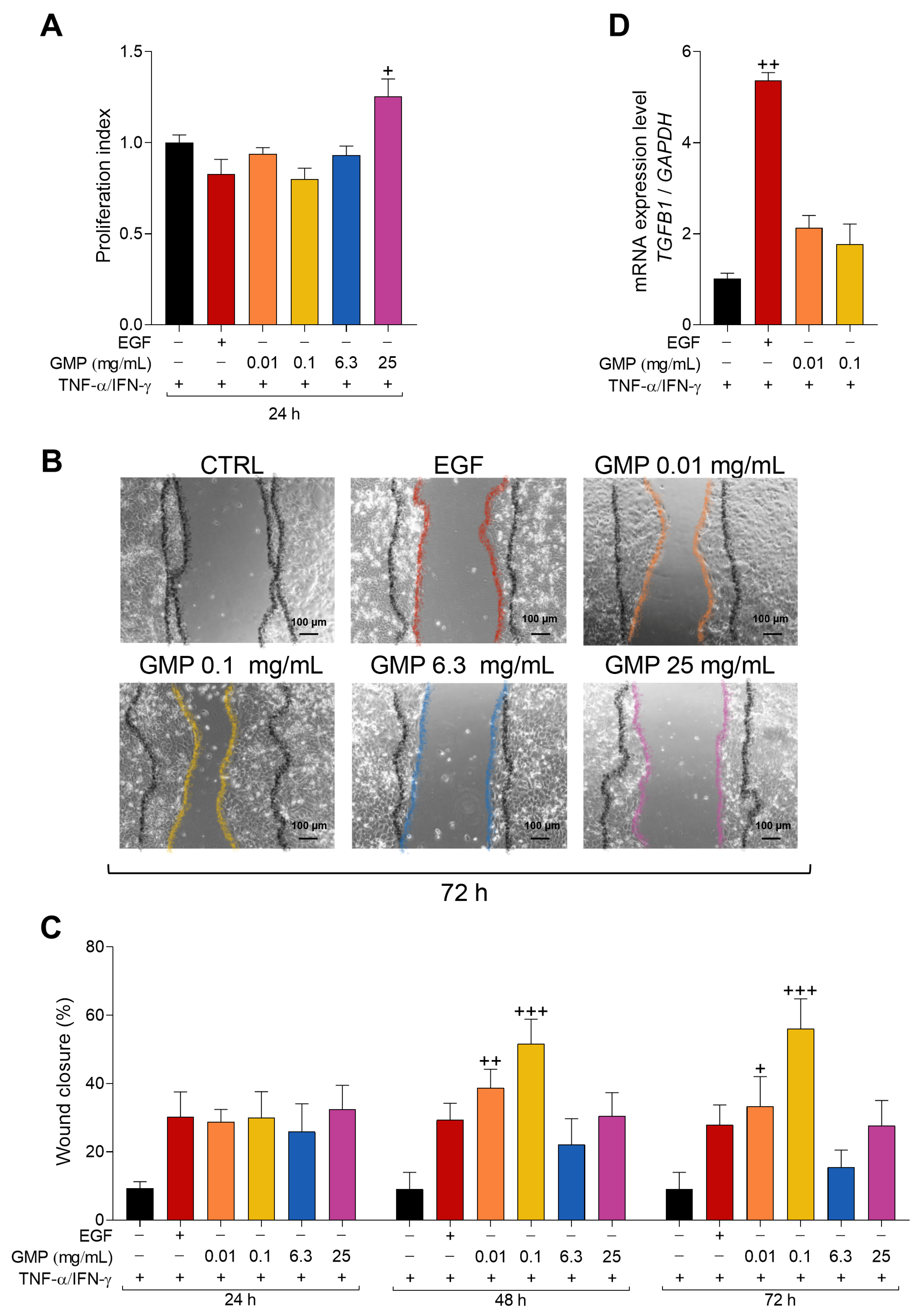
3.6. GMP Improves Wound Healing in an In Vitro AD Model of Keratinocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gallegos-Alcalá, P.; Jiménez, M.; Cervantes-García, D.; Salinas, E. The Keratinocyte as a Crucial Cell in the Predisposition, Onset, Progression, Therapy and Study of the Atopic Dermatitis. Int. J. Mol. Sci. 2021, 22, 10661. [Google Scholar] [CrossRef] [PubMed]
- Hadi, H.A.; Tarmizi, A.I.; Khalid, K.A.; Gajdács, M.; Aslam, A.; Jamshed, S. The Epidemiology and Global Burden of Atopic Dermatitis: A Narrative Review. Life 2021, 11, 936. [Google Scholar] [CrossRef]
- Barbarot, S.; Auziere, S.; Gadkari, A.; Girolomoni, G.; Puig, L.; Simpson, E.L.; Margolis, D.J.; de Bruin-Weller, M.; Eckert, L. Epidemiology of Atopic Dermatitis in Adults: Results from an International Survey. Allergy 2018, 73, 1284–1293. [Google Scholar] [CrossRef]
- Tsuge, M.; Ikeda, M.; Matsumoto, N.; Yorifuji, T.; Tsukahara, H. Current Insights into Atopic March. Children 2021, 8, 1067. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Bao, W.; Zhou, J.; Zhao, Q.-L.; Hong, S.-Z.; Yang, B.-C.; Ren, J.; Wang, P.; Yin, B.; Chu, C.-C.; et al. Global Burden, Incidence and Disability-Adjusted Life-Years for Dermatitis: A Systematic Analysis Combined with Socioeconomic Development Status, 1990–2019. Front. Cell. Infect. Microbiol. 2022, 12, 861053. [Google Scholar] [CrossRef]
- Jahnz-Rozyk, K.; Targowski, T.; Paluchowska, E.; Owczarek, W.; Kucharczyk, A. Serum Thymus and Activation-Regulated Chemokine, Macrophage-Derived Chemokine and Eotaxin as Markers of Severity of Atopic Dermatitis. Allergy 2005, 60, 685–688. [Google Scholar] [CrossRef]
- Cayrol, C.; Girard, J.-P. IL-33: An Alarmin Cytokine with Crucial Roles in Innate Immunity, Inflammation and Allergy. Curr. Opin. Immunol. 2014, 31, 31–37. [Google Scholar] [CrossRef]
- Lee, E.B.; Kim, K.W.; Hong, J.Y.; Jee, H.M.; Sohn, M.H.; Kim, K.E. Increased Serum Thymic Stromal Lymphopoietin in Children with Atopic Dermatitis. Pediatr. Allergy Immunol. 2010, 21, e457–e460. [Google Scholar] [CrossRef]
- Fedenko, E.S.; Elisyutina, O.G.; Filimonova, T.M.; Boldyreva, M.N.; Burmenskaya, O.V.; Rebrova, O.Y.; Yarilin, A.A.; Khaitov, R.M. Cytokine Gene Expression in the Skin and Peripheral Blood of Atopic Dermatitis Patients and Healthy Individuals. Self/Nonself 2011, 2, 120–124. [Google Scholar] [CrossRef]
- Choi, D.I.; Park, J.H.; Choi, J.Y.; Piao, M.S.; Suh, M.S.; Lee, J.B.; Yun, S.J.; Lee, S.C. Keratinocytes-Derived Reactive Oxygen Species Play an Active Role to Induce Type 2 Inflammation of the Skin: A Pathogenic Role of Reactive Oxygen Species at the Early Phase of Atopic Dermatitis. Ann. Dermatol. 2021, 33, 26–36. [Google Scholar] [CrossRef]
- Marek-Jozefowicz, L.; Nedoszytko, B.; Grochocka, M.; Żmijewski, M.A.; Czajkowski, R.; Cubała, W.J.; Slominski, A.T. Molecular Mechanisms of Neurogenic Inflammation of the Skin. Int. J. Mol. Sci. 2023, 24, 5001. [Google Scholar] [CrossRef]
- Zhao, Y.; Bao, L.; Chan, L.S.; DiPietro, L.A.; Chen, L. Aberrant Wound Healing in an Epidermal Interleukin-4 Transgenic Mouse Model of Atopic Dermatitis. PLoS ONE 2016, 11, e0146451. [Google Scholar] [CrossRef]
- Crowe, M.J.; Doetschman, T.; Greenhalgh, D.G. Delayed Wound Healing in Immunodeficient TGF-Β1 Knockout Mice. J. Investig. Dermatol. 2000, 115, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Arkwright, P.D.; David, T.J.; Chase, J.M.; Babbage, S.; Pravica, V.; Hutchinson, I.V. Atopic Dermatitis Is Associated with a Low-Producer Transforming Growth Factor Β1 Cytokine Genotype. J. Allergy Clin. Immunol. 2001, 108, 281–284. [Google Scholar] [CrossRef]
- Weidinger, S.; Novak, N. Atopic Dermatitis. Lancet 2016, 387, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Punia, H.; Tokas, J.; Malik, A.; Sangwan, S.; Baloda, S.; Singh, N.; Singh, S.; Bhuker, A.; Singh, P.; Yashveer, S.; et al. Identification and Detection of Bioactive Peptides in Milk and Dairy Products: Remarks about Agro-Foods. Molecules 2020, 25, 3328. [Google Scholar] [CrossRef]
- Jollés, J.; Alais, C.; Jollès, P. The Tryptic Peptide with the Rennin-Sensitive Linkage of Cow’s κ-Casein. BBA Protein Struct. 1968, 168, 591–593. [Google Scholar] [CrossRef] [PubMed]
- Cordova-Davalos, L.E.; Jimenez, M.; Salinas, E. Glycomacropeptide Bioactivity and Health: A Review Highlighting Action Mechanisms and Signaling Pathways. Nutrients 2019, 11, 598. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chen, B.; Du, M.; Song, J.; Cheng, X.; Wang, X.; Mao, X. Casein Glycomacropeptide Hydrolysates Exert Cytoprotective Effect against Cellular Oxidative Stress by Up-Regulating HO-1 Expression in HepG2 Cells. Nutrients 2017, 9, 31. [Google Scholar] [CrossRef]
- Cheng, X.; Gao, D.-X.; Song, J.-J.; Ren, F.-Z.; Mao, X.-Y. Casein Glycomacropeptide Hydrolysate Exerts Cytoprotection against H2O2-Induced Oxidative Stress in RAW 264.7 Macrophages via ROS-Dependent Heme Oxygenase-1 Expression. RSC Adv. 2015, 5, 4511–4523. [Google Scholar] [CrossRef]
- Muñoz, F.C.; Cervantes, M.M.; Cervantes-Garcia, D.; Jimenez, M.; Ventura-Juarez, J.; Salinas, E. Glycomacropeptide Attenuates Inflammation, Pruritus, and Th2 Response Associated with Atopic Dermatitis Induced by 2,4-Dinitrochlorobenzene in Rat. J. Immunol. Res. 2017, 2017, 6935402. [Google Scholar] [CrossRef]
- Jiménez, M.; Muñoz, F.C.; Cervantes-García, D.; Cervantes, M.M.; Hernández-Mercado, A.; Barrón-García, B.; Moreno Hernández-Duque, J.L.; Rodríguez-Carlos, A.; Rivas-Santiago, B.; Salinas, E. Protective Effect of Glycomacropeptide on the Atopic Dermatitis-Like Dysfunctional Skin Barrier in Rats. J. Med. Food 2020, 23, 1216–1224. [Google Scholar] [CrossRef]
- Jiménez, M.; Cervantes-Garcia, D.; Munoz, Y.H.; Garcia, A.; Haro, L.M., Jr.; Salinas, E. Novel Mechanisms Underlying the Therapeutic Effect of Glycomacropeptide on Allergy: Change in Gut Microbiota, Upregulation of TGF-Beta, and Inhibition of Mast Cells. Int. Arch. Allergy Immunol. 2016, 171, 217–226. [Google Scholar] [CrossRef]
- Chabance, B.; Marteau, P.; Rambaud, J.C.; Migliore-Samour, D.; Boynard, M.; Perrotin, P.; Guillet, R.; Jollès, P.; Fiat, A.M. Casein Peptide Release and Passage to the Blood in Humans during Digestion of Milk or Yogurt. Biochimie 1998, 80, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, X.; Broderick, M.; Fein, H. Measurement of Nitric Oxide Production in Biological Systems by Using Griess Reaction Assay. Sensors 2003, 3, 276–284. [Google Scholar] [CrossRef]
- Nourooz-Zadeh, J.; Tajaddini-Sarmadi, J.; Wolff, S.P. Measurement of Plasma Hydroperoxide Concentrations by the Ferrous Oxidation-Xylenol Orange Assay in Conjunction with Triphenylphosphine. Anal. Biochem. 1994, 220, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative CT Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Curiel, I.; Trujillo, V.; Montoya-Rosales, A.; Rincon, K.; Rivas-Calderon, B.; deHaro-Acosta, J.; Marin-Luevano, P.; Lozano-Lopez, D.; Enciso-Moreno, J.A.; Rivas-Santiago, B. 1,25-Dihydroxyvitamin D3 Induces LL-37 and HBD-2 Production in Keratinocytes from Diabetic Foot Ulcers Promoting Wound Healing: An In Vitro Model. PLoS ONE 2014, 9, e111355. [Google Scholar] [CrossRef]
- Lin, K.Y.; Chung, C.H.; Ciou, J.S.; Su, P.F.; Wang, P.W.; Shieh, D.B.; Wang, T.C. Molecular Damage and Responses of Oral Keratinocyte to Hydrogen Peroxide. BMC Oral. Health 2019, 19, 10. [Google Scholar] [CrossRef]
- Onami, K.; Kimura, Y.; Ito, Y.; Yamauchi, T.; Yamasaki, K.; Aiba, S. Nonmetal Haptens Induce ATP Release from Keratinocytes through Opening of Pannexin Hemichannels by Reactive Oxygen Species. J. Investig. Dermatol. 2014, 134, 1951–1960. [Google Scholar] [CrossRef]
- Kim, H.J.; Baek, J.; Lee, J.R.; Roh, J.Y.; Jung, Y. Optimization of Cytokine Milieu to Reproduce Atopic Dermatitis-Related Gene Expression in HaCaT Keratinocyte Cell Line. Immune Netw. 2018, 18, e9. [Google Scholar] [CrossRef]
- Kahremany, S.; Hofmann, L.; Harari, M.; Gruzman, A.; Cohen, G. Pruritus in Psoriasis and Atopic Dermatitis: Current Treatments and New Perspectives. Pharmacol. Rep. 2021, 73, 443–453. [Google Scholar] [CrossRef]
- Santoro, M.M.; Gaudino, G. Cellular and Molecular Facets of Keratinocyte Reepithelization during Wound Healing. Exp. Cell. Res. 2005, 304, 274–286. [Google Scholar] [CrossRef]
- Furue, M.; Terao, H.; Rikihisa, W.; Urabe, K.; Kinukawa, N.; Nose, Y.; Koga, T. Clinical Dose and Adverse Effects of Topical Steroids in Daily Management of Atopic Dermatitis. Br. J. Dermatol. 2003, 148, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Montanari, C.; Ceccarani, C.; Corsello, A.; Zuvadelli, J.; Ottaviano, E.; Dei Cas, M.; Banderali, G.; Zuccotti, G.; Borghi, E.; Verduci, E. Glycomacropeptide Safety and Its Effect on Gut Microbiota in Patients with Phenylketonuria: A Pilot Study. Nutrients 2022, 14, 1883. [Google Scholar] [CrossRef] [PubMed]
- Hoefle, A.S.; Bangert, A.M.; Rist, M.J.; Gedrich, K.; Lee, Y.-M.; Skurk, T.; Danier, J.; Schwarzenbolz, U.; Daniel, H. Postprandial Metabolic Responses to Ingestion of Bovine Glycomacropeptide Compared to a Whey Protein Isolate in Prediabetic Volunteers. Eur. J. Nutr. 2019, 58, 2067–2077. [Google Scholar] [CrossRef] [PubMed]
- Hvas, C.L.; Dige, A.; Bendix, M.; Wernlund, P.G.; Christensen, L.A.; Dahlerup, J.F.; Agnholt, J. Casein Glycomacropeptide for Active Distal Ulcerative Colitis: A Randomized Pilot Study. Eur. J. Clin. Investig. 2016, 46, 555–563. [Google Scholar] [CrossRef]
- Mikkelsen, T.L.; Rasmussen, E.; Olsen, A.; Barkholt, V.; Frøkiær, H. Immunogenicity of κ-Casein and Glycomacropeptide. J. Dairy Sci. 2006, 89, 824–830. [Google Scholar] [CrossRef]
- Wernlund, P.G.; Hvas, C.L.; Dahlerup, J.F.; Bahl, M.I.; Licht, T.R.; Knudsen, K.E.B.; Agnholt, J.S. Casein glycomacropeptide is well tolerated in healthy adults and changes neither high-sensitive C-reactive protein, gut microbiota nor faecal butyrate: A restricted randomised trial. Br. J. Nutr. 2021, 125, 1374–1385. [Google Scholar] [CrossRef]
- Foisy-Sauvé, M.; Ahmarani, L.; Delvin, E.; Sané, A.T.; Spahis, S.; Levy, E. Glycomacropeptide Prevents Iron/Ascorbate-Induced Oxidative Stress, Inflammation and Insulin Sensitivity with an Impact on Lipoprotein Production in Intestinal Caco-2/15 Cells. Nutrients 2020, 12, 1175. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Cheng, X.; Du, M.; Chen, B.; Mao, X. Upregulation of Heme Oxygenase-1 Mediates the Anti-Inflammatory Activity of Casein Glycomacropeptide (GMP) Hydrolysates in LPS-Stimulated Macrophages. Food Funct. 2017, 8, 2475–2484. [Google Scholar] [CrossRef] [PubMed]
- Sivaranjani, N.; Venkata Rao, S.; Rajeev, G. Role of Reactive Oxygen Species and Antioxidants in Atopic Dermatitis. J. Clin. Diagn. Res. 2013, 7, 2683–2685. [Google Scholar] [CrossRef] [PubMed]
- Salimi, M.; Barlow, J.L.; Saunders, S.P.; Xue, L.; Gutowska-Owsiak, D.; Wang, X.; Huang, L.-C.; Johnson, D.; Scanlon, S.T.; McKenzie, A.N.J.; et al. A Role for IL-25 and IL-33–Driven Type-2 Innate Lymphoid Cells in Atopic Dermatitis. J. Exp. Med. 2013, 210, 2939–2950. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Siracusa, M.C.; Saenz, S.A.; Noti, M.; Monticelli, L.A.; Sonnenberg, G.F.; Hepworth, M.R.; Van Voorhees, A.S.; Comeau, M.R.; Artis, D. TSLP Elicits IL-33-Independent Innate Lymphoid Cell Responses to Promote Skin Inflammation. Sci. Transl. Med. 2013, 5, 170ra16. [Google Scholar] [CrossRef]
- Ito, T.; Wang, Y.-H.; Duramad, O.; Hori, T.; Delespesse, G.J.; Watanabe, N.; Qin, F.X.-F.; Yao, Z.; Cao, W.; Liu, Y.-J. TSLP-Activated Dendritic Cells Induce an Inflammatory T Helper Type 2 Cell Response through OX40 Ligand. J. Exp. Med. 2005, 202, 1213–1223. [Google Scholar] [CrossRef]
- Rochman, Y.; Dienger-Stambaugh, K.; Richgels, P.K.; Lewkowich, I.P.; Kartashov, A.V.; Barski, A.; Hershey, G.K.K.; Leonard, W.J.; Singh, H. TSLP Signaling in CD4 + T Cells Programs a Pathogenic T Helper 2 Cell State. Sci. Signal. 2018, 11, eaam8858. [Google Scholar] [CrossRef]
- Qiu, Z.; Zhu, Z.; Liu, X.; Chen, B.; Yin, H.; Gu, C.; Fang, X.; Zhu, R.; Yu, T.; Mi, W.; et al. A dysregulated sebum-microbial metabolite-IL-33 axis initiates skin inflammation in atopic dermatitis. J. Exp. Med. 2022, 219, e20212397. [Google Scholar] [CrossRef]
- Yamaguchi, J.; Aihara, M.; Kobayashi, Y.; Kambara, T.; Ikezawa, Z. Quantitative Analysis of Nerve Growth Factor (NGF) in the Atopic Dermatitis and Psoriasis Horny Layer and Effect of Treatment on NGF in Atopic Dermatitis. J. Dermatol. Sci. 2009, 53, 48–54. [Google Scholar] [CrossRef]
- Roggenkamp, D.; Falkner, S.; Stab, F.; Petersen, M.; Schmelz, M.; Neufang, G. Atopic Keratinocytes Induce Increased Neurite Outgrowth in a Coculture Model of Porcine Dorsal Root Ganglia Neurons and Human Skin Cells. J. Investig. Dermatol. 2012, 132, 1892–1900. [Google Scholar] [CrossRef]
- Nakamura, N.; Tamagawa-Mineoka, R.; Yasuike, R.; Masuda, K.; Matsunaka, H.; Murakami, Y.; Yokosawa, E.; Katoh, N. Stratum Corneum Interleukin-33 Expressions Correlate with the Degree of Lichenification and Pruritus in Atopic Dermatitis Lesions. Clin. Immunol. 2019, 201, 1–3. [Google Scholar] [CrossRef]
- Wilson, S.R.; Thé, L.; Batia, L.M.; Beattie, K.; Katibah, G.E.; McClain, S.P.; Pellegrino, M.; Estandian, D.M.; Bautista, D.M. The Epithelial Cell-Derived Atopic Dermatitis Cytokine TSLP Activates Neurons to Induce Itch. Cell 2013, 155, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Ostlere, L.S.; Cowen, T.; Rustin, M.H.A. Neuropeptides in the Skin of Patients with Atopic Dermatitis. Clin. Exp. Dermatol. 1995, 20, 462–467. [Google Scholar] [CrossRef]
- Järvikallio, A.; Harvima, I.T.; Naukkarinen, A. Mast Cells, Nerves and Neuropeptides in Atopic Dermatitis and Nummular Eczema. Arch. Dermatol. Res. 2003, 295, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Katsuno, M.; Aihara, M.; Kojima, M.; Osuna, H.; Hosoi, J.; Nakamura, M.; Toyoda, M.; Matsuda, H.; Ikezawa, Z. Neuropeptides Concentrations in the Skin of a Murine (NC/Nga Mice) Model of Atopic Dermatitis. J. Dermatol. Sci. 2003, 33, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Salomon, J.; Baran, E. The Role of Selected Neuropeptides in Pathogenesis of Atopic Dermatitis. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Beken, B.; Serttas, R.; Yazicioglu, M.; Turkekul, K.; Erdogan, S. Quercetin Improves Inflammation, Oxidative Stress, and Impaired Wound Healing in Atopic Dermatitis Model of Human Keratinocytes. Pediatr. Allergy Immunol. Pulmonol. 2020, 33, 69–79. [Google Scholar] [CrossRef]
- Xu, J.; Lamouille, S.; Derynck, R. TGF-β-Induced Epithelial to Mesenchymal Transition. Cell Res. 2009, 19, 156–172. [Google Scholar] [CrossRef]
- Räsänen, K.; Vaheri, A. TGF-Beta1 Causes Epithelial-Mesenchymal Transition in HaCaT Derivatives, but Induces Expression of COX-2 and Migration Only in Benign, Not in Malignant Keratinocytes. J. Dermatol. Sci. 2010, 58, 97–104. [Google Scholar] [CrossRef]
- Roldan, N.R.; Jimenez, M.; Cervantes-Garcia, D.; Marin, E.; Salinas, E. Glycomacropeptide Administration Attenuates Airway Inflammation and Remodeling Associated to Allergic Asthma in Rat. Inflamm. Res. 2016, 65, 273–283. [Google Scholar] [CrossRef]
- López-Posadas, R.; Requena, P.; González, R.; Suárez, M.D.; Zarzuelo, A.; Sánchez De Medina, F.; Martínez-Augustin, O. Bovine Glycomacropeptide Has Intestinal Antiinflammatory Effects in Rats with Dextran Sulfate-Induced Colitis. J. Nutr. 2010, 140, 2014–2019. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhu, C.; Ming, Z.; Cao, J.; Yan, Y.; Zhao, P.; Pang, G.; Deng, Z.; Yao, Y.; Chen, Q. Molecular Mechanisms by Which Casein Glycomacropeptide Maintains Internal Homeostasis in Mice with Experimental Ulcerative Colitis. PLoS ONE 2017, 12, e0181075. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, M.; Werner, S. Oxidative stress in normal and impaired wound repair. Pharmacol. Res. 2008, 58, 165–171. [Google Scholar] [CrossRef] [PubMed]
| Target Genes | NCBI Access Number | Primers |
|---|---|---|
| TSLP | NM_033035.5 | Fw: ATGTTCGCCATGAAAACTAAGGC |
| Rv: GCGACGCCACAATCCTTGTA | ||
| IL33 | NM_033439.4 | Fw: GGAGTGCTTTGCCTTTGGTA |
| Rv: CATTTGAGGGGTGTTGAGAC | ||
| CCL22/MDC | NM_002990.5 | Fw: GCACTCCTGGTTGTCCTCGT |
| Rv: GACGTAATCACGGCAGCAGA | ||
| CCL17/TARC | NM_002987.3 | Fw: GTACTTCAAGGGAGCCATTC |
| Rv: CACTCTCTTGTTGTTGGGGT | ||
| HMOX1 | NM_002133.3 | Fw: AAGACTGCGTTCCTGCTCAAC |
| Rv: AAAGCCCTACAGCAACTGTCG | ||
| cGRP/CALCA | NM_001033952.3 | Fw: TCTAAGCGGTGCGGTAATCTG |
| Rv: CAGTTTGGGGGAACGTGTGA | ||
| NGF | NM_002506.3 | Fw: TGTGGGTTGGGGATAAGACCA |
| Rv: GCTGTCAACGGGATTTGGGT | ||
| TGFB1 | NM_000660.7 | Fw: CTCCCCACCACACCAGCCCT |
| Rv: GCCACAGCAGCGGTAGCAGC | ||
| GADPH | NM_002046.7 | Fw: ATCCCATCACCATCTTCCAG |
| Rv: GGCAGAGATGATGACCCTTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallegos-Alcalá, P.; Jiménez, M.; Cervantes-García, D.; Córdova-Dávalos, L.E.; Gonzalez-Curiel, I.; Salinas, E. Glycomacropeptide Protects against Inflammation and Oxidative Stress, and Promotes Wound Healing in an Atopic Dermatitis Model of Human Keratinocytes. Foods 2023, 12, 1932. https://doi.org/10.3390/foods12101932
Gallegos-Alcalá P, Jiménez M, Cervantes-García D, Córdova-Dávalos LE, Gonzalez-Curiel I, Salinas E. Glycomacropeptide Protects against Inflammation and Oxidative Stress, and Promotes Wound Healing in an Atopic Dermatitis Model of Human Keratinocytes. Foods. 2023; 12(10):1932. https://doi.org/10.3390/foods12101932
Chicago/Turabian StyleGallegos-Alcalá, Pamela, Mariela Jiménez, Daniel Cervantes-García, Laura Elena Córdova-Dávalos, Irma Gonzalez-Curiel, and Eva Salinas. 2023. "Glycomacropeptide Protects against Inflammation and Oxidative Stress, and Promotes Wound Healing in an Atopic Dermatitis Model of Human Keratinocytes" Foods 12, no. 10: 1932. https://doi.org/10.3390/foods12101932