Current Technologies and Uses for Fruit and Vegetable Wastes in a Sustainable System: A Review
Abstract
:1. Introduction
1.1. Global Situation
1.2. Sustainable Development
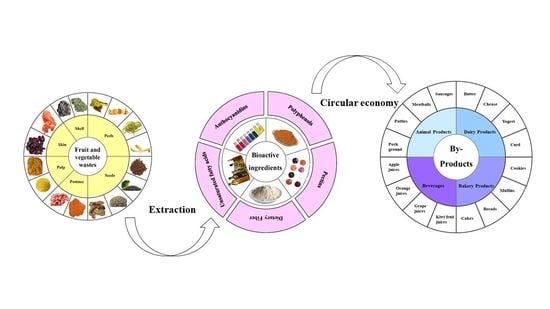
2. Utilization and Technologies to Reduce Fruit and Vegetable Wastes
2.1. Fruit and Vegetable Wastes as an Ingredient in Animal Feed and Food Nutrition
2.2. Current Technologies Used for Fruit and Vegetable Wastes in Food Engineering
2.2.1. Microwave-Assisted Extraction (MAE)
2.2.2. Supercritical Fluid Extraction (SFE)
2.2.3. Ultrasonic-Assisted Extraction (UAE)
2.2.4. Pressurized Liquid Extraction (PLE)
2.2.5. High Hydrostatic Pressure Technique (HHP)
2.2.6. Pulsed Electric Field (PEF)
2.2.7. Enzyme-Assisted Extraction (EAE)
2.2.8. Ionic Liquids Extraction (ILE)
2.3. Industry Examples of Utilization in Food Processing
2.4. Current Technologies for Fruit and Vegetable Wastes in Biorefinery
2.4.1. Anaerobic Digestion (AD)
2.4.2. Fermentation
2.4.3. Incineration
2.4.4. Pyrolysis and Gasification
2.4.5. Hydrothermal Carbonization (HTC)
3. Final Remarks and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matharu, A.S.; de Melo, E.M.; Houghton, J.A. Opportunity for high value-added chemicals from food supply chain wastes. Bioresour. Technol. 2016, 215, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Salim, N.; Singh, A.; Raghavan, V. Potential utilization of fruit and vegetable wastes for food through drying or extraction techniques. Nov. Tech. Nutr. Food Sci. 2017, 1, 1–12. [Google Scholar] [CrossRef]
- FAO. The State of Food and Agriculture 2019. Moving forward on Food Loss and Waste Reduction; FAO: Rome, Italy, 2019. [Google Scholar]
- Lipinski, B.; Hanson, C.; Lomax, J.; Kitinoja, L.; Waite, R.; Searchinger, T. Reducing Food Loss and Waste; World Resources Institute Working Paper 2013; World Resources Institute: Washington, DC, USA, 2013; pp. 1–40. [Google Scholar]
- FAO. Towards the Future We Want: End Hunger and Make the Transition to Sustainable Agricultural and Food Systems. In Proceedings of the Policy Paper for the Rio+ 20 Conference, Rome, Italy, 13–22 June 2012. [Google Scholar]
- Dora, M.; Wesana, J.; Gellynck, X.; Seth, N.; Dey, B.; De Steur, H. Importance of sustainable operations in food loss: Evidence from the Belgian food processing industry. Ann. Oper. Res. 2020, 290, 47–72. [Google Scholar] [CrossRef]
- FAO, Global. Global food losses and food waste—Extent, causes and prevention. SAVE FOOD Initiat. Food Loss Waste Reduct. 2011, 9, 2011. [Google Scholar]
- Laso, J.; Campos, C.; Fernández-Ríos, A.; Hoehn, D.; del Río, A.; Ruiz-Salmón, I.; Cristobal, J.; Quiñones, A.; Amo-Setién, F.J.; del Carmen Ortego, M. Looking for answers to food loss and waste management in spain from a holistic nutritional and economic approach. Sustainability 2020, 13, 125. [Google Scholar] [CrossRef]
- Dou, Z.X.; Ferguson, J.D.; Galligan, D.T.; Kelly, A.M.; Finn, S.M.; Giegengack, R. Assessing US food wastage and opportunities for reduction. Glob. Food Secur. Agric. Policy Econ. Environ. 2016, 8, 19–26. [Google Scholar] [CrossRef]
- De Laurentiis, V.; Corrado, S.; Sala, S. Quantifying household waste of fresh fruit and vegetables in the EU. Waste Manag. 2018, 77, 238–251. [Google Scholar] [CrossRef]
- Jeswani, H.K.; Figueroa-Torres, G.; Azapagic, A. The extent of food waste generation in the UK and its environmental impacts. Sustain. Prod. Consum. 2021, 26, 532–547. [Google Scholar] [CrossRef]
- Gunders, D.; Bloom, J. Wasted: How America Is Losing up to 40 Percent of Its Food from Farm to Fork to Landfill; Natural Resources Defense Council: New York, NY, USA, 2017. [Google Scholar]
- Buzby, J.C.; Farah-Wells, H.; Hyman, J. The Estimated Amount, Value, and Calories of Postharvest Food Losses at the Retail and Consumer Levels in the United States; U.S. Department of Agriculture, Economic Research Service: Washington, DC, USA, 2014.
- Gardas, B.B.; Raut, R.D.; Narkhede, B. Modeling causal factors of post-harvesting losses in vegetable and fruit supply chain: An Indian perspective. Renew. Sustain. Energy Rev. 2017, 80, 1355–1371. [Google Scholar] [CrossRef]
- Gurjar, P.; Verma, A.; Verma, A. Study of Post Harvest Losses and Marketing Channels of Fresh Mangoes in Uttar Pradesh. Agric. Situat. India 2017, 74, 27–33. [Google Scholar]
- Esparza, I.; Jiménez-Moreno, N.; Bimbela, F.; Ancín-Azpilicueta, C.; Gandía, L.M. Fruit and vegetable waste management: Conventional and emerging approaches. J. Environ. Manag. 2020, 265, 110510. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.R.; Fawcett, D.; Sharma, S.B.; Poinern, G.E.J. Progress towards sustainable utilisation and management of food wastes in the global economy. Int. J. Food Sci. 2016, 2016, 3563478. [Google Scholar] [CrossRef] [PubMed]
- Ozoegwu, C.; Eze, C.; Onwosi, C.; Mgbemene, C.; Ozor, P. Biomass and bioenergy potential of cassava waste in Nigeria: Estimations based partly on rural-level garri processing case studies. Renew. Sustain. Energy Rev. 2017, 72, 625–638. [Google Scholar] [CrossRef]
- Redlingshöfer, B.; Soyeux, A. Food losses and wastage as a sustainability indicator of food and farming systems. In Proceedings of the Producing and Reproducing Farming Systems: New Modes of Organisation for Sustainable Food Systems of Tomorrow, 10th European IFSA Symposium, Aarhus, Denmark, 1–4 July 2012. [Google Scholar]
- Ghosh, P.R.; Fawcett, D.; Sharma, S.B.; Poinern, G.E. Production of high-value nanoparticles via biogenic processes using aquacultural and horticultural food waste. Materials 2017, 10, 852. [Google Scholar] [CrossRef] [PubMed]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and vegetable waste: Bioactive compounds, their extraction, and possible utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.M.; Kwon, E.-B.; Lee, B.; Kim, C.Y. Recent trends in controlling the enzymatic browning of fruit and vegetable products. Molecules 2020, 25, 2754. [Google Scholar] [CrossRef]
- Resolution, A. RES/70/1. Transforming Our World: The 2030 Agenda for Sustainable Development; Seventieth United Nations General Assembly; United Nations: New York, NY, USA, 2015. [Google Scholar]
- Panwar, D.; Saini, A.; Panesar, P.S.; Chopra, H.K. Unraveling the scientific perspectives of citrus by-products utilization: Progress towards circular economy. Trends Food Sci. Technol. 2021, 111, 549–562. [Google Scholar] [CrossRef]
- Oltjen, J.; Beckett, J. Role of ruminant livestock in sustainable agricultural systems. J. Anim. Sci. 1996, 74, 1406–1409. [Google Scholar] [CrossRef]
- Aung, M.M.; Chang, Y.S. Traceability in a food supply chain: Safety and quality perspectives. Food Control 2014, 39, 172–184. [Google Scholar] [CrossRef]
- Bryant, C.; Barnett, J. Consumer acceptance of cultured meat: A systematic review. Meat Sci. 2018, 143, 8–17. [Google Scholar] [CrossRef]
- Shahidi, F. Oxidative Stability and Shelf Life of Meat and Meat Products. In Oxidative Stability and Shelf Life of Foods Containing Oils and Fats; Elsevier: Amsterdam, The Netherlands, 2016; pp. 373–389. [Google Scholar]
- Beres, C.; Costa, G.N.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.; Cruz, A.P.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.; Freitas, S.P. Towards integral utilization of grape pomace from winemaking process: A review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Esteban, J.; Ladero, M. Food waste as a source of value-added chemicals and materials: A biorefinery perspective. Int. J. Food Sci. Technol. 2018, 53, 1095–1108. [Google Scholar] [CrossRef]
- Gibson, C. Sensory Evaluation of Meat of Broiler Poultry Birds Fed with Tomato-supplemented Feed. Am. Sci. Res. J. Eng. Technol. Sci. 2018, 47, 145–150. [Google Scholar]
- Guil-Guerrero, J.; Ramos, L.; Moreno, C.; Zúñiga-Paredes, J.; Carlosama-Yepez, M.; Ruales, P. Plant foods by-products as sources of health-promoting agents for animal production: A review focusing on the tropics. Agron. J. 2016, 108, 1759–1774. [Google Scholar] [CrossRef]
- Xing, J.-J.; Cheng, Y.-L.; Chen, P.; Shan, L.; Ruan, R.; Li, D.; Wang, L.-J. Effect of high-pressure homogenization on the extraction of sulforaphane from broccoli (Brassica oleracea) seeds. Powder Technol. 2018, 358, 103–109. [Google Scholar] [CrossRef]
- Marinova, E.M.; Toneva, A.; Yanishlieva, N. Comparison of the antioxidative properties of caffeic and chlorogenic acids. Food Chem. 2009, 114, 1498–1502. [Google Scholar] [CrossRef]
- Jairath, G.; Chatli, M.K.; Biswas, A.K. Comparative study on in vitro and in vivo evaluation of antioxidant potential of apple peel extract and aloe vera gel. J. Food Process. Preserv. 2016, 40, 607–614. [Google Scholar] [CrossRef]
- Ergezer, H.; Serdaroğlu, M. Antioxidant potential of artichoke (Cynara scolymus L.) byproducts extracts in raw beef patties during refrigerated storage. J. Food Meas. Charact. 2018, 12, 982–991. [Google Scholar] [CrossRef]
- Costa, C.; Lucera, A.; Marinelli, V.; Del Nobile, M.A.; Conte, A. Influence of different by-products addition on sensory and physicochemical aspects of Primosale cheese. J. Food Sci. Technol. 2018, 55, 4174–4183. [Google Scholar] [CrossRef]
- Amofa-Diatuo, T.; Anang, D.M.; Barba, F.J.; Tiwari, B.K. Development of new apple beverages rich in isothiocyanates by using extracts obtained from ultrasound-treated cauliflower by-products: Evaluation of physical properties and consumer acceptance. J. Food Compos. Anal. 2017, 61, 73–81. [Google Scholar] [CrossRef]
- Jridi, M.; Souissi, N.; Salem, M.B.; Ayadi, M.; Nasri, M.; Azabou, S. Tunisian date (Phoenix dactylifera L.) by-products: Characterization and potential effects on sensory, textural and antioxidant properties of dairy desserts. Food Chem. 2015, 188, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Ramachandraiah, K.; Chin, K.B. Effect of particle size of persimmon by-product powders on their physicochemical properties and antioxidant activities in porcine patties. J. Food Process Eng. 2018, 41, e12610. [Google Scholar] [CrossRef]
- Rebello, L.P.G.; Ramos, A.M.; Pertuzatti, P.B.; Barcia, M.T.; Castillo-Muñoz, N.; Hermosín-Gutiérrez, I. Flour of banana (Musa AAA) peel as a source of antioxidant phenolic compounds. Food Res. Int. 2014, 55, 397–403. [Google Scholar] [CrossRef]
- Mutua, J.K.; Imathiu, S.; Owino, W. Evaluation of the proximate composition, antioxidant potential, and antimicrobial activity of mango seed kernel extracts. Food Sci. Nutr. 2017, 5, 349–357. [Google Scholar] [CrossRef]
- Adiamo, O.Q.; Ghafoor, K.; Al-Juhaimi, F.; Babiker, E.E.; Ahmed, I.A.M. Thermosonication process for optimal functional properties in carrot juice containing orange peel and pulp extracts. Food Chem. 2018, 245, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Larrosa, M.; Llorach, R.; Espín, J.C.; Tomás-Barberán, F.A. Increase of antioxidant activity of tomato juice upon functionalisation with vegetable byproduct extracts. LWT-Food Sci. Technol. 2002, 35, 532–542. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Cardozo, P.; Calsamiglia, S.; Ferret, A.; Kamel, C. Effects of alfalfa extract, anise, capsicum, and a mixture of cinnamaldehyde and eugenol on ruminal fermentation and protein degradation in beef heifers fed a high-concentrate diet. J. Anim. Sci. 2006, 84, 2801–2808. [Google Scholar] [CrossRef]
- Andrés, A.; Petrón, M.; Adámez, J.; López, M.; Timón, M. Food by-products as potential antioxidant and antimicrobial additives in chill stored raw lamb patties. Meat Sci. 2017, 129, 62–70. [Google Scholar] [CrossRef]
- Moo-Huchin, V.; Estrada-Mota, I.; Estrada-León, R.; Cuevas-Glory, L.F.; Sauri-Duch, E. Chemical composition of crude oil from the seeds of pumpkin (Cucurbita spp.) and mamey sapota (Pouteria sapota Jacq.) grown in Yucatan, Mexico. CYTA-J. Food 2013, 11, 324–327. [Google Scholar] [CrossRef]
- Hwang, I.-W.; Jeong, M.-C.; Chung, S.-K. The physicochemical properties and the antioxidant activities of persimmon peel powders with different particle sizes. J. Korean Soc. Appl. Biol. Chem. 2011, 54, 442–446. [Google Scholar] [CrossRef]
- Bellur Nagarajaiah, S.; Prakash, J. Nutritional composition, acceptability, and shelf stability of carrot pomace-incorporated cookies with special reference to total and β-carotene retention. Cogent Food Agric. 2015, 1, 1039886. [Google Scholar] [CrossRef]
- Casarotti, S.N.; Borgonovi, T.F.; Batista, C.L.; Penna, A.L.B. Guava, orange and passion fruit by-products: Characterization and its impacts on kinetics of acidification and properties of probiotic fermented products. LWT 2018, 98, 69–76. [Google Scholar] [CrossRef]
- Tekgül, Y.; Baysal, T. Comparative evaluation of quality properties and volatile profiles of lemon peels subjected to different drying techniques. J. Food Process Eng. 2018, 41, e12902. [Google Scholar] [CrossRef]
- Inoue, T.; Tsubaki, S.; Ogawa, K.; Onishi, K.; Azuma, J.-I. Isolation of hesperidin from peels of thinned Citrus unshiu fruits by microwave-assisted extraction. Food Chem. 2010, 123, 542–547. [Google Scholar] [CrossRef]
- Caccioni, D.R.; Guizzardi, M.; Biondi, D.M.; Renda, A.; Ruberto, G. Relationship between volatile components of citrus fruit essential oils and antimicrobial action on Penicillium digitatum and Penicillium italicum. Int. J. Food Microbiol. 1998, 43, 73–79. [Google Scholar] [CrossRef]
- Anosa, G.N.; Okoro, O.J. Anticoccidial activity of the methanolic extract of Musa paradisiaca root in chickens. Trop. Anim. Health Prod. 2011, 43, 245–248. [Google Scholar] [CrossRef]
- Bleve, M.; Ciurlia, L.; Erroi, E.; Lionetto, G.; Longo, L.; Rescio, L.; Schettino, T.; Vasapollo, G. An innovative method for the purification of anthocyanins from grape skin extracts by using liquid and sub-critical carbon dioxide. Sep. Purif. Technol. 2008, 64, 192–197. [Google Scholar] [CrossRef]
- Liazid, A.; Guerrero, R.; Cantos, E.; Palma, M.; Barroso, C. Microwave assisted extraction of anthocyanins from grape skins. Food Chem. 2011, 124, 1238–1243. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Chuang, Y.-C.; Hsu, H.-W. The flavonoid, carotenoid and pectin content in peels of citrus cultivated in Taiwan. Food Chem. 2008, 106, 277–284. [Google Scholar] [CrossRef]
- Kazemi, M.; Khodaiyan, F.; Labbafi, M.; Hosseini, S.S.; Hojjati, M. Pistachio green hull pectin: Optimization of microwave-assisted extraction and evaluation of its physicochemical, structural and functional properties. Food Chem. 2019, 271, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Ezzat, S.M.; Adel, R.; Abdel-Sattar, E. Pumpkin bio-wastes as source of functional ingredients. In Mediterranean Fruits Bio-Wastes: Chemistry, Functionality and Technological Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 667–696. [Google Scholar]
- Sengar, A.S.; Rawson, A.; Muthiah, M.; Kalakandan, S.K. Comparison of different ultrasound assisted extraction techniques for pectin from tomato processing waste. Ultrason. Sonochem. 2020, 61, 104812. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Wang, Y.; Wang, L.-J.; Li, D.; Adhikari, B. Optimization of production yield and functional properties of pectin extracted from sugar beet pulp. Carbohydr. Polym. 2013, 95, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Wedamulla, N.E.; Fan, M.; Choi, Y.-J.; Kim, E.-K. Citrus peel as a renewable bioresource: Transforming waste to food additives. J. Funct. Foods 2022, 95, 105163. [Google Scholar] [CrossRef]
- Al-Sayed, H.M.; Ahmed, A.R. Utilization of watermelon rinds and sharlyn melon peels as a natural source of dietary fiber and antioxidants in cake. Ann. Agric. Sci. 2013, 58, 83–95. [Google Scholar] [CrossRef]
- Noh, N.A.N.M.; Karim, L.; Omar, S.R. Value-added products from pumpkin wastes: A review. Malays. J. Sci. Health Technol. 2022, 8, 77–84. [Google Scholar]
- Chau, C.-F.; Chen, C.-H.; Lee, M.-H. Comparison of the characteristics, functional properties, and in vitro hypoglycemic effects of various carrot insoluble fiber-rich fractions. LWT-Food Sci. Technol. 2004, 37, 155–160. [Google Scholar] [CrossRef]
- Javed, A.; Ahmad, A.; Tahir, A.; Shabbir, U.; Nouman, M.; Hameed, A. Potato peel waste—Its nutraceutical, industrial and biotechnological applacations. AIMS Agric. Food 2019, 4, 807–823. [Google Scholar] [CrossRef]
- Elbadrawy, E.; Sello, A. Evaluation of nutritional value and antioxidant activity of tomato peel extracts. Arab. J. Chem. 2016, 9, S1010–S1018. [Google Scholar] [CrossRef]
- Abdolshahi, A.; Majd, M.H.; Rad, J.S.; Taheri, M.; Shabani, A.; Da Silva, J.A.T. Choice of solvent extraction technique affects fatty acid composition of pistachio (Pistacia vera L.) oil. J. Food Sci. Technol. 2015, 52, 2422–2427. [Google Scholar] [CrossRef]
- Kothari, V.; Seshadri, S. In vitro antibacterial activity in seed extracts of Manilkara zapota, Anona squamosa, and Tamarindus indica. Biol. Res. 2010, 43, 165–168. [Google Scholar] [CrossRef] [PubMed]
- El-Safy, F.S.; Salem, R.H.; Abd El-Ghany, M. Chemical and nutritional evaluation of different seed flours as novel sources of protein. World J. Dairy Food Sci. 2012, 7, 59–65. [Google Scholar]
- Milner, J.A.; Romagnolo, D.F. Cancer biology and nutrigenomics. In Bioactive Compounds and Cancer; Springer: Berlin/Heidelberg, Germany, 2010; pp. 25–43. [Google Scholar]
- Yáñez-Ruiz, D.R.; Molina-Alcaide, E. A comparative study of the effect of two-stage olive cake added to alfalfa on digestion and nitrogen losses in sheep and goats. Animal 2007, 1, 227–232. [Google Scholar] [CrossRef]
- Rifna, E.; Misra, N.; Dwivedi, M. Recent advances in extraction technologies for recovery of bioactive compounds derived from fruit and vegetable waste peels: A review. Crit. Rev. Food Sci. Nutr. 2023, 63, 719–752. [Google Scholar] [CrossRef]
- Banerjee, J.; Singh, R.; Vijayaraghavan, R.; MacFarlane, D.; Patti, A.F.; Arora, A. Bioactives from fruit processing wastes: Green approaches to valuable chemicals. Food Chem. 2017, 225, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Niu, G.; Liu, H. Microwave-assisted extraction of tea polyphenols and tea caffeine from green tea leaves. Chem. Eng. Process. Process Intensif. 2003, 42, 129–133. [Google Scholar] [CrossRef]
- Fishman, M.L.; Chau, H.K.; Cooke, P.H.; Hotchkiss Jr, A.T. Global structure of microwave-assisted flash-extracted sugar beet pectin. J. Agric. Food Chem. 2008, 56, 1471–1478. [Google Scholar] [CrossRef]
- Guo, X.; Han, D.; Xi, H.; Rao, L.; Liao, X.; Hu, X.; Wu, J. Extraction of pectin from navel orange peel assisted by ultra-high pressure, microwave or traditional heating: A comparison. Carbohydr. Polym. 2012, 88, 441–448. [Google Scholar] [CrossRef]
- Dorta, E.; Lobo, M.G.; González, M. Improving the efficiency of antioxidant extraction from mango peel by using microwave-assisted extraction. Plant Foods Hum. Nutr. 2013, 68, 190–199. [Google Scholar] [CrossRef]
- Wijngaard, H.; Hossain, M.B.; Rai, D.K.; Brunton, N. Techniques to extract bioactive compounds from food by-products of plant origin. Food Res. Int. 2012, 46, 505–513. [Google Scholar] [CrossRef]
- Hassas-Roudsari, M.; Chang, P.R.; Pegg, R.B.; Tyler, R.T. Antioxidant capacity of bioactives extracted from canola meal by subcritical water, ethanolic and hot water extraction. Food Chem. 2009, 114, 717–726. [Google Scholar] [CrossRef]
- Sabio, E.; Lozano, M.; Montero de Espinosa, V.; Mendes, R.; Pereira, A.; Palavra, A.; Coelho, J. Lycopene and β-carotene extraction from tomato processing waste using supercritical CO2. Ind. Eng. Chem. Res. 2003, 42, 6641–6646. [Google Scholar] [CrossRef]
- Shi, J.; Yi, C.; Xue, S.J.; Jiang, Y.; Ma, Y.; Li, D. Effects of modifiers on the profile of lycopene extracted from tomato skins by supercritical CO2. J. Food Eng. 2009, 93, 431–436. [Google Scholar] [CrossRef]
- Wang, Y.; Ke, L.; Yang, Q.; Peng, Y.; Hu, Y.; Dai, L.; Jiang, L.; Wu, Q.; Liu, Y.; Ruan, R. Biorefinery process for production of bioactive compounds and bio-oil from Camellia oleifera shell. Int. J. Agric. Biol. Eng. 2019, 12, 190–194. [Google Scholar] [CrossRef]
- Bagherian, H.; Ashtiani, F.Z.; Fouladitajar, A.; Mohtashamy, M. Comparisons between conventional, microwave-and ultrasound-assisted methods for extraction of pectin from grapefruit. Chem. Eng. Process. Process Intensif. 2011, 50, 1237–1243. [Google Scholar] [CrossRef]
- Lianfu, Z.; Zelong, L. Optimization and comparison of ultrasound/microwave assisted extraction (UMAE) and ultrasonic assisted extraction (UAE) of lycopene from tomatoes. Ultrason. Sonochem. 2008, 15, 731–737. [Google Scholar] [CrossRef]
- Mendiola, J.A.; Herrero, M.; Cifuentes, A.; Ibañez, E. Use of compressed fluids for sample preparation: Food applications. J. Chromatogr. A 2007, 1152, 234–246. [Google Scholar] [CrossRef]
- Monrad, J.K.; Howard, L.R.; King, J.W.; Srinivas, K.; Mauromoustakos, A. Subcritical solvent extraction of anthocyanins from dried red grape pomace. J. Agric. Food Chem. 2010, 58, 2862–2868. [Google Scholar] [CrossRef]
- Guo, X.; Zhao, W.; Liao, X.; Hu, X.; Wu, J.; Wang, X. Extraction of pectin from the peels of pomelo by high-speed shearing homogenization and its characteristics. LWT-Food Sci. Technol. 2017, 79, 640–646. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, B.; Yang, R.; Zhao, W. Recent developments in the preservation of raw fresh food by pulsed electric field. Food Rev. Int. 2022, 38, 247–265. [Google Scholar] [CrossRef]
- Liu, D.; Vorobiev, E.; Savoire, R.; Lanoisellé, J.-L. Extraction of polyphenols from grape seeds by unconventional methods and extract concentration through polymeric membrane. In Proceedings of the 11th International Congress on Engineering and Food, ICEF 11, Athens, Greece, 22–26 May 2011; Volume III, pp. 1939–1940. [Google Scholar]
- Boateng, I.D. Recent processing of fruits and vegetables using emerging thermal and non-thermal technologies. A critical review of their potentialities and limitations on bioactives, structure, and drying performance. Crit. Rev. Food Sci. Nutr. 2022. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.Z.; Zhang, H.Q. Pulsed electric fields for pasteurization: Food safety and shelf life. In Food Safety Engineering; Springer: Berlin/Heidelberg, Germany, 2020; pp. 553–577. [Google Scholar]
- Gaur, R.; Sharma, A.; Khare, S.; Gupta, M.N. A novel process for extraction of edible oils: Enzyme assisted three phase partitioning (EATPP). Bioresour. Technol. 2007, 98, 696–699. [Google Scholar] [CrossRef] [PubMed]
- Boulila, A.; Hassen, I.; Haouari, L.; Mejri, F.; Amor, I.B.; Casabianca, H.; Hosni, K. Enzyme-assisted extraction of bioactive compounds from bay leaves (Laurus nobilis L.). Ind. Crops Prod. 2015, 74, 485–493. [Google Scholar] [CrossRef]
- Manasa, D.; Srinivas, P.; Sowbhagya, H. Enzyme-assisted extraction of bioactive compounds from ginger (Zingiber officinale Roscoe). Food Chem. 2013, 139, 509–514. [Google Scholar]
- Zuorro, A.; Fidaleo, M.; Lavecchia, R. Enzyme-assisted extraction of lycopene from tomato processing waste. Enzym. Microb. Technol. 2011, 49, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, B.L.; Granato, D.; Nunes, I.L. Uses of ionic liquids to obtain bioactive compounds: Insights from the main international regulations for technological applications. Crit. Rev. Food Sci. Nutr. 2022. [Google Scholar] [CrossRef]
- Idris, A.; Vijayaraghavan, R.; Patti, A.F.; Macfarlane, D.R. Distillable protic ionic liquids for keratin dissolution and recovery. ACS Sustain. Chem. Eng. 2014, 2, 1888–1894. [Google Scholar] [CrossRef]
- Chowdhury, S.A.; Vijayaraghavan, R.; MacFarlane, D. Distillable ionic liquid extraction of tannins from plant materials. Green Chem. 2010, 12, 1023–1028. [Google Scholar] [CrossRef]
- Yanes, E.G.; Gratz, S.R.; Baldwin, M.J.; Robison, S.E.; Stalcup, A.M. Capillary electrophoretic application of 1-alkyl-3-methylimidazolium-based ionic liquids. Anal. Chem. 2001, 73, 3838–3844. [Google Scholar] [CrossRef]
- Trigo, J.P.; Alexandre, E.M.; Saraiva, J.A.; Pintado, M.E. High value-added compounds from fruit and vegetable by-products–Characterization, bioactivities, and application in the development of novel food products. Crit. Rev. Food Sci. Nutr. 2019, 60, 1388–1416. [Google Scholar] [CrossRef]
- Younis, K.; Ahmad, S.; Malik, M.A. Mosambi peel powder incorporation in meat products: Effect on physicochemical properties and shelf life stability. Appl. Food Res. 2021, 1, 100015. [Google Scholar] [CrossRef]
- Abdel-Naeem, H.H.; Elshebrawy, H.A.; Imre, K.; Morar, A.; Herman, V.; Pașcalău, R.; Sallam, K.I. Antioxidant and antibacterial effect of fruit peel powders in chicken patties. Foods 2022, 11, 301. [Google Scholar] [CrossRef] [PubMed]
- Vlaicu, P.A.; Untea, A.E.; Panaite, T.D.; Turcu, R.P. Effect of dietary orange and grapefruit peel on growth performance, health status, meat quality and intestinal microflora of broiler chickens. Ital. J. Anim. Sci. 2020, 19, 1394–1405. [Google Scholar] [CrossRef]
- Doll, C.N.; Pachauri, S. Estimating rural populations without access to electricity in developing countries through night-time light satellite imagery. Energy Policy 2010, 38, 5661–5670. [Google Scholar] [CrossRef]
- Rhodes, C.J. The imperative for regenerative agriculture. Sci. Prog. 2017, 100, 80–129. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.P.T.; Kaushik, R.; Parshetti, G.K.; Mahmood, R.; Balasubramanian, R. Food waste-to-energy conversion technologies: Current status and future directions. Waste Manag. 2015, 38, 399–408. [Google Scholar] [CrossRef]
- Zia, M.; Ahmed, S.; Kumar, A. Anaerobic digestion (AD) of fruit and vegetable market waste (FVMW): Potential of FVMW, bioreactor performance, co-substrates, and pre-treatment techniques. Biomass Convers. Biorefinery 2022, 12, 3573–3592. [Google Scholar] [CrossRef]
- Ren, Y.; Yu, M.; Wu, C.; Wang, Q.; Gao, M.; Huang, Q.; Liu, Y. A comprehensive review on food waste anaerobic digestion: Research updates and tendencies. Bioresour. Technol. 2018, 247, 1069–1076. [Google Scholar] [CrossRef]
- Fermoso, F.G.; Serrano, A.; Alonso-Farinas, B.; Fernández-Bolanos, J.; Borja, R.; Rodríguez-Gutiérrez, G. Valuable compound extraction, anaerobic digestion, and composting: A leading biorefinery approach for agricultural wastes. J. Agric. Food Chem. 2018, 66, 8451–8468. [Google Scholar] [CrossRef]
- Linke, B. Kinetic study of thermophilic anaerobic digestion of solid wastes from potato processing. Biomass Bioenergy 2006, 30, 892–896. [Google Scholar] [CrossRef]
- El Sheikha, A.F.; Ray, R.C. Bioprocessing of horticultural wastes by solid-state fermentation into value-added/innovative bioproducts: A review. Food Rev. Int. 2022. [Google Scholar] [CrossRef]
- Khedkar, M.A.; Nimbalkar, P.R.; Gaikwad, S.G.; Chavan, P.V.; Bankar, S.B. Sustainable biobutanol production from pineapple waste by using Clostridium acetobutylicum B 527: Drying kinetics study. Bioresour. Technol. 2017, 225, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Song, H.B.; Song, Y.; Jeong, I.T.; Kim, J.W. Evaluation of food waste disposal options in terms of global warming and energy recovery: Korea. Int. J. Energy Environ. Eng. 2013, 4, 1. [Google Scholar] [CrossRef]
- Frolov, S.M.; Smetanyuk, V.A.; Sadykov, I.A.; Silantiev, A.S.; Shamshin, I.O.; Aksenov, V.S.; Avdeev, K.A.; Frolov, F.S. Natural gas conversion and liquid/solid organic waste gasification by ultra-superheated steam. Energies 2022, 15, 3616. [Google Scholar] [CrossRef]
- Kim, S.; Lee, Y.; Lin, K.-Y.A.; Hong, E.; Kwon, E.E.; Lee, J. The valorization of food waste via pyrolysis. J. Clean. Prod. 2020, 259, 120816. [Google Scholar] [CrossRef]
- Gonzalez, J.F.; Ramiro, A.; González-García, C.M.; Gañán, J.; Encinar, J.M.; Sabio, E.; Rubiales, J. Pyrolysis of almond shells. Energy applications of fractions. Ind. Eng. Chem. Res. 2005, 44, 3003–3012. [Google Scholar] [CrossRef]
- Niksiar, A.; Nasernejad, B. Activated carbon preparation from pistachio shell pyrolysis and gasification in a spouted bed reactor. Biomass Bioenergy 2017, 106, 43–50. [Google Scholar] [CrossRef]
- Yaman, S. Pyrolysis of biomass to produce fuels and chemical feedstocks. Energy Convers. Manag. 2004, 45, 651–671. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, L.; Dai, L.; Yu, Z.; Liu, Y.; Ruan, R.; Fu, G.; Zhou, Y.; Fan, L.; Duan, D. Microwave-assisted catalytic co-pyrolysis of soybean straw and soapstock for bio-oil production using SiC ceramic foam catalyst. J. Anal. Appl. Pyrolysis 2018, 133, 76–81. [Google Scholar] [CrossRef]



| Area | Type | Quantity (MT/Year) |
|---|---|---|
| North America | Corn stover | 80–100 (dry basis) |
| Vegetable crop residue | 1 (dry basis) | |
| Tomato pomace | 6 × 10−3 (California) | |
| Nutshell and hull | 4 × 10−2 | |
| Starch | 8 | |
| Europe | Tomato pomace | 4 |
| Post-manufacture food waste | 34 | |
| Citrus waste | 0.6 (Spain) | |
| Olive mill residue | 30 (Mediterranean Basin) | |
| Cocoa pods | 20 | |
| Africa | Citrus waste | 0.14 (South Africa) |
| Palm oil residue | 15.8 (Indonesia) | |
| Asia | Food waste | 1.2 (Hong Kong) |
| Citrus residues | 9.4 | |
| South America-Brazil | Apple pomace | 3–4.2 |
| World | Kiwi residue | 0.3 |
| Grape pomace | 5–9 | |
| Banana peels | 9 | |
| Citrus peel waste | 15.6 |
| Bioactive Substance | By-Products | Function | Reference |
|---|---|---|---|
| Sulforaphane | Broccoli seeds | Against carcinogens and inflammation | [33] |
| Caffeic acid | Coffee shells | Antioxidant; Anti-bacterial | [34] |
| Polyphenols | Apple peels | Anti-microbial activity | [35] |
| Artichoke | [36] | ||
| [37] | |||
| Cauliflower | [38] | ||
| Date | [39] | ||
| Persimmon by-products | [40] | ||
| Banana peels | [41] | ||
| Mango kernel | [42] | ||
| Orange (peels, pulp) | [43] | ||
| Onion and carrot peel | Antioxidant | [44] | |
| Eugenol | Allspice | Bacteriostatic | [45] |
| Alfalfa | [46] | ||
| Carotenoids | Tomato (seeds, skins) | Pigment, Radical scavenger | [47] |
| Grape pomace | [48] | ||
| Olive | |||
| Pomegranate pomace | |||
| Persimmon peels | [49] | ||
| Carrot pomace | [50] | ||
| Guava, orange, and passion fruit by-products | [51] | ||
| Lemon peels | [52] | ||
| Flavonoids | Satsuma peels Orange/Lemon (peels, pulp) Banana (peels, roots) Grape (seeds, skin) | Antibacterial, Antioxidant; | [53] |
| [54] | |||
| Anti-parasitic, Antioxidant, | [55] | ||
| Food color additives (such as Anthocyanidins) | [56] | ||
| [57] | |||
| Pectins | Citrus peels | Thickening agent and emulsification, Food additives | [58] |
| Pistachio green hull | [59] | ||
| Pumpkin peels and pulp | [60] | ||
| Tomato wastes | [61] | ||
| Sugar beet (pomace) | [62] | ||
| Dietary Fiber | Grapefruit peels, sweet oranges peels, lemon peels | Binders, Texturizers | [63] |
| Watermelon rinds, tamarind seed | Low-calorie bulking ingredient | [64] | |
| Pumpkin by-products | [65] | ||
| Carrot pomace | [66] | ||
| Potato peel | [67] | ||
| Unsaturated fatty acids | Tomato seeds | Antioxidant | [68] |
| Pistachio pomace | [69] | ||
| Saponins | Sapota seeds | Antibacterial | [70] |
| Amino acids and proteins | Kinnow mandarin waste, pineapple peels, papaya peels, | Protein supplementation | [71] |
| Glycosides | Banana stem, apple peels | Anti-cancer, Antioxidant | [72] |
| Category | Product Modified | By-Products | Storage Conditions | Key Findings |
|---|---|---|---|---|
| Animal Products | Beef meatballs, Sausages | Pomegranate peels | 8 days (4 °C) | Antioxidant; Anti-bacterial; Antibacterial |
| 6 months (−18 °C) | ||||
| 2 months (−18 °C) | ||||
| Mosambi peels | 2 months (−18 °C) | |||
| Lamb meat, Patties | Tomato pomace Grape pomace Olive pomace Tomato pamace Pomegranate pomace | 7 days (2 °C) | Antioxidant; Anti-bacterial | |
| Chicken meat, Patties, Chickens thigh | Grape pomace, Grapefruit peels, lemon peels Orange and grapefruit peels | 14 days (4 °C) 3 months (−18 °C) NA | Antioxidant; Anti-bacteria; Meat qualities and microflora | |
| Pork ground, Meatballs, Sausages | Persimmon seeds Mango peels Grape seeds | 12 days (3 °C) 10 days (4 °C) 12 days (4 °C) | Antioxidant | |
| Shrimp | Pomegranate peels | 10 days (4 °C) | Antioxidant; Anti-bacterial; Meat flavor; Color | |
| Tuna | Pomegranate peels | 10 days (4 °C, 12 °C) | Antibacterial | |
| Dairy Products | Butter | Tomato peel and seeds | 2 months (4 °C) | Antioxidant; Anti-bacterial; Flavor; Texture |
| Curd | Pomegranate peels | 15 days (5 °C) | Antioxidant; Anti-bacterial; Flavor; Texture | |
| Cheese | Tomato peels and grape pomace | NA | Antioxidant; Texture | |
| Fermented milk | Grape pomace Olive pomace Grape pomace | 28 days (4 °C); 50 days (5 °C) | Antioxidant; Anti-bacterial; Flavor | |
| Yogurt | Apricot and apple pomace Orange peels Pineapple peels | 2 months (−20 °C) 21 days (4 °C) 28 days (4 °C) | Antibacterial; Flavor; Texture; Shorten fermentation time | |
| Beverages | Apple juice | Pomegranate peel | NA | Antioxidant; Antibacterial, Color; Flavor |
| Carrot juice | Orange pulp and peels | NA | NA | |
| Orange juice | Banana peels | 30 days (5 °C) | Antioxidant; Color; Flavor | |
| Bakery Products | Cookies | Grape pomace Pineapple central axis Defatted mango kernel | NA | Antioxidant; Color; Flavor, Taste; Texture |
| Biscuits | Grape leaves Rowanberry, Blackcurrant, Elderberry pomace | 2 months | Antioxidant; Color; Flavor, Taste; Texture | |
| Bread | Mango peels Pumpkin pomace Grape pomace | NA | Antioxidant; Color; Flavor; Taste; Texture | |
| Muffins | Raspberry pomace Cranberry pomace Grape peels | NA | Color; Flavor; Taste; Texture | |
| Cakes | Orange peels Guava seeds Guava pomace Peach palm peels | 8 days (25 °C) | Antioxidant; Color; Flavor; Taste; Texture |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Luan, Y.; Zhao, Y.; Liu, J.; Duan, Z.; Ruan, R. Current Technologies and Uses for Fruit and Vegetable Wastes in a Sustainable System: A Review. Foods 2023, 12, 1949. https://doi.org/10.3390/foods12101949
Zhu Y, Luan Y, Zhao Y, Liu J, Duan Z, Ruan R. Current Technologies and Uses for Fruit and Vegetable Wastes in a Sustainable System: A Review. Foods. 2023; 12(10):1949. https://doi.org/10.3390/foods12101949
Chicago/Turabian StyleZhu, Yingdan, Yueting Luan, Yingnan Zhao, Jiali Liu, Zhangqun Duan, and Roger Ruan. 2023. "Current Technologies and Uses for Fruit and Vegetable Wastes in a Sustainable System: A Review" Foods 12, no. 10: 1949. https://doi.org/10.3390/foods12101949