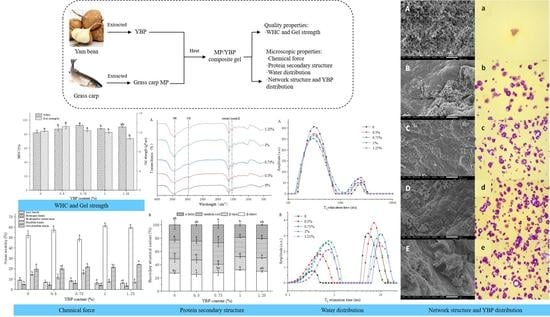
Yam Bean (Pachyrhizus erosus L. Urban) Powder Improves Grass Carp Myofibrillar Protein Gel by Forming Disulfide Bonds, Hydrogen Bonds, and Proper Microstructure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Myofibrillar Protein (MP) from Grass Carp
2.3. Extraction of Dry Matter from Yam Bean into Powder
2.4. Preparation of MP/YBP Composite Gel
2.5. Water-Holding Capacity (WHC) of Complex Gel
2.6. Textural Profile Analysis (TPA) of Complex Gel
2.7. Scanning Electron Microscopy (SEM) of Complex Gel
2.8. Light Microscopy Observation of MP/YBP Composite Gel Double-Stained with PAS (Periodic Acid-Schiff Stain)/Naphthol Yellow S
2.9. Chemical Force of Complex Gel
2.10. Fourier Transform Infrared Spectroscopy (FTIR) of Complex Gel
2.11. Low-Field Nuclear Magnetic Resonance (LF-NMR) of Complex Gel
2.12. Magnetic Resonance Imaging (MRI) of Complex Gel
2.13. Statistical Analysis
3. Results and Discussion
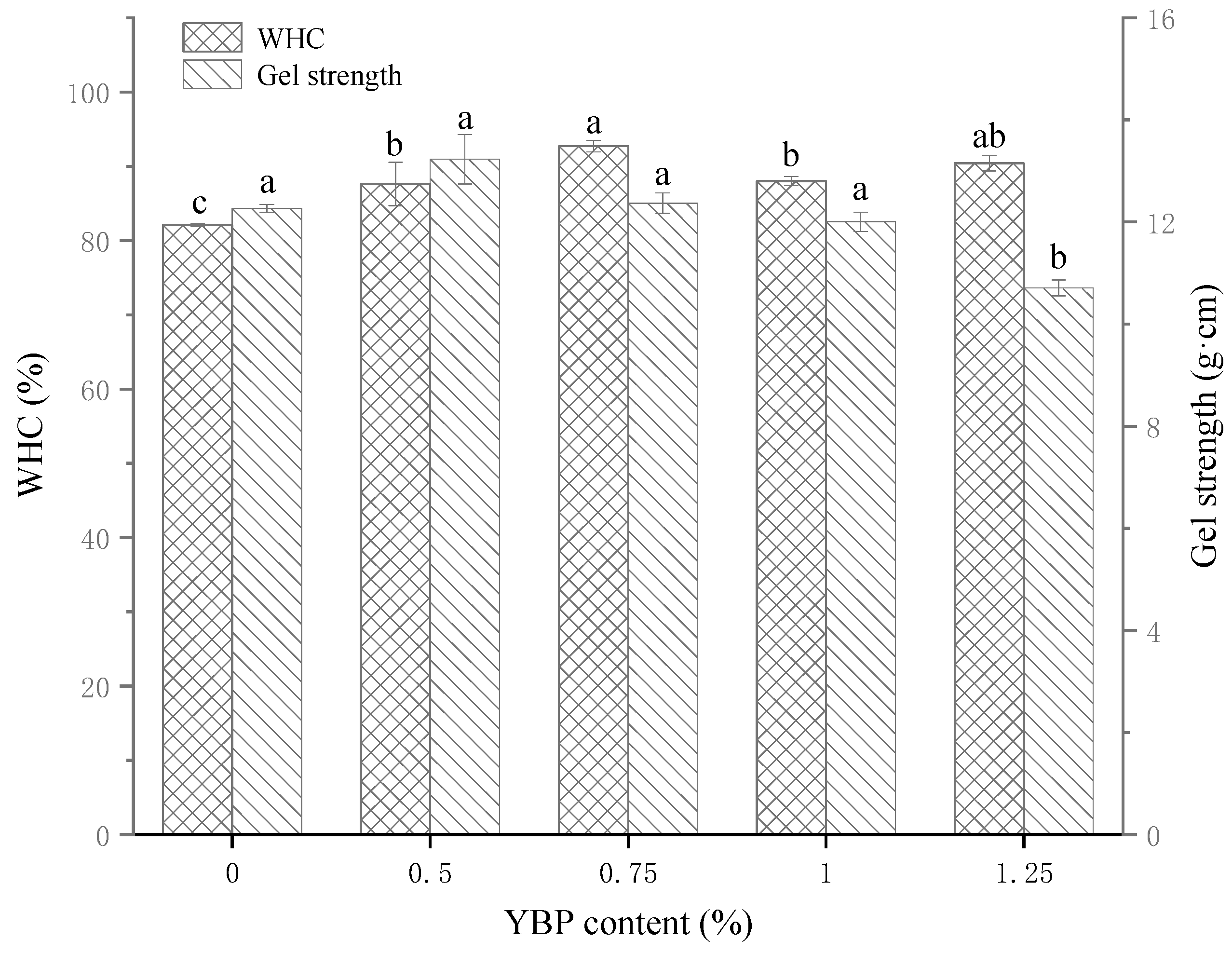
3.1. WHC and Gel Strength of MP/YBP Composite Gel
3.2. Microstructure of MP/YBP Composite Gel and Spatial Distribution of YBP in Composite Gel
3.2.1. Network Structure
3.2.2. YBP Distribution
3.3. Chemical Force of MP/YBP Composite Gel
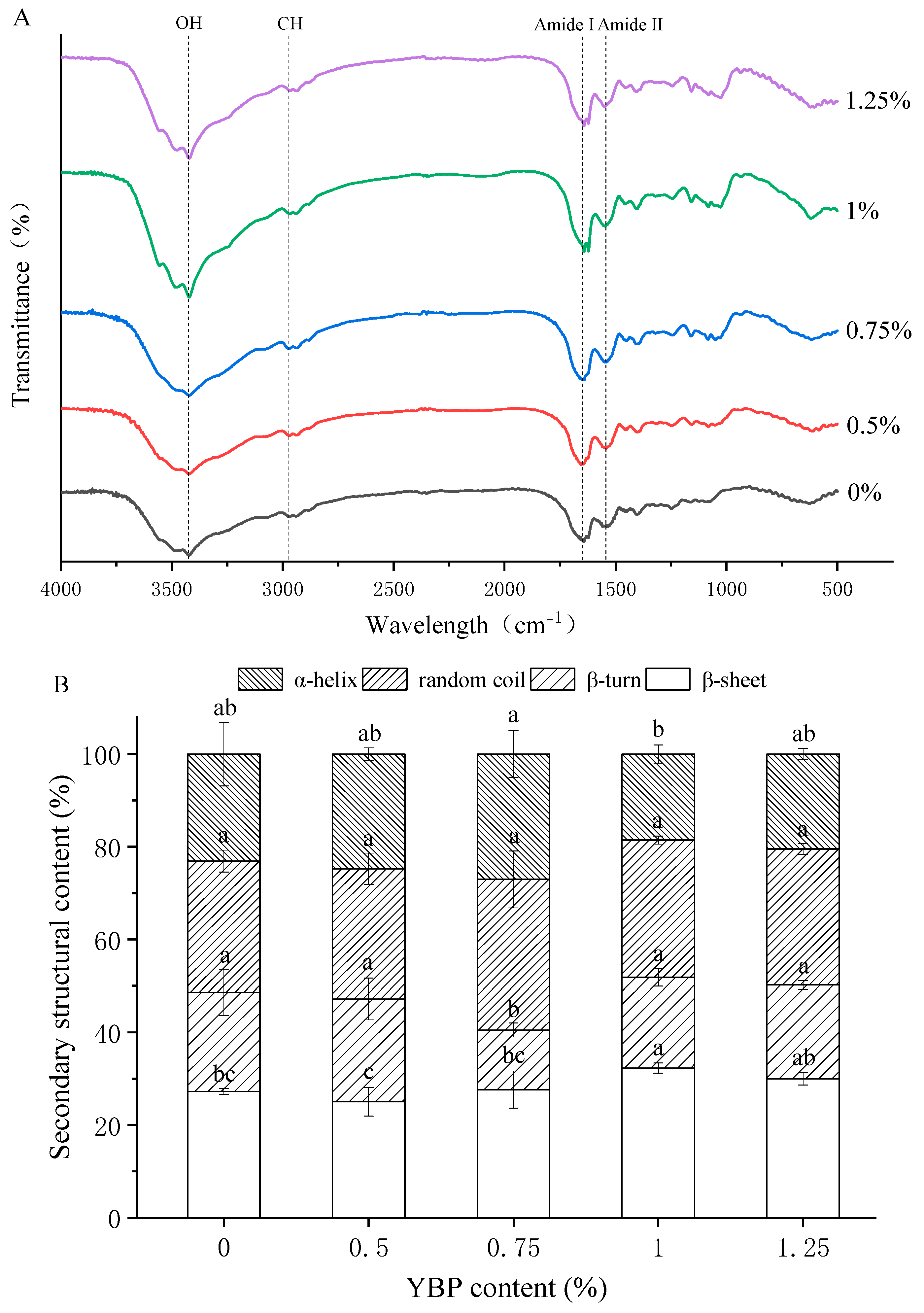
3.4. Molecular Structures of MP/YBP Composite Gel
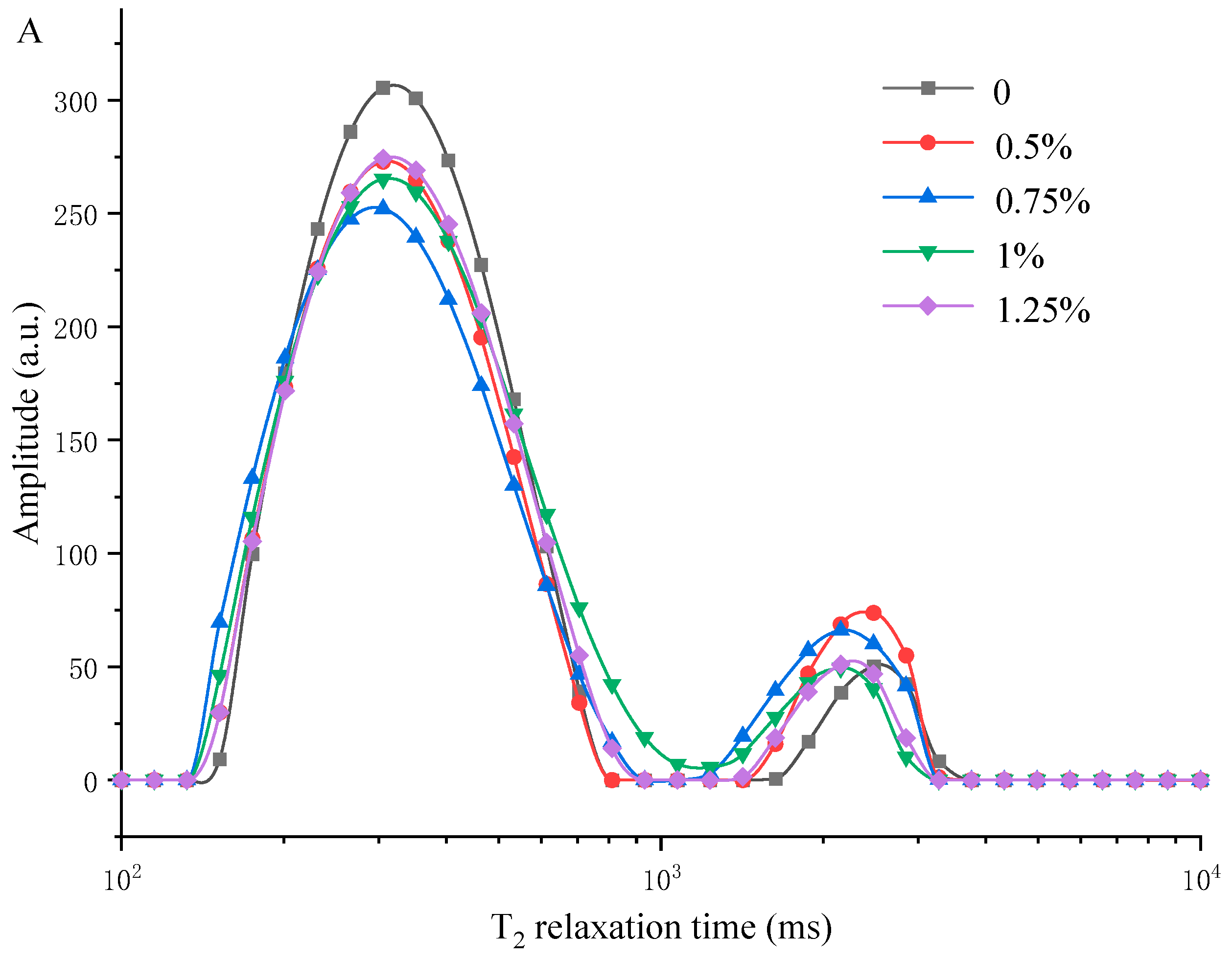
3.5. Measuring Water Distribution of MP/YBP Composite Gel Using LF-NMR and MRI
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Qiu, C.; Xia, W.; Jiang, Q. Effect of high hydrostatic pressure (HHP) on myofibril-bound serine proteinases and myofibrillar protein in silver carp (Hypophthalmichthys molitrix). Food Res. Int. 2013, 52, 199–205. [Google Scholar] [CrossRef]
- Luo, Y.K. Comparison of gel properties of surimi from Alaska pollock and three freshwater fish species: Effects of thermal processing and protein concentration. J. Food Sci. 2001, 66, 548–554. [Google Scholar] [CrossRef]
- Sun, X.D.; Holley, R.A. Factors Influencing Gel Formation by Myofibrillar Proteins in Muscle Foods. Compr. Rev. Food Sci. F. 2011, 10, 33–51. [Google Scholar] [CrossRef]
- Barrera, A.M.; Ramírez, J.A.; González-Cabriales, J.J.; Vázquez, M. Effect of pectins on the gelling properties of surimi from silver carp. Food Hydrocoll. 2002, 16, 441–447. [Google Scholar] [CrossRef]
- Li, C.T. Myofibrillar protein extracts from spent hen meat to improve whole muscle processed meats. Meat Sci. 2006, 72, 581–583. [Google Scholar] [CrossRef]
- Ramírez, J.A.; Uresti, R.M.; Velazquez, G.; Vázquez, M. Food hydrocolloids as additives to improve the mechanical and functional properties of fish products: A review. Food Hydrocoll. 2011, 25, 1842–1852. [Google Scholar] [CrossRef]
- Fogaça, F.H.S.; Trinca, L.A.; Bombo, Á.J.; Silvia Sant’Ana, L. Optimization of the Surimi Production from Mechanically Recovered Fish Meat (MRFM) Using Response Surface Methodology. J. Food Qual. 2013, 36, 209–216. [Google Scholar] [CrossRef]
- Karayannakidis, P.D.; Zotos, A.; Petridis, D.; Taylor, K.D.A. The Effect of Washing, Microbial Transglutaminase, Salts and Starch Addition on the Functional Properties of Sardine (Sardina Pilchardus) Kamaboko Gels. Food Sci. Technol. Int. 2008, 14, 167–177. [Google Scholar] [CrossRef]
- Fan, M.; Hu, T.; Zhao, S.; Xiong, S.; Xie, J.; Huang, Q. Gel characteristics and microstructure of fish myofibrillar protein/cassava starch composites. Food Chem. 2017, 218, 221–230. [Google Scholar] [CrossRef]
- Contreras-Jimenez, B.; Vazquez-Contreras, G.; de Los Angeles Cornejo-Villegas, M.; Del Real-Lopez, A.; Rodriguez-Garcia, M.E. Structural, morphological, chemical, vibrational, pasting, rheological, and thermal characterization of isolated jicama (Pachyrhizus spp.) starch and jicama starch added with Ca(OH)2. Food Chem. 2019, 283, 83–91. [Google Scholar] [CrossRef]
- Noman, A.S.M.; Hoque, M.A.; Haque, M.M.; Pervin, F.; Karim, M.R. Nutritional and anti-nutritional components in Pachyrhizus erosus L. tuber. Food Chem. 2007, 102, 1112–1118. [Google Scholar] [CrossRef]
- Stevenson, D.G.; Jane, J.-l.; Inglett, G.E. Characterisation of Jícama (Mexican Potato) (Pachyrhizus erosus L. Urban) Starch from Taproots Grown in USA and Mexico. Starch-Stärke 2007, 59, 132–140. [Google Scholar] [CrossRef]
- Yang, W.; Zhao, B.; Gao, A.; Huang, Y.; Xie, H.; Gong, J.; Ni, S. Overview of Pharmacological Research on Pachyrhizus erosus Urban (in Chinese). J. Anhui Agri. Sci. 2011, 39, 20391–20392. [Google Scholar]
- Zhou, F.; Zhao, M.; Zhao, H.; Sun, W.; Cui, C. Effects of oxidative modification on gel properties of isolated porcine myofibrillar protein by peroxyl radicals. Meat Sci. 2014, 96, 1432–1439. [Google Scholar] [CrossRef]
- Badrie, N.; Mellowes, W.A. Extrusion processing of cassava: Formulation of snacks. Cassava Flour Starch Prog. Res. Dev. 2002, 35, 304–312. [Google Scholar]
- Arfat, Y.A.; Benjakul, S. Gelling characteristics of surimi from yellow stripe trevally (Selaroides leptolepis). Int. Aquat. Res. 2012, 4, 5. [Google Scholar] [CrossRef]
- Sutton, D.S.; Ellis, M.; Lan, Y.; McKeith, F.K.; Wilson, E.R. Influence of slaughter weight and stress gene genotype on the water-holding capacity and protein gel characteristics of three porcine muscles. Meat Sci. 1997, 46, 173–180. [Google Scholar] [CrossRef]
- Xia, M.; Chen, Y.; Guo, J.; Huang, H.; Wang, L.; Wu, W.; Xiong, G.; Sun, W. Water distribution and textual properties of heat-induced pork myofibrillar protein gel as affected by sarcoplasmic protein. LWT-Food Sci. Technol. 2019, 103, 308–315. [Google Scholar] [CrossRef]
- Xia, X.; Kong, B.; Xiong, Y.; Ren, Y. Decreased gelling and emulsifying properties of myofibrillar protein from repeatedly frozen-thawed porcine longissimus muscle are due to protein denaturation and susceptibility to aggregation. Meat Sci. 2010, 85, 481–486. [Google Scholar] [CrossRef]
- Jia, R.; Katano, T.; Yoshimoto, Y.; Gao, Y.; Watanabe, Y.; Nakazawa, N.; Osako, K.; Okazaki, E. Sweet potato starch with low pasting temperature to improve the gelling quality of surimi gels after freezing. Food Hydrocoll. 2018, 81, 467–473. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, S.-M.; Xie, B.-J.; Xiong, S.-B. Contribution of protein conformation and intermolecular bonds to fish and pork gelation properties. Food Hydrocoll. 2011, 25, 898–906. [Google Scholar] [CrossRef]
- Huang, X.; Sun, L.; Liu, L.; Wang, G.; Luo, P.; Tang, D.; Huang, Q. Study on the mechanism of mulberry polyphenols inhibiting oxidation of beef myofibrillar protein. Food Chem. 2022, 372, 131241. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Bertram, H.C. Designing healthier comminuted meat products: Effect of dietary fibers on water distribution and texture of a fat-reduced meat model system. Meat Sci. 2017, 133, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xiong, Y.; Bakry, A.M.; Xiong, S.; Yin, T.; Zhang, B.; Huang, J.; Liu, Z.; Huang, Q. Effect of yeast β-glucan on gel properties, spatial structure and sensory characteristics of silver carp surimi. Food Hydrocoll. 2019, 88, 256–264. [Google Scholar] [CrossRef]
- Yao, J.; Zhou, Y.; Chen, X.; Ma, F.; Li, P.; Chen, C. Effect of sodium alginate with three molecular weight forms on the water holding capacity of chicken breast myosin gel. Food Chem. 2018, 239, 1134–1142. [Google Scholar] [CrossRef]
- Sun, F.; Huang, Q.; Hu, T.; Xiong, S.; Zhao, S. Effects and mechanism of modified starches on the gel properties of myofibrillar protein from grass carp. Int. J. Biol. Macromol. 2014, 64, 17–24. [Google Scholar] [CrossRef]
- Zhuang, X.; Wang, L.; Jiang, X.; Chen, Y.; Zhou, G. The effects of three polysaccharides on the gelation properties of myofibrillar protein: Phase behaviour and moisture stability. Meat Sci. 2020, 170, 108228. [Google Scholar] [CrossRef]
- Li, X.; Fan, M.; Huang, Q.; Zhao, S.; Xiong, S.; Zhang, B.; Yin, T. Effect of wet-media milling on the physicochemical properties of tapioca starch and their relationship with the texture of myofibrillar protein gel. Food Hydrocoll. 2020, 109, 106082. [Google Scholar] [CrossRef]
- Zhuang, X.; Han, M.; Bai, Y.; Liu, Y.; Xing, L.; Xu, X.-l.; Zhou, G.-h. Insight into the mechanism of myofibrillar protein gel improved by insoluble dietary fiber. Food Hydrocoll. 2018, 74, 219–226. [Google Scholar] [CrossRef]
- Xu, J.; Fan, Y.; Chen, Q.; Sun, F.; Li, M.; Kong, B.; Xia, X. Effects of kappa-carrageenan gum on 3D printability and rheological properties of pork pastes. Meat Sci. 2023, 197, 109078. [Google Scholar] [CrossRef]
- Ayadi, M.A.; Kechaou, A.; Makni, I.; Attia, H. Influence of carrageenan addition on turkey meat sausages properties. J. Food Eng. 2009, 93, 278–283. [Google Scholar] [CrossRef]
- Yang, H.; Park, J.W. Effects of Starch Properties and Thermal-processing Conditions on Surimi–Starch Gels. LWT-Food Sci. Technol. 1998, 31, 344–353. [Google Scholar] [CrossRef]
- Fan, M.; Huang, Q.; Zhong, S.; Li, X.; Xiong, S.; Xie, J.; Yin, T.; Zhang, B.; Zhao, S. Gel properties of myofibrillar protein as affected by gelatinization and retrogradation behaviors of modified starches with different crosslinking and acetylation degrees. Food Hydrocoll. 2019, 96, 604–616. [Google Scholar] [CrossRef]
- Zhuang, X.; Jiang, X.; Han, M.; Kang, Z.-L.; Zhao, L.; Xu, X.-L.; Zhou, G.-H. Influence of sugarcane dietary fiber on water states and microstructure of myofibrillar protein gels. Food Hydrocoll. 2016, 57, 253–261. [Google Scholar] [CrossRef]
- Kong, C.S.; Ogawa, H.; Iso, N. Compression Properties of Fish-Meat Gel as Affected by Gelatinization of Added Starch. J. Food Sci. 1999, 64, 283–286. [Google Scholar] [CrossRef]
- Li, N.; Niu, M.; Zhang, B.; Zhao, S.; Xiong, S.; Xie, F. Effects of concurrent ball milling and octenyl succinylation on structure and physicochemical properties of starch. Carbohydr. Polym. 2017, 155, 109–116. [Google Scholar] [CrossRef]
- Cao, H.; Fan, D.; Jiao, X.; Huang, J.; Zhao, J.; Yan, B.; Zhou, W.; Zhang, W.; Zhang, H. Effects of microwave combined with conduction heating on surimi quality and morphology. J. Food Eng. 2018, 228, 1–11. [Google Scholar] [CrossRef]
- Zheng, H.; Han, M.; Bai, Y.; Xu, X.; Zhou, G. Combination of high pressure and heat on the gelation of chicken myofibrillar proteins. Innov. Food Sci. Emerg. Technol. 2019, 52, 122–130. [Google Scholar] [CrossRef]
- Jia, D.; Huang, Q.; Xiong, S. Chemical interactions and gel properties of black carp actomyosin affected by MTGase and their relationships. Food Chem. 2016, 196, 1180–1187. [Google Scholar] [CrossRef]
- Li, X.; Fan, M.; Huang, Q.; Zhao, S.; Xiong, S.; Yin, T.; Zhang, B. Effect of micro- and nano-starch on the gel properties, microstructure and water mobility of myofibrillar protein from grass carp. Food Chem. 2022, 366, 130579. [Google Scholar] [CrossRef]
- Rashid, I.; Omari, M.H.A.; Leharne, S.A.; Chowdhry, B.Z.; Badwan, A. Starch gelatinization using sodium silicate: FTIR, DSC, XRPD, and NMR studies. Starch-Stärke 2012, 64, 713–728. [Google Scholar] [CrossRef]
- Kačuráková, M.; Capek, P.; Sasinková, V.; Wellner, N.; Ebringerová, A. FT-IR study of plant cell wall model compounds: Pectic polysaccharides and hemicelluloses. Carbohydr. Polym. 2000, 43, 195–203. [Google Scholar] [CrossRef]
- Kaddour, A.A.; Mondet, M.; Cuq, B. Description of Chemical Changes Implied During Bread Dough Mixing by FT-ATR Mid-Infrared Spectroscopy. Cereal Chem. 2008, 85, 673–678. [Google Scholar] [CrossRef]
- Carbonaro, M.; Nucara, A. Secondary structure of food proteins by Fourier transform spectroscopy in the mid-infrared region. Amino Acids 2010, 38, 679–690. [Google Scholar] [CrossRef]
- Nawrocka, A.; Szymańska-Chargot, M.; Miś, A.; Ptaszyńska, A.A.; Kowalski, R.; Waśko, P.; Gruszecki, W.I. Influence of dietary fibre on gluten proteins structure—A study on model flour with application of FT-Raman spectroscopy. J. Raman Spectrosc. 2015, 46, 309–316. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Wang, J.-z.; Zhang, C.-h.; Sun, H.-m.; Wang, C.-q.; Xie, X.-l. Effects of Oxidation on Water Distribution and Physicochemical Properties of Porcine Myofibrillar Protein Gel. Food Biophys. 2014, 9, 169–178. [Google Scholar] [CrossRef]


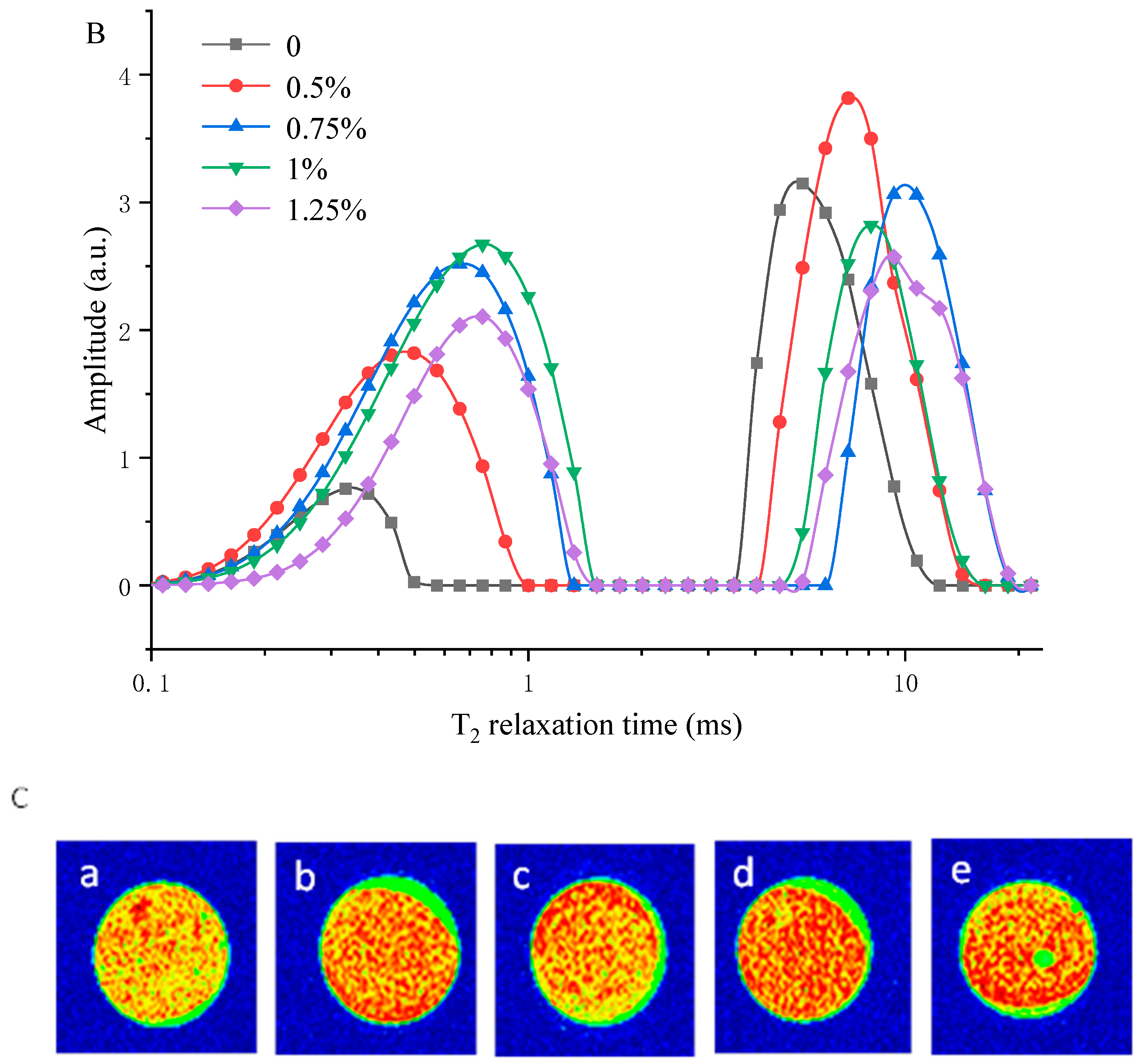
| YBP Content (%) | PT21 (%) | PT22a (%) | PT22b (%) | PT23 (%) |
|---|---|---|---|---|
| 0 | 0.40 ± 0.32 b | 0.59 ± 0.08 a | 92.47 ± 0.25 a | 6.54 ± 0.02 b |
| 0.5 | 0.58 ± 0.14 ab | 0.62 ± 0.15 a | 89.28 ± 1.80 ab | 9.52 ± 1.64 ab |
| 0.75 | 0.83 ± 0.21 a | 0.58 ± 0.14 a | 86.49 ± 3.62 b | 12.09 ± 3.57 a |
| 1 | 0.66 ± 0.31 ab | 0.71 ± 0.16 a | 87.24 ± 4.21 b | 11.39 ± 4.36 ab |
| 1.25 | 0.47 ± 0.19 ab | 0.66 ± 0.18 a | 89.19 ± 2.44 ab | 9.68 ± 2.48 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, G.; Yang, K.; Wu, D.; Lu, W.; Chi, R.; Ma, J.; Sun, W. Yam Bean (Pachyrhizus erosus L. Urban) Powder Improves Grass Carp Myofibrillar Protein Gel by Forming Disulfide Bonds, Hydrogen Bonds, and Proper Microstructure. Foods 2023, 12, 2072. https://doi.org/10.3390/foods12102072
Song G, Yang K, Wu D, Lu W, Chi R, Ma J, Sun W. Yam Bean (Pachyrhizus erosus L. Urban) Powder Improves Grass Carp Myofibrillar Protein Gel by Forming Disulfide Bonds, Hydrogen Bonds, and Proper Microstructure. Foods. 2023; 12(10):2072. https://doi.org/10.3390/foods12102072
Chicago/Turabian StyleSong, Geyao, Kun Yang, Di Wu, Weiwei Lu, Rongshuo Chi, Jing Ma, and Weiqing Sun. 2023. "Yam Bean (Pachyrhizus erosus L. Urban) Powder Improves Grass Carp Myofibrillar Protein Gel by Forming Disulfide Bonds, Hydrogen Bonds, and Proper Microstructure" Foods 12, no. 10: 2072. https://doi.org/10.3390/foods12102072