Identification of Eight High Yielding Strains via Morpho-Molecular Characterization of Thirty-Three Wild Strains of Calocybe indica
Abstract
:1. Introduction
2. Materials and Methods
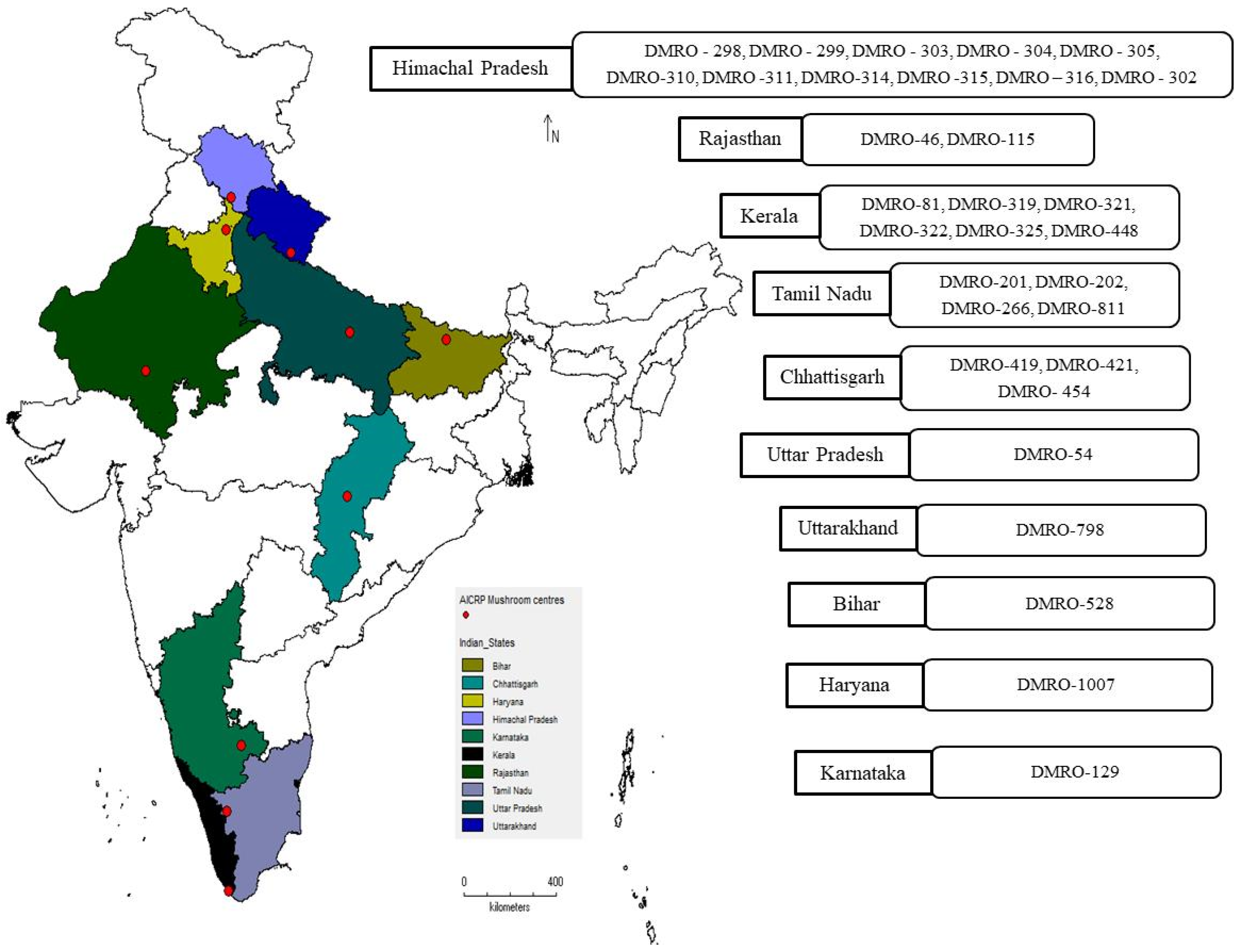
2.1. Strains
2.2. Molecular Identification of the Different Strains of C. indica
2.3. Agronomical Evaluation
2.4. Morphological Characterization
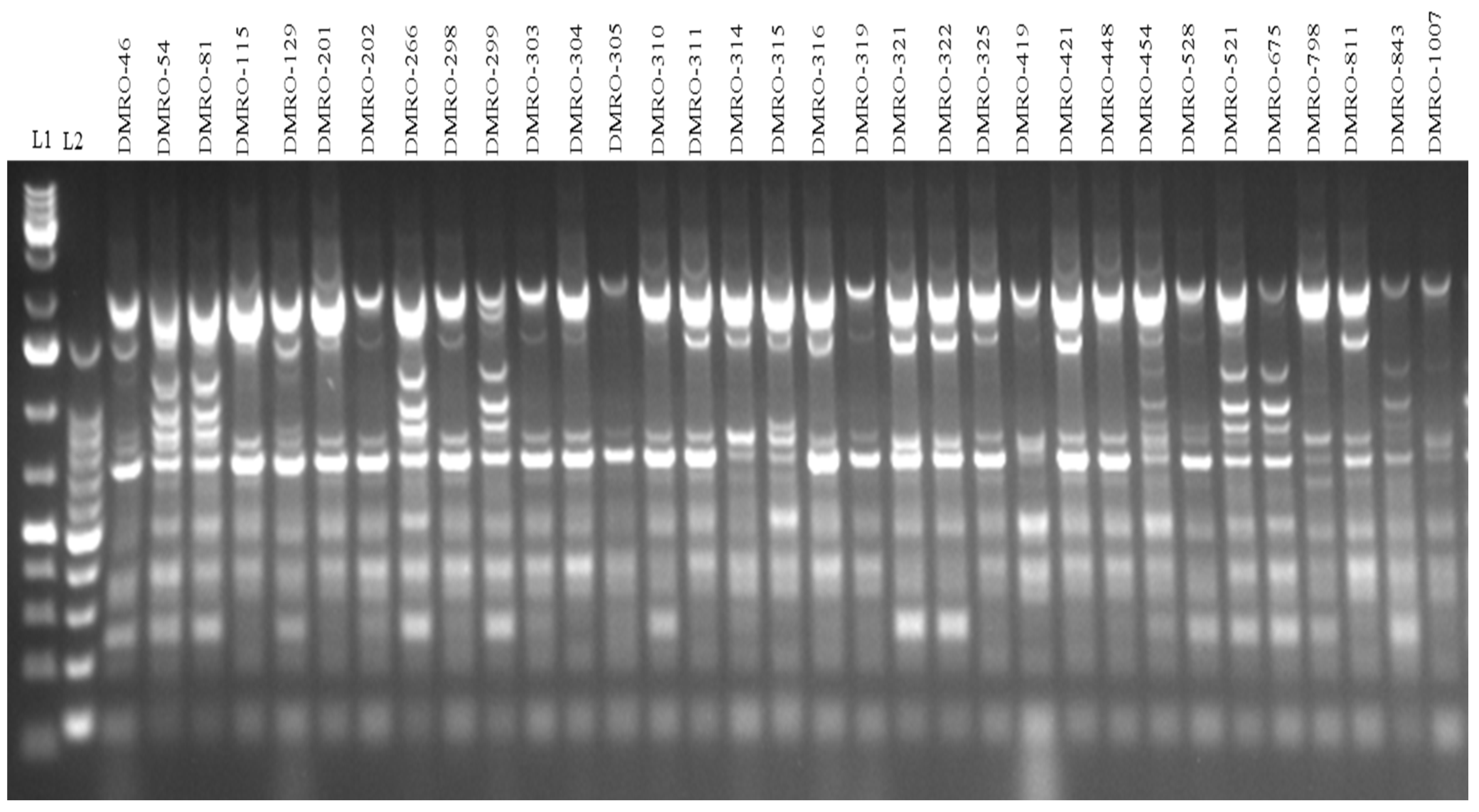
2.5. Genetic Diversity Analysis Using SRAP Markers
2.6. Proximate Analysis of High Yielding Strains of C. indica
2.7. Statistical and Software Analysis
3. Results and Discussion
3.1. Molecular Identification and Morphological Yield Analysis of the Thirty-Three Strains of C. indica
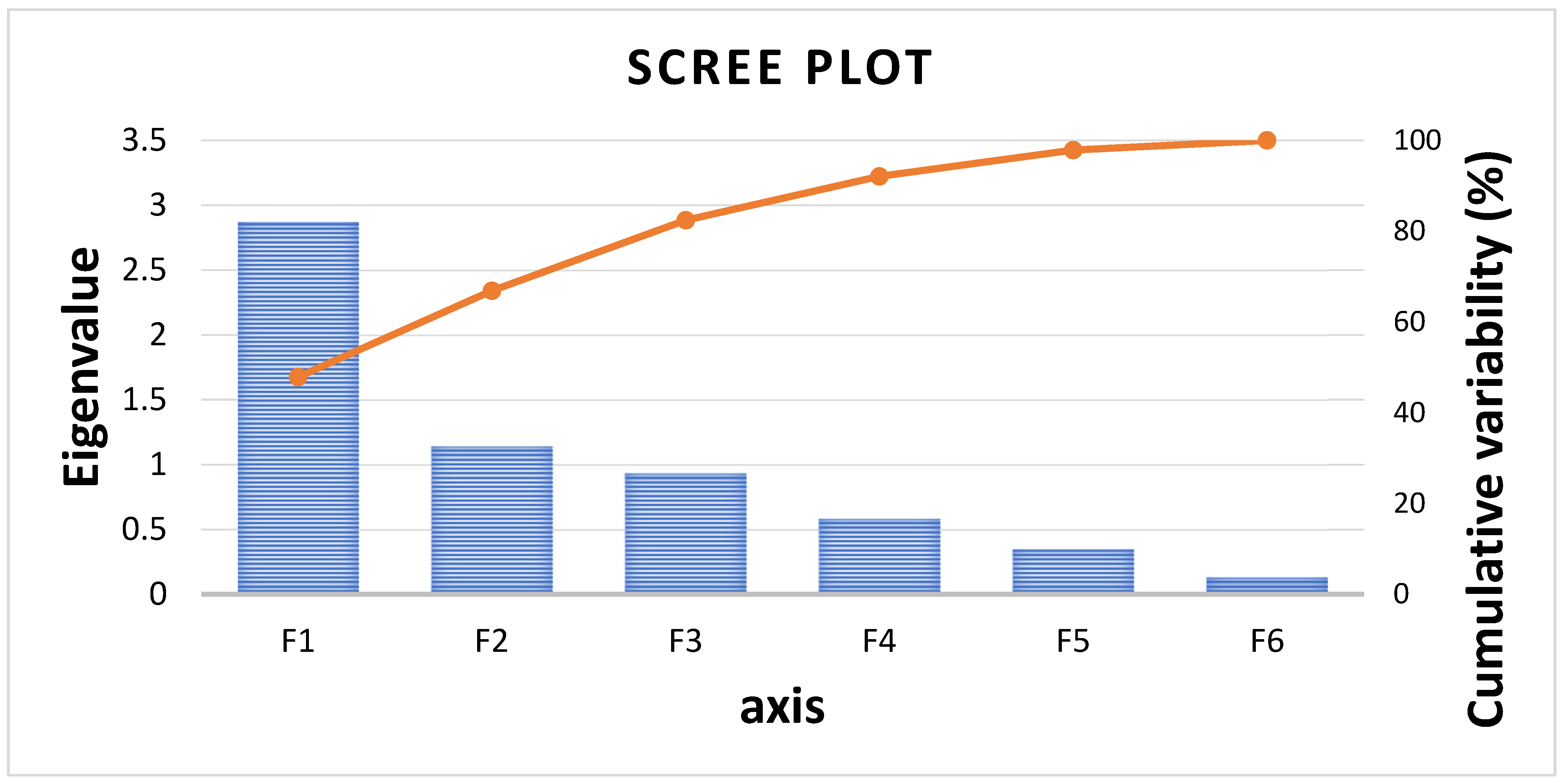
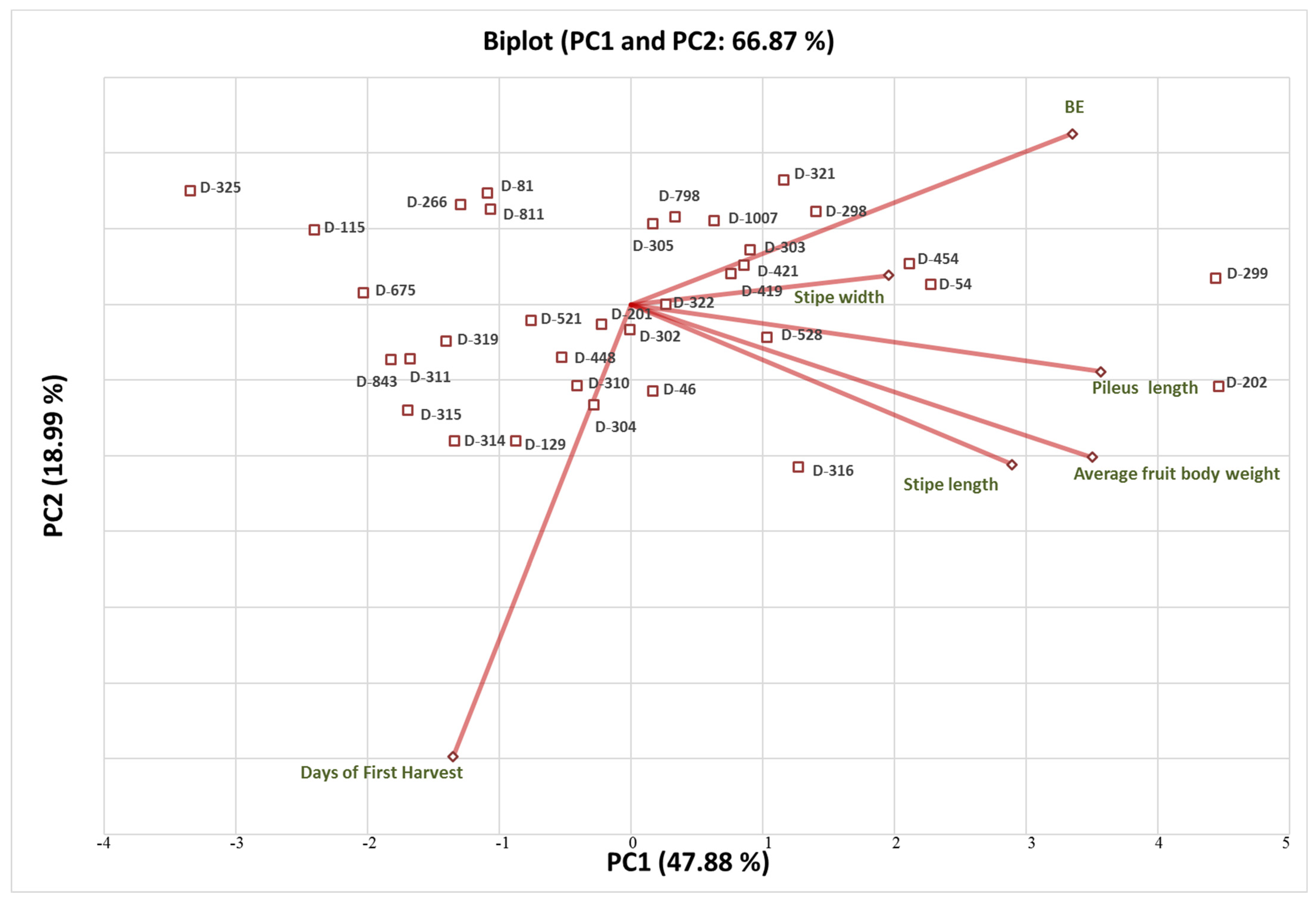
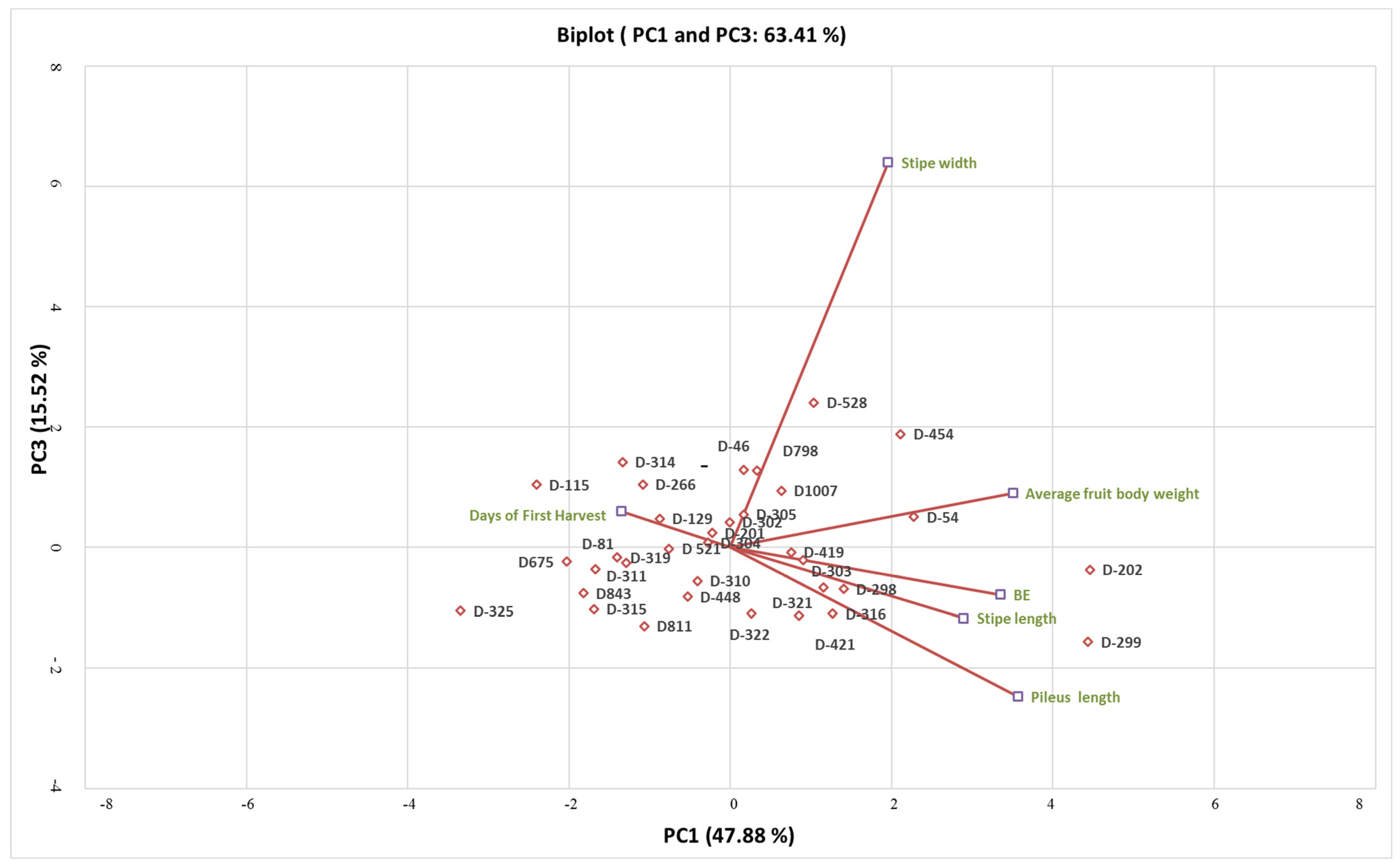
3.2. Principal Component Analysis
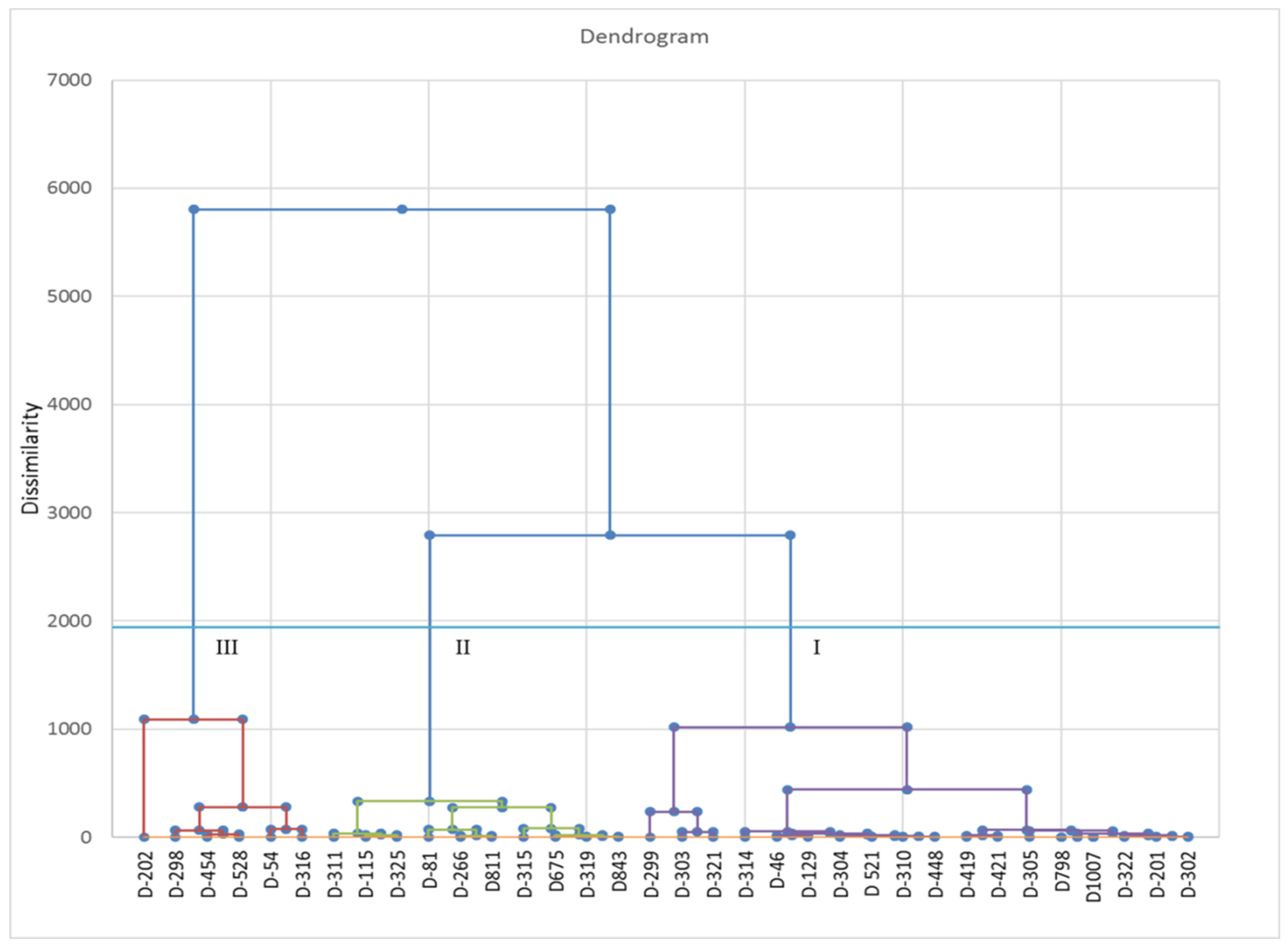
3.3. Correlation and Cluster Analysis of the Thirty-Three Strains of C. indica
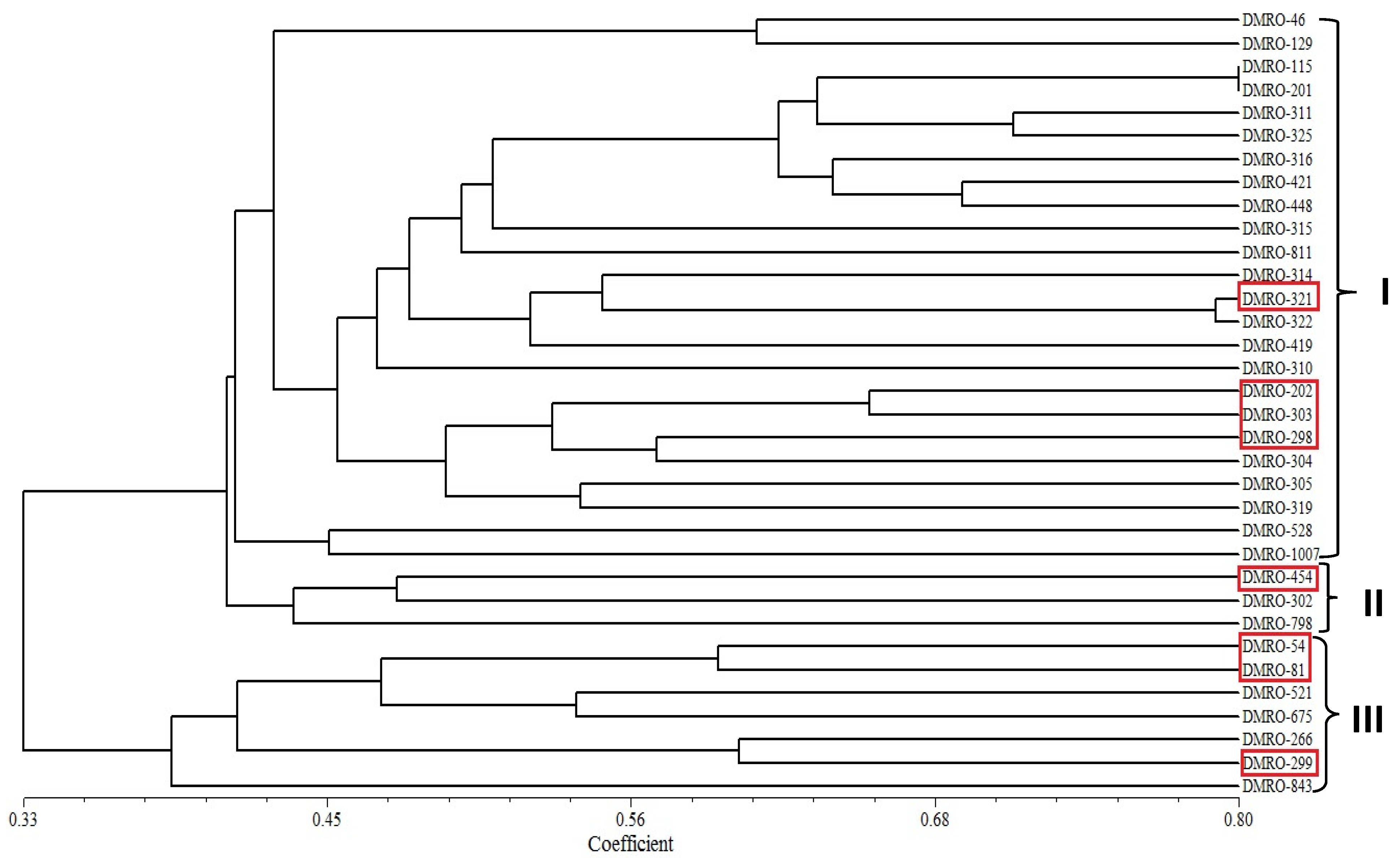
3.4. Phylogenetic Analysis of Different Strains of C. indica
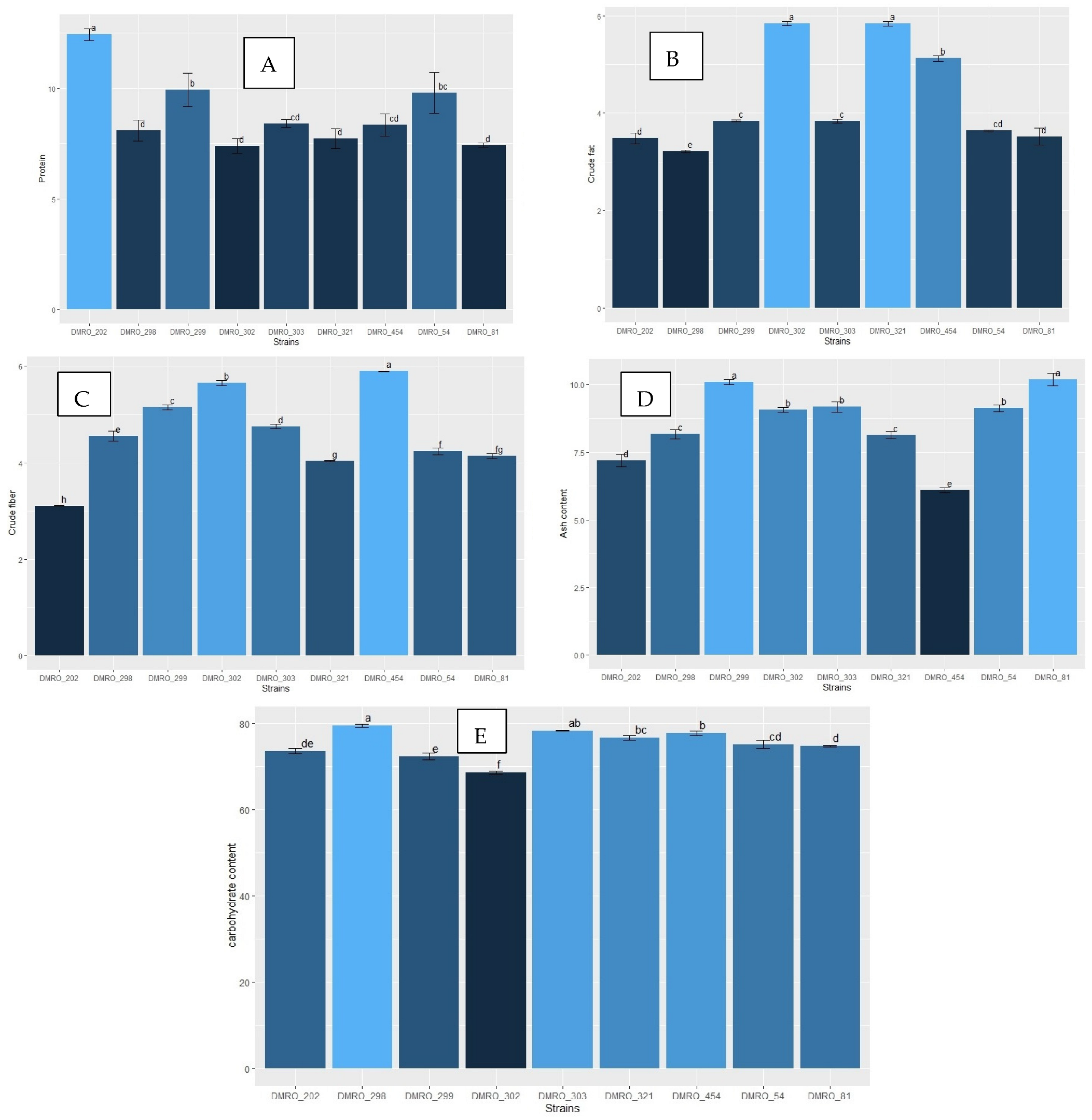
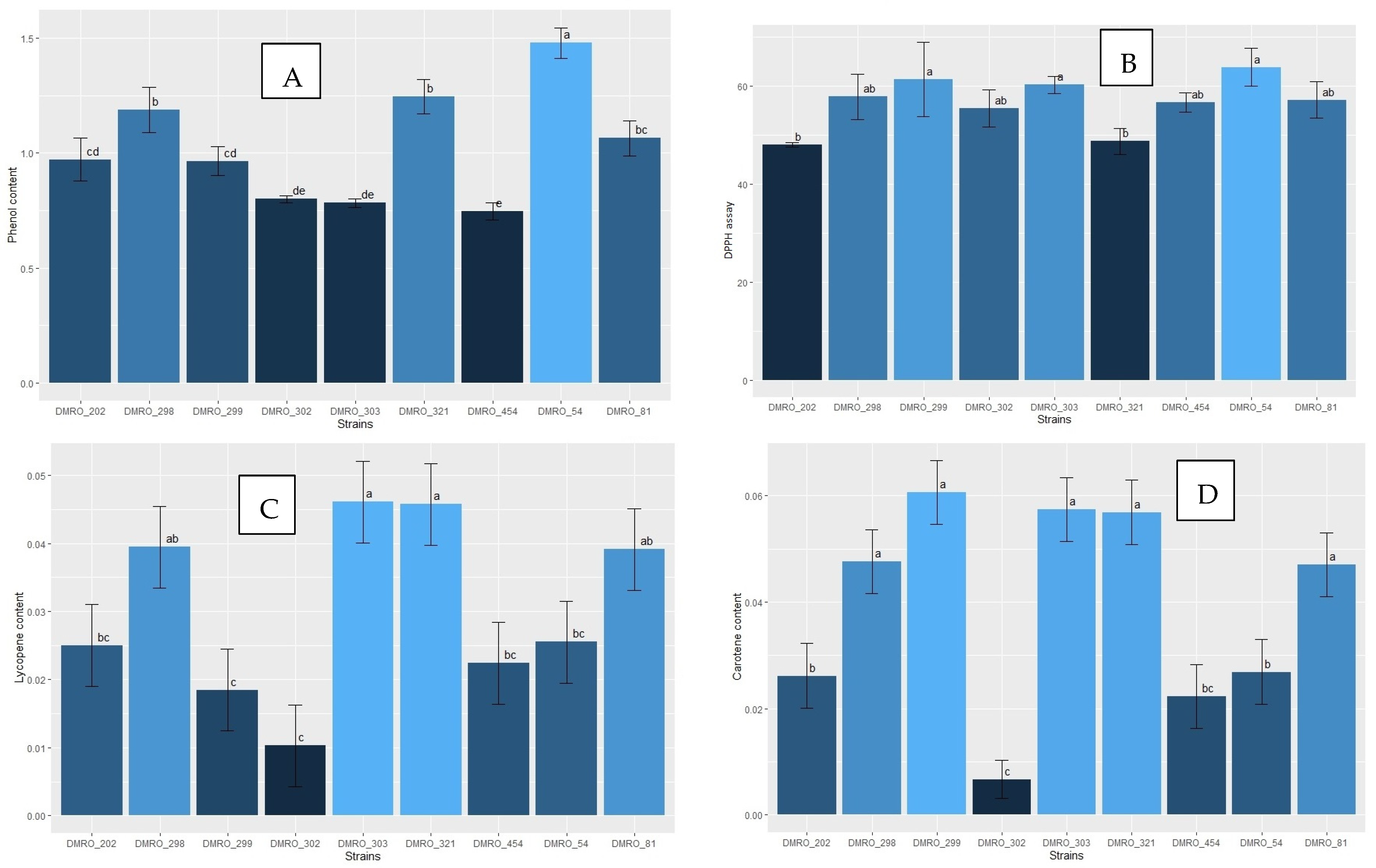
3.5. Nutritional Analysis of the Eight High Yielding Strains of C. indica
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alam, N.; Amin, R.; Khan, A.; Ara, I.; Shim, M.J.; Lee, M.W.; Lee, T.S. Nutritional analysis of cultivated mushrooms in Bangladesh—Pleurotus ostreatus, Pleurotus sajor-caju, Pleurotus florida and Calocybe indica. Mycobiology 2008, 36, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.P.; Singh, H. Advances in the cultivation technology of tropical mushrooms in India. JNKVV Res. J. 2014, 48, 120–135. [Google Scholar]
- Ghosh, S.; Acharya, K. Milky mushroom: A healthy nutritious diet. Food Res. Int. 2022, 156, 111113. [Google Scholar] [CrossRef] [PubMed]
- Kamal, S.; Barh, A.; Sharma, K.; Sharma, V.P. Mushroom Biology and Advances. In Agricultural Biotechnology: Latest Research and Trends; Kumar Srivastava, D., Kumar Thakur, A., Kumar, P., Eds.; Springer: Singapore, 2021. [Google Scholar] [CrossRef]
- Chakraborty, U.; Sikdar, S.R. Intergeneric protoplast fusion between Calocybe indica (milky mushroom) and Pleurotus florida aids in the qualitative and quantitative improvement of sporophore of the milky mushroom. World J. Microbiol. Biotechnol. 2010, 26, 213–225. [Google Scholar] [CrossRef]
- Krishnamoorthy, A.S.; Balan, V. A comprehensive review of tropical milky white mushroom (Calocybe indica P&C). Mycobiology 2015, 43, 184–194. [Google Scholar]
- Shashikant, M.; Bains, A.; Chawla, P.; Fogarasi, M.; Fogarasi, S. The current status, bioactivity, food, and pharmaceutical approaches of Calocybe indica: A review. Antioxidants 2022, 11, 1145. [Google Scholar] [CrossRef]
- Krishnamoorthy, A.S.; Muthuswamy, M.T.; Nakkeeran, S. Technique for commercial production of milky mushroom Calocybe indica P&C. Indian J. Mush. 2000, 18, 19–23. [Google Scholar]
- Rathore, H.; Sharma, A.; Prasad, S.; Kumar, A.; Shama, S.; Singh, A. Yield, nutritional composition and antioxidant properties of Calocybe indica cultivated on wheat straw basal substrate supplemented with nitrogenous tree leaves. Waste Biomass Valorization 2020, 11, 807–815. [Google Scholar] [CrossRef]
- Sharma, V.P.; Kumar, S.; Sharma, S. Technologies Developed by ICAR-DMR for Commercial Use; Indian Council of Agricultural Research (ICAR)-Directorate of Mushroom Research: Solan, India, 2020. [Google Scholar]
- Sharma, V.P.; Heera, G.; Kumar, S.; Nath, M. Development and identification of high yielding strain of Calocybe indica based on the multilocation trials. Mushroom Res. 2020, 29, 47–50. [Google Scholar]
- Sharma, J.P.; Kumar, S. Evaluation of strains of milky mushroom (Calocybe indica) for cultivation in Jharkhand. Mushroom Res. 2008, 17, 31–33. [Google Scholar]
- Dhakad, P.K.; Chandra, R.; Yadav, M.K.; Patar, U.R. Comparative study on growth parameters and yield potential of five strains of milky mushroom (Calocybe indica). J. Pure Appl. Microbiol. 2015, 9, 2333–2337. [Google Scholar]
- Bhupathi, P.; Subbaiah, K.A. Comparison of colony morphology, sporophore characters and yield performance of wild and cultivated milky mushroom isolates. J. Pure Appl. Microbiol. 2019, 13, 2405–2419. [Google Scholar] [CrossRef]
- Singh, K.; Sharma, S.; Kaur, R.; Sodhi, H.S. Evaluation of Calocybe indica strains for lignocellulolytic enzymes and mushroom yield potential. Indian J. Hortic. 2020, 77, 671–675. [Google Scholar] [CrossRef]
- Alzahib, R.H.; Migdadi, H.M.; Ghamdi, A.A.A.; Alwahibi, M.S.; Afzal, M.; Elharty, E.H.; Alghamdi, S.S. Exploring genetic variability among and within Hail tomato landraces based on sequence-related amplified polymorphism markers. Diversity 2021, 13, 135. [Google Scholar] [CrossRef]
- Li, G.; Quiros, C.F. Sequence-related amplified polymorphism (SRAP), a new marker system based on a simple PCR reaction: Its application to mapping and gene tagging in Brassica. Theor. Appl. Genet. 2001, 103, 455–461. [Google Scholar] [CrossRef]
- Fu, L.Z.; Zhang, H.Y.; Wu, X.Q.; Li, H.B.; Wei, H.; Wu, Q.Q.; Wang, L.A. Evaluation of genetic diversity in Lentinula edodes strains using RAPD, ISSR and SRAP markers. World J. Microbiol. Biotechnol. 2010, 26, 709–716. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.R.; Li, C.; Bian, Y.B.; Xiao, Y. Evaluating genetic diversity and constructing core collections of Chinese Lentinula edodes cultivars using ISSR and SRAP markers. J. Basic Microbiol. 2015, 55, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Ma, B.; Luo, X.; Zheng, L.; Xu, X.; Yang, Z. Molecular diversity of Auricularia polytricha revealed by inter-simple sequence repeat and sequence-related amplified polymorphism markers. Curr. Microbiol. 2008, 56, 240–245. [Google Scholar] [CrossRef]
- Tang, L.; Xiao, Y.; Li, L.; Guo, Q.; Bian, Y. Analysis of genetic diversity among Chinese Auricularia auricula cultivars using combined ISSR and SRAP markers. Curr. Microbiol. 2010, 61, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Lu, L.; Wang, P.; Fang, M.; Zhang, Y.; Chen, Y.; Zhang, W.; Kong, X.; Lu, J.; Honda, Y. Development of a molecular marker for fruiting body pattern in Auricularia auricula-judae. Mycobiology 2018, 46, 72–78. [Google Scholar] [CrossRef]
- Du, J.; Guo, H.-B.; Li, Q.; Forsythe, A.; Chen, X.-H.; Yu, X.-D. Genetic diversity of Lepista nuda (Agaricales, Basidiomycota) in Northeast China as indicated by SRAP and ISSR markers. PLoS ONE 2018, 13, e0202761. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.S.; Xu, B.L.; Liu, L.D.; Yuan, Q.Q.; Dong, H.X.; Cheng, X.H.; Lin, D.L. Analysis of genetic diversity among Chinese Pleurotus citrinopileatus Singer cultivars using two molecular marker systems (ISSRs and SRAPs) and morphological traits. World J. Microbiol. Biotechnol. 2012, 28, 2237–2248. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Liu, Y.; Li, H.; Zhao, S.; Wang, S.; Liu, Y.; Wu, D.; Xu, F. Genetic diversity of Pleurotus pulmonarius revealed by RAPD, ISSR, and SRAP fingerprinting. Curr. Microbiol. 2014, 68, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Barh, A.; Kamal, S.; Sharma, V.P.; Sharma, K.; Kumari, B.; Nath, M. Identification and morpho-molecular characterization of low spore strain in oyster mush-room. Mol. Biol. Rep. 2023. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ding, F.; Song, H.; Chen, M.; Hu, D. Analysis of genetic diversity among Chinese Cyclocybe chaxingu strains using ISSR and SRAP markers. PeerJ 2022, 10, e14037. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Singh, G.; Pandey, P.; Mishra, P. Cultural, physiological characteristics and yield attributes of strains of milky mushroom (Calocybe indica). J. Mycol. Plant Pathol. 2011, 41, 67. [Google Scholar]
- White, T.J.; Bruns, T.D.; Lee, S.S.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Application; Innis, M.A., Gelf, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Sharma, V.; Kumar, S. Spawn production technology. In Mushrooms: Cultivation, Marketing and Consumption; Singh, M., Vijay, B., Kamal, S., Wakchaure, G.C., Eds.; Indian Council of Agricultural Research (ICAR)-Directorate of Mushroom Research: Solan, India, 2011; pp. 31–42. [Google Scholar]
- Nath, M.; Barh, A.; Sharma, A.; Bijla, S.; Bairwa, R.K.; Kamal, S.; Sharma, V. Impact of the different casing material on the yield of Calocybe indica in polyethylene bag and bed system. Mushroom Res. 2022, 31, 181–186. [Google Scholar] [CrossRef]
- Sharma, V.P.; Kumari, B.; Barh, A.; Kamal, S.; Kashyap, R.; Annepu, S.K. Biochemical profiling and cultivation of medicinal fungus Isaria cicadae (Ascomycetes) from India. Int. J. Med. Mushrooms 2021, 23, 91–101. [Google Scholar] [CrossRef]
- Sheoran, O.P.; Tonk, D.S.; Kaushik, L.S.; Hasija, R.C.; Pannu, R.S. Statistical Software Package for Agricultural Research Workers. In Recent Advances in Information Theory, Statistics & Computer Applications; Hooda, D.S., Hasija, R.C., Eds.; Department of Mathematics Statistics, CCS HAU: Haryana, India, 1998; pp. 139–143. [Google Scholar]
- Vidal, N.P.; Manful, C.F.; Pham, T.H.; Stewart, P.; Keough, D.; Thomas, R.H. The use of XLSTAT in conducting principal component analysis (PCA) when evaluating the relationships between sensory and quality attributes in grilled foods. MethodsX 2020, 7, 100835. [Google Scholar] [CrossRef]
- Rohlf, F.J. NTSYS-pc: Numerical Taxonomy and Multivariate Analysis System; Version 2.0; State University of New York: Stony Brook, NY, USA, 1998. [Google Scholar]
- Bellemain, E.; Carlsen, T.; Brochmann, C.; Coissac, E.; Taberlet, P.; Kauserud, H. ITS as an environmental DNA barcode for fungi: An in silico approach reveals potential PCR biases. BMC Microbiol. 2010, 10, 189. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, K.; Latha, R.; Thirukumaran, K. Assessment of Milky Mushroom Varieties in Kanyakumari District, India. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 1617–1623. [Google Scholar] [CrossRef]
- Kerketta, A.; Singh, H.K.; Shukla, C.S. Cultivation of milky mushroom collected from different region of Chhattisgarh state. Int. J. Chem. Stud. 2018, 6, 1418–1421. [Google Scholar]
- Barh, A.; Sharma, V.P.; Annepu, S.K.; Kumari, B.; Kamal, S.; Shirur, M.; Sharma, K. Selection of superior transgressive segregants from NBS-5 strain of white button mushroom. Mushroom Res. 2020, 29, 155–163. [Google Scholar] [CrossRef]
- Du, P.; Cui, B.K.; Dai, Y.C. Genetic diversity of wild Auricularia polytricha in Yunnan Province of South-western China revealed by sequence-related amplified polymorphism (SRAP) analysis. J. Med. Plants Res. 2011, 5, 1374–1381. [Google Scholar]
- Pedneault, K.; Angers, P.; Avis, T.J.; Gosselin, A.; Tweddell, R.J. Fatty acid profiles of polar and non-polar lipids of Pleurotus ostreatus and P. cornucopiae var. ‘citrino-pileatus’ grown at different temperatures. Mycol. Res. 2007, 111, 1228–1234. [Google Scholar] [CrossRef] [PubMed]
- Sumathy, R.; Kumuthakalavalli, R.; Krishnamoorthy, A.S. Proximate, vitamin, aminoacid and mineral composition of milky mushroom, Calocybe Indica (P&C). Var. Apk2 commonly cultivated in Tamil nadu. J. Nat. Prod. Plant Resour. 2015, 5, 38–43. [Google Scholar]
- Pushpa, H.; Purushothoma, K.B. Nutritional analysis of wild and cultivated edible medicinal mushrooms. World J. Dairy Food Sci. 2010, 5, 140–144. [Google Scholar]
- Manikandan, K. Nutritional and medicinal values of mushrooms. In Mushrooms: Cultivation, Marketing and Consumption; Singh, M., Vijay, B., Kamal, S., Wakchaure, G.C., Eds.; Indian Council of Agricultural Research (ICAR)-Directorate of Mushroom Research: Solan, India, 2011; pp. 11–14. [Google Scholar]
- Dhakad, P.K.; Chandra, R.; Yadav, M.K.; Patar, U.R. Comparative Study on Nutraceuticals of Five Strains of Milky Mushroom (Calocybe indica). Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 645–648. [Google Scholar] [CrossRef]
- Vishwakarma, P.; Singh, P.; Tripathi, N.N. Nutritional and antioxidant properties of wild edible macrofungi from North-Eastern Uttar Pradesh, India. Indian J. Tradit. Knowl. 2016, 15, 143–148. [Google Scholar]
- Saranya, V.; Madhanraj, P.; Panneerselvam, A. Cultivation, composting, biochemical and molecular characterization of Calocybe indica (C and A). Asian J. Pharm Res. 2011, 1, 55–57. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nath, M.; Barh, A.; Sharma, A.; Verma, P.; Bairwa, R.K.; Kamal, S.; Sharma, V.P.; Annepu, S.K.; Sharma, K.; Bhatt, D.; et al. Identification of Eight High Yielding Strains via Morpho-Molecular Characterization of Thirty-Three Wild Strains of Calocybe indica. Foods 2023, 12, 2119. https://doi.org/10.3390/foods12112119
Nath M, Barh A, Sharma A, Verma P, Bairwa RK, Kamal S, Sharma VP, Annepu SK, Sharma K, Bhatt D, et al. Identification of Eight High Yielding Strains via Morpho-Molecular Characterization of Thirty-Three Wild Strains of Calocybe indica. Foods. 2023; 12(11):2119. https://doi.org/10.3390/foods12112119
Chicago/Turabian StyleNath, Manoj, Anupam Barh, Annu Sharma, Parul Verma, Rakesh Kumar Bairwa, Shwet Kamal, Ved Prakash Sharma, Sudheer Kumar Annepu, Kanika Sharma, Deepesh Bhatt, and et al. 2023. "Identification of Eight High Yielding Strains via Morpho-Molecular Characterization of Thirty-Three Wild Strains of Calocybe indica" Foods 12, no. 11: 2119. https://doi.org/10.3390/foods12112119






