Plant Antimicrobials for Food Quality and Safety: Recent Views and Future Challenges
Abstract
:1. Introduction
2. Classification and Antimicrobial Activity of Plant Antimicrobials
2.1. Polyphenols
2.1.1. Phenolic Acids
2.1.2. Stilbenes, Tannins, and Lignins
2.1.3. Flavonoids
2.2. Terpenes and Essential Oils
2.3. Glucosinolate Derivatives
2.4. Alkaloids and Thiols
2.5. Modes of Action
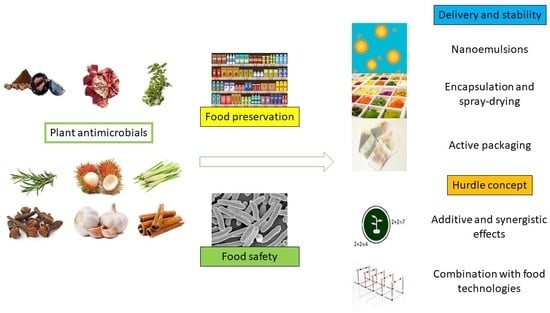
3. Plant Antimicrobials for Food Quality and Safety
3.1. Plant Antimicrobials as Food Preservatives
3.1.1. Applications in Plant Foods
3.1.2. Applications in Animal-Based Foods
| Food Matrix | Plant Antimicrobial | Concentration/Conditions | Antimicrobial Effect | Data from Ref.* |
|---|---|---|---|---|
| Mandarins | Pomegranate peel extract | Dipping in 25 g L−1 extract for 2 min | Reduction of lesion diameter and infection rate (80–90%) caused by P. italicum and P. digitatum | [118] |
| Fresh-cut lettuce | Pepper EO | 3–5 µL mL−1 addition in salad dressing | Reduction of P. fluorescens biomass by 30–40% | [129] |
| Concentrated apple juice | Thymol, carvacrol | MIC of 0.1–0.16 mM, treatment time 9 days | Reduction of Z. rouxii load by 99% | [134] |
| Pork burgers | Ethanolic extract of cranberry pomace | 2% extract-16 days of storage | Bacteriostatic effect on B. thermospacta and P. putida during cold storage | [140] |
| Snakehead fillets | Grape seed extract | 0.52 mg GAE mL−1 for 20 min | Decrease of Aeromonas spp. abundance by 37% and reduction of 1 log cfu g−1 of total viable counts during cold storage | [146] |
| Quark cheese | Arbutus unedo L. leaf extracts | 0.1 g 100 g−1 cheese, 8 days of cold storage | Reduction of total aerobic mesophilic bacteria and yeasts by 2–3 log cfu g−1 | [150] |
3.2. Use of Plant Antimicrobials for Food Safety
3.2.1. Effect on Viability of Foodborne Pathogens
3.2.2. Effect on Biofilm-Producing Strains
3.2.3. Effect on Microbial Toxins
4. Stabilization Techniques
4.1. Nano-Emulsions
4.2. Spray-Drying and Encapsulation
4.2.1. Spray-Drying Process
4.2.2. Other Encapsulation Techniques of Plant Antimicrobials
4.2.3. Challenges Associated with Spray-Drying and Encapsulation Techniques
4.3. Active Packaging
5. Combining Effects and Hurdle Technologies
5.1. Additive or Synergistic Effects
5.2. Hurdle Technologies
6. Regulation and Safety Issues of Plant Extracts
6.1. Heavy Metals and Crop-Protection Residues
6.2. Mycotoxins
6.3. Regulation
7. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bouarab Chibane, L.; Degraeve, P.; Ferhout, H.; Bouajila, J.; Oulahal, N. Plant antimicrobial polyphenols as potential natural food preservatives. J. Sci. Food Agric. 2019, 99, 1457–1474. [Google Scholar] [CrossRef] [PubMed]
- Olszewska, M.A.; Gędas, A.; Simões, M. Antimicrobial polyphenol-rich extracts: Applications and limitations in the food industry. Food Res. Int. 2020, 134, 109214. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Georgescu, C.; Turcuş, V.; Olah, N.K.; Mathe, E. An overview of natural antimicrobials role in food. Eur. J. Med. Chem. 2018, 143, 922–935. [Google Scholar] [CrossRef] [PubMed]
- Petruzzi, L.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Microbial spoilage of foods: Fundamentals. In The Microbiological Quality of Food; Bevilacqua, A., Corbo, M.R., Sinigaglia, M., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 1–21. [Google Scholar] [CrossRef]
- Joardder, M.U.; Masud, M.H. Food Preservation in Developing Countries: Challenges and Solutions; Springer: Berlin, Germany, 2019; pp. 1–239. [Google Scholar]
- McClements, D.J.; Das, A.K.; Dhar, P.; Nanda, P.K.; Chatterjee, N. Nanoemulsion-based technologies for delivering natural plant-based antimicrobials in foods. Front. Sustain. Food Syst. 2021, 5, 643208. [Google Scholar] [CrossRef]
- Favela-González, K.M.; Hernández-Almanza, A.Y.; De la Fuente-Salcido, N.M. The value of bioactive compounds of cruciferous vegetables (Brassica) as antimicrobials and antioxidants: A review. J. Food Biochem. 2020, 44, e13414. [Google Scholar] [CrossRef] [PubMed]
- Saeed, F.; Afzaal, M.; Tufail, T.; Ahmad, A. Use of natural antimicrobial agents: A safe preservation approach. In Active Antimicrobial Food Packaging; Var, I., Uzunlu, S., Eds.; IntechOpen: London, UK, 2019; Volume 18, pp. 7–24. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources Added to Food (EFSA ANS Panel); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; et al. Refined Exposure Assessment of Extracts of Rosemary (E 392) from Its Use as Food Additive. EFSA J. 2018, 16, e05373. [Google Scholar]
- Christopoulou, S.D.; Androutsopoulou, C.; Hahalis, P.; Kotsalou, C.; Vantarakis, A.; Lamari, F.N. Rosemary extract and essential oil as drink ingredients: An evaluation of their chemical composition, genotoxicity, antimicrobial, antiviral, and antioxidant properties. Foods 2021, 10, 3143. [Google Scholar] [CrossRef]
- Rathod, N.B.; Ranveer, R.C.; Benjakul, S.; Kim, S.K.; Pagarkar, A.U.; Patange, S.; Ozogul, F. Recent developments of natural antimicrobials and antioxidants on fish and fishery food products. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4182–4210. [Google Scholar] [CrossRef]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V.; Stefan, G. Plant polyphenols as antioxidant and antibacterial agents for shelf-life extension of meat and meat products: Classification, structures, sources, and action mechanisms. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1243–1268. [Google Scholar] [CrossRef]
- Pateiro, M.; Munekata, P.E.; Sant’Ana, A.S.; Domínguez, R.; Rodríguez-Lázaro, D.; Lorenzo, J.M. Application of essential oils as antimicrobial agents against spoilage and pathogenic microorganisms in meat products. Int. J. Food Microbiol. 2021, 337, 108966. [Google Scholar] [CrossRef]
- Patrignani, F.; Siroli, L.; Serrazanetti, D.I.; Gardini, F.; Lanciotti, R. Innovative strategies based on the use of essential oils and their components to improve safety, shelf-life and quality of minimally processed fruits and vegetables. Trends Food Sci. Technol. 2015, 46, 311–319. [Google Scholar] [CrossRef]
- Ceruso, M.; Clement, J.A.; Todd, M.J.; Zhang, F.; Huang, Z.; Anastasio, A.; Pepe, T.; Liu, Y. The inhibitory effect of plant extracts on growth of the foodborne pathogen, Listeria monocytogenes. Antibiotics 2020, 9, 319. [Google Scholar] [CrossRef] [PubMed]
- Kalogianni, A.I.; Lazou, T.; Bossis, I.; Gelasakis, A.I. Natural phenolic compounds for the control of oxidation, bacterial spoilage, and foodborne pathogens in meat. Foods 2020, 9, 794. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. The inhibitory effect of plant essential oils on foodborne pathogenic bacteria in food. Crit. Rev. Food Sci. Nutr. 2019, 59, 3281–3292. [Google Scholar] [CrossRef] [PubMed]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Makhuvele, R.; Naidu, K.; Gbashi, S.; Thipe, V.C.; Adebo, O.A.; Njobeh, P.B. The use of plant extracts and their phytochemicals for control of toxigenic fungi and mycotoxins. Heliyon 2020, 6, e05291. [Google Scholar] [CrossRef] [PubMed]
- Sakarikou, C.; Kostoglou, D.; Simões, M.; Giaouris, E. Exploitation of plant extracts and phytochemicals against resistant Salmonella spp. in biofilms. Food Res. Int. 2020, 128, 108806. [Google Scholar] [CrossRef]
- Qian, W.; Liu, M.; Fu, Y.; Zhang, J.; Liu, W.; Li, J.; Li, X.; Li, Y.; Wang, T. Antimicrobial mechanism of luteolin against Staphylococcus aureus and Listeria monocytogenes and its antibiofilm properties. Microb. Pathogen. 2020, 142, 104056. [Google Scholar] [CrossRef]
- Somrani, M.; Inglés, M.-C.; Debbabi, H.; Abidi, F.; Palop, A. Garlic, onion, and cinnamon essential oil anti-biofilms’ effect against Listeria monocytogenes. Foods 2020, 9, 567. [Google Scholar] [CrossRef]
- Castro-Rosas, J.; Ferreira-Grosso, C.R.; Gómez-Aldapa, C.A.; Rangel-Vargas, E.; Rodríguez-Marín, M.L.; Guzmán-Ortiz, F.A.; Falfan-Cortes, R.N. Recent advances in microencapsulation of natural sources of antimicrobial compounds used in food—A review. Food Res. Int. 2017, 102, 575–587. [Google Scholar] [CrossRef]
- Pinto, L.; Bonifacio, M.A.; De Giglio, E.; Santovito, E.; Cometa, S.; Bevilacqua, A.; Baruzzi, F. Biopolymer hybrid materials: Development, characterization, and food packaging applications. Food Packag. Shelf Life 2021, 28, 100676. [Google Scholar] [CrossRef]
- Iordache, A.M.; Nechita, C.; Voica, C.; Roba, C.; Botoran, O.R.; Ionete, R.E. Assessing the health risk and the metal content of thirty-four plant essential oils using the ICP-MS technique. Nutrients 2022, 14, 2363. [Google Scholar] [CrossRef] [PubMed]
- Ałtyn, I.; Twarużek, M. Mycotoxin contamination concerns of herbs and medicinal plants. Toxins 2020, 12, 182. [Google Scholar] [CrossRef] [PubMed]
- Fillâtre, Y.; Gray, F.X.; Roy, C. Pesticides in essential oils: Occurrence and concentration in organic and conventional orange essential oils from eleven geographical origins. Anal. Chim. Acta 2017, 992, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Current understanding of modes of action of multicomponent bioactive phytochemicals: Potential for nutraceuticals and antimicrobials. Annu. Rev. Food Sci. Technol. 2022, 13, 337–359. [Google Scholar] [CrossRef]
- Gutiérrez-del-Río, I.; Fernández, J.; Lombó, F. Plant nutraceuticals as antimicrobial agents in food preservation: Terpenoids, polyphenols and thiols. Int. J. Antimicrob. Agents 2018, 52, 309–315. [Google Scholar] [CrossRef]
- Villalobos-Delgado, L.H.; Nevárez-Moorillon, G.V.; Caro, I.; Quinto, E.J.; Mateo, J. Natural antimicrobial agents to improve foods shelf-life. In Food Quality and Shelf-Life; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 125–157. [Google Scholar] [CrossRef]
- Bae, J.Y.; Seo, Y.H.; Oh, S.W. Antibacterial activities of polyphenols against foodborne pathogens and their application as antibacterial agents. Food Sci. Biotechnol. 2022, 31, 985–997. [Google Scholar] [CrossRef]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef]
- Xu, J.G.; Hu, H.X.; Chen, J.Y.; Xue, Y.S.; Kodirkhonov, B.; Han, B.Z. Comparative study on inhibitory effects of ferulic acid and p-coumaric acid on Salmonella Enteritidis biofilm formation. World J. Microbiol. Biotechnol. 2022, 38, 136. [Google Scholar] [CrossRef]
- Li, J.; Zhao, N.; Xu, R.; Li, G.; Dong, H.; Wang, B.; Li, Z.; Fan, M.; Wei, X. Deciphering the antibacterial activity and mechanism of p-coumaric acid against Alicyclobacillus acidoterrestris and its application in apple juice. Int. J. Food Microbiol. 2022, 378, 109822. [Google Scholar] [CrossRef]
- Shi, Y.G.; Zhu, Y.J.; Shao, S.Y.; Zhang, R.R.; Wu, Y.; Zhu, C.M.; Liang, X.R.; Cai, W.Q. Alkyl ferulate esters as multifunctional food additives: Antibacterial activity and mode of action against Escherichia coli in vitro. J. Agric. Food Chem. 2018, 66, 12088–12101. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.G.; Zhang, R.R.; Zhu, C.M.; Liang, X.R.; Ettelaie, R.; Jiang, L.; Lin, S. On the mechanism behind enhanced antibacterial activity of alkyl gallate esters against foodborne pathogens and its application in Chinese icefish preservation. Food Microbiol. 2021, 99, 103817. [Google Scholar] [CrossRef] [PubMed]
- Patzke, H.; Schieber, A. Growth-inhibitory activity of phenolic compounds applied in an emulsifiable concentrate-ferulic acid as a natural pesticide against Botrytis cinerea. Food Res. Int. 2018, 113, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Li, W.; Li, Q.; Wang, L.; Cao, J.; Jiang, W. Defense responses, induced by p-coumaric acid and methyl p-coumarate, of jujube (Ziziphus jujuba Mill.) fruit against black spot rot caused by Alternaria alternata. J. Agric. Food Chem. 2019, 67, 2801–2810. [Google Scholar] [CrossRef]
- Li, W.; Yuan, S.; Sun, J.; Li, Q.; Jiang, W.; Cao, J. Ethyl p-coumarate exerts antifungal activity in vitro and in vivo against fruit Alternaria alternata via membrane-targeted mechanism. Int. J. Food Microbiol. 2018, 278, 26–35. [Google Scholar] [CrossRef]
- Vestergaard, M.; Ingmer, H. Antibacterial and antifungal properties of resveratrol. Int. J. Antimicrob. Agents 2019, 53, 716–723. [Google Scholar] [CrossRef]
- Cai, X.; Qi, J.; Xu, Z.; Huang, L.; Li, Y.; Ren, X.; Kong, Q. Three stilbenes make difference to the antifungal effects on ochratoxin A and its precursor production of Aspergillus carbonarius. Food Microbiol. 2022, 103, 103967. [Google Scholar] [CrossRef]
- Puljula, E.; Walton, G.; Woodward, M.J.; Karonen, M. Antimicrobial activities of ellagitannins against Clostridiales perfringens, Escherichia coli, Lactobacillus plantarum and Staphylococcus aureus. Molecules 2020, 25, 3714. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, Y.; Jia, Y.; Zhang, M.; Huang, Y.; Li, C.; Li, K. Persimmon oligomeric proanthocyanidins exert antibacterial activity through damaging the cell membrane and disrupting the energy metabolism of Staphylococcus aureus. ACS Food Sci. Technol. 2020, 1, 35–44. [Google Scholar] [CrossRef]
- Peng, M.; Jiang, C.; Jing, H.; Du, X.; Fan, X.; Zhang, Y.; Wang, H. Comparison of different extraction methods on yield, purity, antioxidant, and antibacterial activities of proanthocyanidins from chokeberry (Aronia melanocarpa). J. Food Meas. Charact. 2022, 16, 2049–2059. [Google Scholar] [CrossRef]
- Wang, G.; Pang, T.; Xia, Y.; Liu, X.; Li, S.; Parvez, A.M.; Kong, F.; Si, C. Subdivision of bamboo kraft lignin by one-step ethanol fractionation to enhance its water-solubility and antibacterial performance. Int. J. Biol. Macromol. 2019, 133, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Zhou, M.; Liu, Z.; Chen, Y.; Li, R. Inhibition effects of low concentrations of epigallocatechin gallate on the biofilm formation and hemolytic activity of Listeria monocytogenes. Food Control 2018, 85, 119–126. [Google Scholar] [CrossRef]
- Xiong, L.G.; Chen, Y.J.; Tong, J.W.; Huang, J.A.; Li, J.; Gong, Y.S.; Liu, Z.H. Tea polyphenol epigallocatechin gallate inhibits Escherichia coli by increasing endogenous oxidative stress. Food Chem. 2017, 217, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Cetin-Karaca, H.; Newman, M.C. Antimicrobial efficacy of plant phenolic compounds against Salmonella and Escherichia coli. Food Biosci. 2015, 11, 8–16. [Google Scholar] [CrossRef]
- Amin, M.U.; Khurram, M.; Khattak, B.; Khan, J. Antibiotic additive and synergistic action of rutin, morin and quercetin against methicillin resistant Staphylococcus aureus. BMC Complement. Altern. Med. 2015, 15, 59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xu, L.; Zhang, L.; Guo, Y.; Qi, X.; He, L. Effects of quercetin on postharvest blue mold control in kiwifruit. Sci. Hortic. 2018, 228, 18–25. [Google Scholar] [CrossRef]
- Li, X.M.; Li, Z.Y.; Wang, Y.D.; Wang, J.Q.; Yang, P.L. Quercetin inhibits the proliferation and aflatoxins biosynthesis of Aspergillus flavus. Toxins 2019, 11, 154. [Google Scholar] [CrossRef]
- Cha, J.D.; Moon, S.E.; Kim, J.Y.; Jung, E.K.; Lee, Y.S. Antibacterial activity of sophoraflavanone G isolated from the roots of Sophora flavescens against methicillin-resistant Staphylococcus aureus. Phytother. Res. 2009, 23, 1326–1331. [Google Scholar] [CrossRef]
- Kalli, S.; Araya-Cloutier, C.; Chapman, J.; Sanders, J.W.; Vincken, J.P. Prenylated (iso) flavonoids as antifungal agents against the food spoiler Zygosaccharomyces parabailii. Food Control 2022, 132, 108434. [Google Scholar] [CrossRef]
- Batiha, G.E.S.; Hussein, D.E.; Algammal, A.M.; George, T.T.; Jeandet, P.; Al-Snafi, A.E.; Tiwari, A.; Pagnossa, G.P.; Lima, C.M.; Thorat, N.D.; et al. Application of natural antimicrobials in food preservation: Recent views. Food Control 2021, 126, 108066. [Google Scholar] [CrossRef]
- Bajalan, I.; Rouzbahani, R.; Pirbalouti, A.G.; Maggi, F. Antioxidant and antibacterial activities of the essential oils obtained from seven Iranian populations of Rosmarinus officinalis. Ind. Crops Prod. 2017, 107, 305–311. [Google Scholar] [CrossRef]
- Rathore, S.; Mukhia, S.; Kapoor, S.; Bhatt, V.; Kumar, R.; Kumar, R. Seasonal variability in essential oil composition and biological activity of Rosmarinus officinalis L. accessions in the western Himalaya. Sci. Rep. 2022, 12, 3305. [Google Scholar] [CrossRef] [PubMed]
- Micić, D.; Đurović, S.; Riabov, P.; Tomić, A.; Šovljanski, O.; Filip, S.; Tosti, T.; Dojčinović, B.; Božović, R.; Jovanović, D.; et al. Rosemary essential oils as a promising source of bioactive compounds: Chemical composition, thermal properties, biological activity, and gastronomical perspectives. Foods 2021, 10, 2734. [Google Scholar] [CrossRef] [PubMed]
- Dammak, I.; Hamdi, Z.; El Euch, S.K.; Zemni, H.; Mliki, A.; Hassouna, M.; Lasram, S. Evaluation of antifungal and anti-ochratoxigenic activities of Salvia officinalis, Lavandula dentata and Laurus nobilis essential oils and a major monoterpene constituent 1, 8-cineole against Aspergillus carbonarius. Ind. Crops Prod. 2019, 128, 85–93. [Google Scholar] [CrossRef]
- Sakkas, H.; Economou, V.; Gousia, P.; Bozidis, P.; Sakkas, V.A.; Petsios, S.; Mpekoulis, G.; Ilia, A.; Papadopoulou, C. Antibacterial efficacy of commercially available essential oils tested against drug-resistant Gram-positive pathogens. Appl. Sci. 2018, 8, 2201. [Google Scholar] [CrossRef]
- Valdivieso-Ugarte, M.; Plaza-Diaz, J.; Gomez-Llorente, C.; Gómez, E.L.; Sabés-Alsina, M.; Gil, Á. In vitro examination of antibacterial and immunomodulatory activities of cinnamon, white thyme, and clove essential oils. J. Funct. Foods 2021, 81, 104436. [Google Scholar] [CrossRef]
- Reyes-Jurado, F.; Cervantes-Rincón, T.; Bach, H.; López-Malo, A.; Palou, E. Antimicrobial activity of Mexican oregano (Lippia berlandieri), thyme (Thymus vulgaris), and mustard (Brassica nigra) essential oils in gaseous phase. Ind. Crops Prod. 2019, 131, 90–95. [Google Scholar] [CrossRef]
- Císarová, M.; Hleba, L.; Medo, J.; Tančinová, D.; Mašková, Z.; Čuboň, J.; Kováčik, A.; Foltinová, D.; Božike, M.; Klouček, P. The in vitro and in situ effect of selected essential oils in vapour phase against bread spoilage toxicogenic aspergilli. Food Control 2020, 110, 107007. [Google Scholar] [CrossRef]
- Marchese, A.; Arciola, C.R.; Barbieri, R.; Silva, A.S.; Nabavi, S.F.; Tsetegho Sokeng, A.J.; Izadi, M.; Jafari, N.J.; Suntar, I.; Daglia, M.; et al. Update on monoterpenes as antimicrobial agents: A particular focus on p-cymene. Materials 2017, 10, 947. [Google Scholar] [CrossRef]
- Pinto, L.; Bonifacio, M.A.; De Giglio, E.; Cometa, S.; Logrieco, A.F.; Baruzzi, F. Unravelling the antifungal effect of red thyme oil (Thymus vulgaris L.) compounds in vapor phase. Molecules 2020, 25, 4761. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhang, L.F.; Xu, J.G.; Hu, Q.P. Comparison study on antioxidant, DNA damage protective and antibacterial activities of eugenol and isoeugenol against several foodborne pathogens. Food Nutr. Res. 2017, 61, 1353356. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Cai, N.; Chen, J.; Wan, C. Clove essential oil as an alternative approach to control postharvest blue mold caused by Penicillium italicum in citrus fruit. Biomolecules 2019, 9, 197. [Google Scholar] [CrossRef] [PubMed]
- Cava-Roda, R.; Taboada-Rodríguez, A.; López-Gómez, A.; Martínez-Hernández, G.B.; Marín-Iniesta, F. Synergistic antimicrobial activities of combinations of vanillin and essential oils of cinnamon bark, cinnamon leaves, and cloves. Foods 2021, 10, 1406. [Google Scholar] [CrossRef] [PubMed]
- Yoplac, I.; Vargas, L.; Robert, P.; Hidalgo, A. Characterization and antimicrobial activity of microencapsulated citral with dextrin by spray drying. Heliyon 2021, 7, e06737. [Google Scholar] [CrossRef]
- Zheng, S.; Jing, G.; Wang, X.; Ouyang, Q.; Jia, L.; Tao, N. Citral exerts its antifungal activity against Penicillium digitatum by affecting the mitochondrial morphology and function. Food Chem. 2015, 178, 76–81. [Google Scholar] [CrossRef]
- Tang, X.; Shao, Y.L.; Tang, Y.J.; Zhou, W.W. Antifungal activity of essential oil compounds (geraniol and citral) and inhibitory mechanisms on grain pathogens (Aspergillus flavus and Aspergillus ochraceus). Molecules 2018, 23, 2108. [Google Scholar] [CrossRef]
- Quintieri, L.; Fancello, F.; Caputo, L.; Sorrentino, A.; Zara, S.; Lippolis, V.; Cervellieri, S.; Fanelli, F.; Corvino, A.; Pace, B.; et al. Effect of gaseous citral on table grapes contaminated by Rhizopus oryzae ITEM 18876. Foods 2022, 11, 2478. [Google Scholar] [CrossRef]
- Siddiqua, S.; Anusha, B.A.; Ashwini, L.S.; Negi, P.S. Antibacterial activity of cinnamaldehyde and clove oil: Effect on selected foodborne pathogens in model food systems and watermelon juice. J. Food Sci. Technol. 2015, 52, 5834–5841. [Google Scholar] [CrossRef]
- Sun, Q.; Li, J.; Sun, Y.; Chen, Q.; Zhang, L.; Le, T. The antifungal effects of cinnamaldehyde against Aspergillus niger and its application in bread preservation. Food Chem. 2020, 317, 126405. [Google Scholar] [CrossRef]
- Xu, L.; Tao, N.; Yang, W.; Jing, G. Cinnamaldehyde damaged the cell membrane of Alternaria alternata and induced the degradation of mycotoxins in vivo. Ind. Crops Prod. 2018, 112, 427–433. [Google Scholar] [CrossRef]
- Song, H.J.; Ku, K.M. Optimization of allyl isothiocyanate sanitizing concentration for inactivation of Salmonella typhimurium on lettuce based on its phenotypic and metabolome changes. Food Chem. 2021, 364, 130438. [Google Scholar] [CrossRef] [PubMed]
- Olaimat, A.N.; Al-Holy, M.A.; Ghoush, M.A.; Al-Nabulsi, A.A.; Holley, R.A. Control of Salmonella enterica and Listeria monocytogenes in hummus using allyl isothiocyanate. Int. J. Food Microbiol. 2018, 278, 73–80. [Google Scholar] [CrossRef]
- de Melo Nazareth, T.; Alonso-Garrido, M.; Stanciu, O.; Mañes, J.; Manyes, L.; Meca, G. Effect of allyl isothiocyanate on transcriptional profile, aflatoxin synthesis, and Aspergillus flavus growth. Food Res. Int. 2020, 128, 108786. [Google Scholar] [CrossRef] [PubMed]
- de Melo Nazareth, T.; Quiles, J.M.; Torrijos, R.; Luciano, F.B.; Manes, J.; Meca, G. Antifungal and antimycotoxigenic activity of allyl isothiocyanate on barley under different storage conditions. LWT 2019, 112, 108237. [Google Scholar] [CrossRef]
- Yang, C.X.; Wu, H.T.; Li, X.X.; Wu, H.Y.; Niu, T.X.; Wang, X.N.; Lian, R.; Zhang, G.L.; Hou, H.M. Comparison of the inhibitory potential of benzyl isothiocyanate and phenethyl isothiocyanate on Shiga toxin-producing and enterotoxigenic Escherichia coli. LWT 2020, 118, 108806. [Google Scholar] [CrossRef]
- Wu, H.Y.; Xu, Y.H.; Wei, L.N.; Bi, J.R.; Hou, H.M.; Hao, H.S.; Zhang, G.L. Inhibitory effects of 3-(methylthio) propyl isothiocyanate in comparison with benzyl isothiocyanate on Listeria monocytogenes. J. Food Meas. Charact. 2022, 16, 1768–1775. [Google Scholar] [CrossRef]
- Yang, B.; Li, L.; Geng, H.; Zhang, C.; Wang, G.; Yang, S.; Gao, S.; Zhao, Y.; Xing, F. Inhibitory effect of allyl and benzyl isothiocyanates on ochratoxin a producing fungi in grape and maize. Food Microbiol. 2021, 100, 103865. [Google Scholar] [CrossRef]
- Nowicki, D.; Maciąg-Dorszyńska, M.; Bogucka, K.; Szalewska-Pałasz, A.; Herman-Antosiewicz, A. Various modes of action of dietary phytochemicals, sulforaphane and phenethyl isothiocyanate, on pathogenic bacteria. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y.; Bi, Y.; Wang, T.; Dong, Y.; Yang, Q.; Zhang, T. 2-Phenylethyl isothiocyanate exerts antifungal activity against Alternaria alternata by affecting membrane integrity and mycotoxin production. Toxins 2020, 12, 124. [Google Scholar] [CrossRef]
- Wu, T.L.; Hu, Y.M.; Sun, Y.; Zhang, Z.J.; Wu, Z.R.; Zhao, W.B.; Tang, C.; Du, S.S.; He, Y.H.; Ma, Y.; et al. Insights into the mode of action of 2-(4-methoxyphenyl) ethyl isothiocyanate on Aspergillus niger. Food Control 2022, 136, 108871. [Google Scholar] [CrossRef]
- Othman, L.; Sleiman, A.; Abdel-Massih, R.M. Antimicrobial activity of polyphenols and alkaloids in middle eastern plants. Front. Microbiol. 2019, 10, 911. [Google Scholar] [CrossRef]
- El-Zahar, K.M.; Al-Jamaan, M.E.; Al-Mutairi, F.R.; Al-Hudiab, A.M.; Al-Einzi, M.S.; Mohamed, A.A.Z. Antioxidant, antibacterial, and antifungal activities of the ethanolic extract obtained from Berberis vulgaris roots and leaves. Molecules 2022, 27, 6114. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Zhe, T.; Li, F.; Zhang, Y.; Yu, M.; Li, R.; Wang, L. Sustainable films containing AIE-active berberine-based nanoparticles: A promising antibacterial food packaging. Food Hydrocoll. 2022, 123, 107147. [Google Scholar] [CrossRef]
- He, M.; Wang, Y.; Hong, M.; Li, T. Berberine as a promising natural compound to control Penicillium italicum causing blue mold of citrus fruit. Sci. Hortic. 2022, 305, 111370. [Google Scholar] [CrossRef]
- Marchese, A.; Barbieri, R.; Sanches-Silva, A.; Daglia, M.; Nabavi, S.F.; Jafari, N.J.; Izadi, M.; Ajami, M.; Nabavi, S.M. Antifungal and antibacterial activities of allicin: A review. Trends Food Sci. Technol. 2016, 52, 49–56. [Google Scholar] [CrossRef]
- Jin, Z.; Li, L.; Zheng, Y.; An, P. Diallyl disulfide, the antibacterial component of garlic essential oil, inhibits the toxicity of Bacillus cereus ATCC 14579 at sub-inhibitory concentrations. Food Control 2021, 126, 108090. [Google Scholar] [CrossRef]
- Tang, Y.; Li, F.; Gu, D.; Wang, W.; Huang, J.; Jiao, X. Antimicrobial effect and the mechanism of diallyl trisulfide against Campylobacter jejuni. Antibiotics 2021, 10, 246. [Google Scholar] [CrossRef]
- Pernin, A.; Guillier, L.; Dubois-Brissonnet, F. Inhibitory activity of phenolic acids against Listeria monocytogenes: Deciphering the mechanisms of action using three different models. Food Microbiol. 2019, 80, 18–24. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, X.; Wu, H.; Wang, H.; Bian, H.; Zhu, Y.; Xu, W.; Liu, F.; Wang, D.; Fu, L. Antibacterial activity and action mode of chlorogenic acid against Salmonella enteritidis, a foodborne pathogen in chilled fresh chicken. World J. Microbiol. Biotechnol. 2020, 36, 24. [Google Scholar] [CrossRef]
- Ansari, M.A.; Anurag, A.; Fatima, Z.; Hameed, S. Natural phenolic compounds: A potential antifungal agent. In Microbial Pathogens and Strategies for Combating Them: Science, Technology and Education, 1st ed.; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2013; pp. 1189–1195. [Google Scholar]
- Morales, J.; Mendoza, L.; Cotoras, M. Alteration of oxidative phosphorylation as a possible mechanism of the antifungal action of p-coumaric acid against Botrytis cinerea. J. Appl. Microbiol. 2017, 123, 969–976. [Google Scholar] [CrossRef]
- Zhu, C.; Lei, M.; Andargie, M.; Zeng, J.; Li, J. Antifungal activity and mechanism of action of tannic acid against Penicillium digitatum. Physiol. Mol. Plant Pathol. 2019, 107, 46–50. [Google Scholar] [CrossRef]
- You, S.; Xie, Y.; Zhuang, X.; Chen, H.; Qin, Y.; Cao, J.; Lan, T. Effect of high antioxidant activity on bacteriostasis of lignin from sugarcane bagasse. Biochem. Eng. J. 2022, 180, 108335. [Google Scholar] [CrossRef]
- Luo, J.; Xu, F.; Zhang, X.; Shao, X.; Wei, Y.; Wang, H. Transcriptome analysis of Penicillium italicum in response to the flavonoids from Sedum aizoon L. World J. Microbiol. Biotechnol. 2020, 36, 62. [Google Scholar] [CrossRef] [PubMed]
- Churklam, W.; Chaturongakul, S.; Ngamwongsatit, B.; Aunpad, R. The mechanisms of action of carvacrol and its synergism with nisin against Listeria monocytogenes on sliced bologna sausage. Food Control 2020, 108, 106864. [Google Scholar] [CrossRef]
- Stratakos, A.C.; Sima, F.; Ward, P.; Linton, M.; Kelly, C.; Pinkerton, L.; Stef, L.; Pet, I.; Corcionivoschi, N. The in vitro effect of carvacrol, a food additive, on the pathogenicity of O157 and non-O157 Shiga-toxin producing Escherichia coli. Food Control 2018, 84, 290–296. [Google Scholar] [CrossRef]
- Barbosa, L.N.; Alves, F.C.B.; Andrade, B.F.M.T.; Albano, M.; Rall, V.L.M.; Fernandes, A.A.H.; Buzalaf, M.A.R.; de Lima Leite, A.; de Pontes, L.G.; dos Santos, L.D.; et al. Proteomic analysis and antibacterial resistance mechanisms of Salmonella enteritidis submitted to the inhibitory effect of Origanum vulgare essential oil, thymol and carvacrol. J. Proteomics 2020, 214, 103625. [Google Scholar] [CrossRef]
- Han, Y.; Sun, Z.; Chen, W. Antimicrobial susceptibility and antibacterial mechanism of limonene against Listeria monocytogenes. Molecules 2019, 25, 33. [Google Scholar] [CrossRef]
- Gupta, A.; Jeyakumar, E.; Lawrence, R. Strategic approach of multifaceted antibacterial mechanism of limonene traced in Escherichia coli. Sci. Rep. 2021, 11, 13816. [Google Scholar] [CrossRef]
- Han, Y.; Chen, W.; Sun, Z. Antimicrobial activity and mechanism of limonene against Staphylococcus aureus. J. Food Saf. 2021, 41, e12918. [Google Scholar] [CrossRef]
- Jeyakumar, G.E.; Lawrence, R. Mechanisms of bactericidal action of eugenol against Escherichia coli. J. Herb. Med. 2021, 26, 100406. [Google Scholar] [CrossRef]
- Bai, X.; Li, X.; Liu, X.; Xing, Z.; Su, R.; Wang, Y.; Xia, X.; Shi, C. Antibacterial effect of eugenol on Shigella flexneri and its mechanism. Foods 2022, 11, 2565. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, S.; Du, S.; Chen, S.; Sun, H. Antifungal activity of thymol and carvacrol against postharvest pathogens Botrytis cinerea. J. Food Sci. Technol. 2019, 56, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Li, Z.; Wang, X. UHPLC-HRMS-based untargeted lipidomics reveal mechanism of antifungal activity of carvacrol against Aspergillus flavus. Foods 2022, 11, 93. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Hu, M.; Zhang, Y.; Niu, C.; Yue, T.; Yuan, Y.; Wang, Z. Antifungal activity and mechanism of citral, limonene and eugenol against Zygosaccharomyces rouxii. LWT 2019, 106, 50–56. [Google Scholar] [CrossRef]
- Jiang, N.; Wang, L.; Jiang, D.; Wang, M.; Liu, H.; Yu, H.; Yao, W. Transcriptomic analysis of inhibition by eugenol of ochratoxin A biosynthesis and growth of Aspergillus carbonarius. Food Control 2022, 135, 108788. [Google Scholar] [CrossRef]
- Luciano, F.B.; Holley, R.A. Enzymatic inhibition by allyl isothiocyanate and factors affecting its antimicrobial action against Escherichia coli O157: H7. Int. J. Food Microbiol. 2009, 131, 240–245. [Google Scholar] [CrossRef]
- Li, P.; Zhao, Y.M.; Wang, C.; Zhu, H.P. Antibacterial activity and main action pathway of benzyl isothiocyanate extracted from papaya seeds. J. Food Sci. 2021, 86, 169–176. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Y.; Xu, Y.; Chen, T.; Li, B.; Zhang, Z.; Tian, S. Application and mechanism of benzyl-isothiocyanate, a natural antimicrobial agent from cruciferous vegetables, in controlling postharvest decay of strawberry. Postharvest Biol. Technol. 2021, 180, 111604. [Google Scholar] [CrossRef]
- Boberek, J.M.; Stach, J.; Good, L. Genetic evidence for inhibition of bacterial division protein FtsZ by berberine. PLoS ONE 2010, 5, e13745. [Google Scholar] [CrossRef]
- Xu, C.; Wang, F.; Huang, F.; Yang, M.; He, D.; Deng, L. Targeting effect of berberine on type I fimbriae of Salmonella typhimurium and its effective inhibition of biofilm. Appl. Microbiol. Biotechnol. 2021, 105, 1563–1573. [Google Scholar] [CrossRef]
- Arshad, M.S.; Batool, S.A. Natural antimicrobials, their sources and food safety. In Food Additives; Karunaratne, D.N., Pamunuwa, G., Eds.; IntechOpen: London, UK, 2017; Volume 87, pp. 87–104. [Google Scholar] [CrossRef]
- Belgacem, I.; Li Destri Nicosia, M.G.; Pangallo, S.; Abdelfattah, A.; Benuzzi, M.; Agosteo, G.E.; Schena, L. Pomegranate peel extracts as safe natural treatments to control plant diseases and increase the shelf-life and safety of fresh fruits and vegetables. Plants 2021, 10, 453. [Google Scholar] [CrossRef] [PubMed]
- Givi, F.; Gholami, M.; Massah, A. Application of pomegranate peel extract and essential oil as a safe botanical preservative for the control of postharvest decay caused by Penicillium italicum and Penicillium digitatum on “Satsuma” mandarin. J. Food Saf. 2019, 39, e12639. [Google Scholar] [CrossRef]
- Gómez-Maldonado, D.; Lobato-Calleros, C.; Aguirre-Mandujano, E.; Leyva-Mir, S.G.; Robles-Yerena, L.; Vernon-Carter, E.J. Antifungal activity of mango kernel polyphenols on mango fruit infected by anthracnose. LWT 2020, 126, 109337. [Google Scholar] [CrossRef]
- Hernández, A.; Ruiz-Moyano, S.; Galván, A.I.; Merchán, A.V.; Nevado, F.P.; Aranda, E.; Serradilla, M.J.; de Guía Córdoba, M.; Martín, A. Anti-fungal activity of phenolic sweet orange peel extract for controlling fungi responsible for post-harvest fruit decay. Fungal Biol. 2021, 125, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wu, H.; Shi, F.; Wang, H.; Chen, K.; Feng, J.; Jia, W. Antifungal activity screening for mint and thyme essential oils against Rhizopus stolonifer and their application in postharvest preservation of strawberry and peach fruits. J. Appl. Microbiol. 2021, 130, 1993–2007. [Google Scholar] [CrossRef] [PubMed]
- Sumalan, R.M.; Kuganov, R.; Obistioiu, D.; Popescu, I.; Radulov, I.; Alexa, E.; Negrea, M.; Salimzoda, A.F.; Sumalan, R.L.; Cocan, I. Assessment of mint, basil, and lavender essential oil vapor-phase in antifungal protection and lemon fruit quality. Molecules 2020, 25, 1831. [Google Scholar] [CrossRef]
- Pinto, L.; Cefola, M.; Bonifacio, M.A.; Cometa, S.; Bocchino, C.; Pace, B.; De Giglio, E.; Palumbo, M.; Sada, A.; Logrieco, A.F.; et al. Effect of red thyme oil (Thymus vulgaris L.) vapours on fungal decay, quality parameters and shelf-life of oranges during cold storage. Food Chem. 2021, 336, 127590. [Google Scholar] [CrossRef]
- Arellano, S.; Zhu, L.; Dev Kumar, G.; Law, B.; Friedman, M.; Ravishankar, S. Essential oil microemulsions inactivate antibiotic-resistant bacteria on iceberg lettuce during 28-day storage at 4 °C. Molecules 2022, 27, 6699. [Google Scholar] [CrossRef]
- Kara, M.; Soylu, E.M. Assessment of glucosinolate-derived isothiocyanates as potential natural antifungal compounds against citrus sour rot disease agent Geotrichum citri-aurantii. J. Phytopathol. 2020, 168, 279–289. [Google Scholar] [CrossRef]
- Santos, M.I.S.; Marques, C.; Mota, J.; Pedroso, L.; Lima, A. Applications of essential oils as antibacterial agents in minimally processed fruits and vegetables—A review. Microorganisms 2022, 10, 760. [Google Scholar] [CrossRef]
- Wong, J.X.; Ramli, S.; Desa, S.; Chen, S.N. Use of Centella asiatica extract in reducing microbial contamination and browning effect in fresh cut fruits and vegetables during storage: A potential alternative of synthetic preservatives. LWT 2021, 151, 112229. [Google Scholar] [CrossRef]
- Wieczyńska, J.; Cavoski, I. Antimicrobial, antioxidant and sensory features of eugenol, carvacrol and trans-anethole in active packaging for organic ready-to-eat iceberg lettuce. Food Chem. 2018, 259, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Myszka, K.; Schmidt, M.T.; Majcher, M.; Juzwa, W.; Czaczyk, K. β-Caryophyllene-rich pepper essential oils suppress spoilage activity of Pseudomonas fluorescens KM06 in fresh-cut lettuce. LWT Food Sci. Technol. 2017, 83, 118–126. [Google Scholar] [CrossRef]
- Amiri, A.; Ramezanian, A.; Mortazavi, S.M.H.; Hosseini, S.M.H.; Yahia, E. Shelf-life extension of pomegranate arils using chitosan nanoparticles loaded with Satureja hortensis essential oil. J. Sci. Food Agric. 2021, 101, 3778–3786. [Google Scholar] [CrossRef]
- Awad, A.H.R.; Parmar, A.; Ali, M.R.; El-Mogy, M.M.; Abdelgawad, K.F. Extending the shelf-life of fresh-cut green bean pods by ethanol, ascorbic Acid, and essential oils. Foods 2021, 10, 1103. [Google Scholar] [CrossRef] [PubMed]
- Maleš, I.; Pedisić, S.; Zorić, Z.; Elez-Garofulić, I.; Repajić, M.; You, L.; Vladimir-Knežević, S.; Butorac, D.; Dragović-Uzelac, V. The medicinal and aromatic plants as ingredients in functional beverage production. J. Funct. Foods 2022, 96, 105210. [Google Scholar] [CrossRef]
- da Cruz Almeida, E.T.; de Souza, G.T.; de Sousa Guedes, J.P.; Barbosa, I.M.; de Sousa, C.P.; Castellano, L.R.C.; Magnani, M.; de Souza, E.L. Mentha piperita L. essential oil inactivates spoilage yeasts in fruit juices through the perturbation of different physiological functions in yeast cells. Food Microbiol. 2019, 82, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, H. Assessment of different antimicrobials to inhibit the growth of Zygosaccharomyces rouxii cocktail in concentrated apple juice. Food Microbiol. 2020, 91, 103549. [Google Scholar] [CrossRef]
- Lee, S.; Kim, H.; Beuchat, L.R.; Kim, Y.; Ryu, J.H. Synergistic antimicrobial activity of oregano and thyme thymol essential oils against Leuconostoc citreum in a laboratory medium and tomato juice. Food Microbiol. 2020, 90, 103489. [Google Scholar] [CrossRef]
- Fernandez, M.V.; Bengardino, M.; Jagus, R.J.; Agüero, M.V. Enrichment and preservation of a vegetable smoothie with an antioxidant and antimicrobial extract obtained from beet by-products. LWT 2020, 117, 108622. [Google Scholar] [CrossRef]
- Maqsood, S.; Abushelaibi, A.; Manheem, K.; Al Rashedi, A.; Kadim, I.T. Lipid oxidation, protein degradation, microbial and sensorial quality of camel meat as influenced by phenolic compounds. LWT Food Sci. Technol. 2015, 63, 953–959. [Google Scholar] [CrossRef]
- Nowak, A.; Czyzowska, A.; Efenberger, M.; Krala, L. Polyphenolic extracts of cherry (Prunus cerasus L.) and blackcurrant (Ribes nigrum L.) leaves as natural preservatives in meat products. Food Microbiol. 2016, 59, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Casaburi, A.; Di Martino, V.; Ercolini, D.; Parente, E.; Villani, F. Antimicrobial activity of Myrtus communis L. water-ethanol extract against meat spoilage strains of Brochothrix thermosphacta and Pseudomonas fragi in vitro and in meat. Ann. Microbiol. 2015, 65, 841–850. [Google Scholar] [CrossRef]
- Tamkutė, L.; Gil, B.M.; Carballido, J.R.; Pukalskienė, M.; Venskutonis, P.R. Effect of cranberry pomace extracts isolated by pressurized ethanol and water on the inhibition of food pathogenic/spoilage bacteria and the quality of pork products. Food Res. Int. 2019, 120, 38–51. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J.; Guo, X. Effects of antimicrobial and antioxidant activities of spice extracts on raw chicken meat quality. Food Sci. Hum. Wellness 2016, 5, 39–48. [Google Scholar] [CrossRef]
- Shahbazi, Y.; Shavisi, N.; Mohebi, E. Effects of Ziziphora clinopodioides essential oil and nisin, both separately and in combination, to extend shelf-life and control Escherichia coli O157:H7 and Staphylococcus aureus in raw beef patty during refrigerated storage. J. Food Saf. 2016, 36, 227–236. [Google Scholar] [CrossRef]
- Karam, L.; Chehab, R.; Osaili, T.M.; Savvaidis, I.N. Antimicrobial effect of thymol and carvacrol added to a vinegar-based marinade for controlling spoilage of marinated beef (Shawarma) stored in air or vacuum packaging. Int. J. Food Microbiol. 2020, 332, 108769. [Google Scholar] [CrossRef] [PubMed]
- Olatunde, O.O.; Benjakul, S.; Huda, N.; Zhang, B.; Deng, S. Ethanolic Noni (Morinda citrifolia L.) leaf extract dechlorophyllised using sedimentation process: Antioxidant, antibacterial properties and efficacy in extending the shelf-life of striped catfish slices. Int. J. Food Sci. Technol. 2021, 56, 2804–2819. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Della Tan, S.L.; Shiekh, K.A.; Benjakul, S.; Nirmal, N.P. Ethanolic guava leaf extracts with different chlorophyll removal processes: Anti-melanosis, antibacterial properties and the impact on qualities of Pacific white shrimp during refrigerated storage. Food Chem. 2021, 341, 128251. [Google Scholar] [CrossRef]
- Li, Y.; Zhuang, S.; Liu, Y.; Zhang, L.; Liu, X.; Cheng, H.; Liu, J.; Shu, R.; Luo, Y. Effect of grape seed extract on quality and microbiota community of container-cultured snakehead (Channa argus) fillets during chilled storage. Food Microbiol. 2020, 91, 103492. [Google Scholar] [CrossRef]
- Van Haute, S.; Raes, K.; Van Der Meeren, P.; Sampers, I. The effect of cinnamon, oregano and thyme essential oils in marinade on the microbial shelf-life of fish and meat products. Food Control 2016, 68, 30–39. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, D.; Lv, J.; Li, Q.; Kong, C.; Luo, Y. Effect of cinnamon essential oil on bacterial diversity and shelf-life in vacuum-packaged common carp (Cyprinus carpio) during refrigerated storage. Int. J. Food Microbiol. 2017, 249, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Roila, R.; Valiani, A.; Ranucci, D.; Ortenzi, R.; Servili, M.; Veneziani, G.; Branciari, R. Antimicrobial efficacy of a polyphenolic extract from olive oil by-product against “Fior di latte” cheese spoilage bacteria. Int. J. Food Microbiol. 2019, 295, 49–53. [Google Scholar] [CrossRef]
- Derbassi, N.; Pedrosa, M.C.; Heleno, S.; Fernandes, F.; Dias, M.I.; Calhelha, R.C.; Rodrigues, P.; Carocho, M.; Ferreira, I.C.F.R.; Barros, L. Arbutus unedo leaf extracts as potential dairy preservatives: Case study on quark cheese. Food Funct. 2022, 13, 5442–5454. [Google Scholar] [CrossRef] [PubMed]
- Milanović, V.; Sabbatini, R.; Garofalo, C.; Cardinali, F.; Pasquini, M.; Aquilanti, L.; Osimani, A. Evaluation of the inhibitory activity of essential oils against spoilage yeasts and their potential application in yogurt. Int. J. Food Microbiol. 2021, 341, 109048. [Google Scholar] [CrossRef] [PubMed]
- Novais, C.; Molina, A.K.; Abreu, R.M.; Santo-Buelga, C.; Ferreira, I.C.; Pereira, C.; Barros, L. Natural food colorants and preservatives: A review, a demand, and a challenge. J. Agric. Food Chem. 2022, 70, 2789–2805. [Google Scholar] [CrossRef]
- Schirone, M.; Visciano, P.; Tofalo, R.; Suzzi, G. Foodborne pathogens: Hygiene and safety. Front. Microbiol. 2019, 10, 1974. [Google Scholar] [CrossRef]
- Kachur, K.; Suntres, Z. The antibacterial properties of phenolic isomers, carvacrol and thymol. Crit. Rev. Food Sci. Nutr. 2020, 60, 3042–3053. [Google Scholar] [CrossRef]
- Guan, P.; Wang, X.; Dong, Z.; Song, M.; Zhu, H.; Suo, B. Cinnamaldehyde inactivates Listeria monocytogenes at a low temperature in ground pork by disturbing the expression of stress regulatory genes. Food Biosci. 2023, 51, 102277. [Google Scholar] [CrossRef]
- Bai, J.; Li, J.; Chen, Z.; Bai, X.; Yang, Z.; Wang, Z.; Yang, Y. Antibacterial activity and mechanism of clove essential oil against foodborne pathogens. LWT 2023, 173, 114249. [Google Scholar] [CrossRef]
- Sepahvand, S.; Amiri, S.; Radi, M.; Akhavan, H.R. Antimicrobial activity of thymol and thymol-nanoemulsion against three food-borne pathogens inoculated in a sausage model. Food Bioproc. Tech. 2021, 14, 1936–1945. [Google Scholar] [CrossRef]
- Saraiva, C.; Silva, A.C.; García-Díez, J.; Cenci-Goga, B.; Grispoldi, L.; Silva, A.F.; Almeida, J.M. Antimicrobial activity of Myrtus communis L. and Rosmarinus officinalis L. essential oils against Listeria monocytogenes in cheese. Foods 2021, 10, 1106. [Google Scholar] [CrossRef]
- Ahmed, L.I.; Ibrahim, N.; Abdel-Salam, A.B.; Fahim, K.M. Potential application of ginger, clove and thyme essential oils to improve soft cheese microbial safety and sensory characteristics. Food Biosci. 2021, 42, 101177. [Google Scholar] [CrossRef]
- Rossi, C.; Chaves-López, C.; Možina, S.S.; Di Mattia, C.; Scuota, S.; Luzzi, I.; Jenič, T.; Paparella, A.; Serio, A. Salmonella enterica adhesion: Effect of Cinnamomum zeylanicum essential oil on lettuce. LWT 2019, 111, 16–22. [Google Scholar] [CrossRef]
- Brnawi, W.I.; Hettiarachchy, N.S.; Horax, R.; Kumar-Phillips, G.; Ricke, S. Antimicrobial activity of leaf and bark cinnamon essential oils against Listeria monocytogenes and Salmonella typhimurium in broth system and on celery. J. Food Process. Preserv. 2019, 43, e13888. [Google Scholar] [CrossRef]
- Dai, J.; Li, C.; Cui, H.; Lin, L. Unraveling the anti-bacterial mechanism of Litsea cubeba essential oil against E. coli O157: H7 and its application in vegetable juices. Int. J. Food Microbiol. 2021, 338, 108989. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.; Coimbra, A.T.; Silva, L.; Duarte, A.P.; Ferreira, S. Melissa officinalis essential oil as an antimicrobial agent against Listeria monocytogenes in watermelon juice. Food Microbiol. 2023, 109, 104105. [Google Scholar] [CrossRef]
- Gerardi, C.; Pinto, L.; Baruzzi, F.; Giovinazzo, G. Comparison of antibacterial and antioxidant properties of red (cv. Negramaro) and white (cv. Fiano) skin pomace extracts. Molecules 2021, 26, 5918. [Google Scholar] [CrossRef]
- Tian, L.; Fu, J.; Wu, M.; Liao, S.; Jia, X.; Wang, J.; Yang, S.; Liu, Z.; Liu, Z.; Xue, Z.; et al. Evaluation of gallic acid on membrane damage of Yersinia enterocolitica and its application as a food preservative in pork. Int. J. Food Microbiol. 2022, 374, 109720. [Google Scholar] [CrossRef]
- Phuong, N.N.M.; Le, T.T.; Van Camp, J.; Raes, K. Evaluation of antimicrobial activity of rambutan (Nephelium lappaceum L.) peel extracts. Int. J. Food Microbiol. 2020, 321, 108539. [Google Scholar] [CrossRef]
- Belgacem, I.; Schena, L.; Teixidó, N.; Romeo, F.V.; Ballistreri, G.; Abadias, M. Effectiveness of a pomegranate peel extract (PGE) in reducing Listeria monocytogenes in vitro and on fresh-cut pear, apple and melon. Eur. Food Res. Technol. 2020, 246, 1765–1772. [Google Scholar] [CrossRef]
- Nicolau-Lapeña, I.; Aguiló-Aguayo, I.; Bobo, G.; Viñas, I.; Anguera, M.; Abadias, M. Ferulic acid application to control growth Listeria monocytogenes and Salmonella enterica on fresh-cut apples and melon, and its effect in quality parameters. Postharvest Biol. Technol. 2022, 186, 111831. [Google Scholar] [CrossRef]
- Bombelli, A.; Araya-Cloutier, C.; Vincken, J.P.; Abee, T.; den Besten, H.M. Impact of food-relevant conditions and food matrix on the efficacy of prenylated isoflavonoids glabridin and 6, 8-diprenylgenistein as potential natural preservatives against Listeria monocytogenes. Int. J. Food Microbiol. 2023, 390, 110109. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, S.; Hao, G.; Zhao, L.; Lü, X.; Wang, H.; Wang, L.; Zhang, J.; Ge, W. Antimicrobial activity and mechanism of isothiocyanate from Moringa oleifera seeds against Bacillus cereus and Cronobacter sakazakii and its application in goat milk. Food Control 2022, 139, 109067. [Google Scholar] [CrossRef]
- Dogruyol, H.; Mol, S.; Cosansu, S. Increased thermal sensitivity of Listeria monocytogenes in sous-vide salmon by oregano essential oil and citric acid. Food Microbiol. 2020, 90, 103496. [Google Scholar] [CrossRef]
- Orizano-Ponce, E.; Char, C.; Sepúlveda, F.; Ortiz-Viedma, J. Heat sensitization of Escherichia coli by the natural antimicrobials vanillin and emulsified citral in blended carrot-orange juice. Food Microbiol. 2022, 107, 104058. [Google Scholar] [CrossRef]
- Cheng, S.; Su, R.; Song, L.; Bai, X.; Yang, H.; Li, Z.; Li, Z.; Zhan, X.; Xia, X.; Lü, X.; et al. Citral and trans-cinnamaldehyde, two plant-derived antimicrobial agents can induce Staphylococcus aureus into VBNC state with different characteristics. Food Microbiol. 2023, 112, 104241. [Google Scholar] [CrossRef]
- de Candia, S.; Quintieri, L.; Caputo, L.; Baruzzi, F. Antimicrobial activity of processed spices used in traditional Southern Italian sausage processing. J. Food Process. Preserv. 2017, 41, e13022. [Google Scholar] [CrossRef]
- Palomares-Navarro, J.J.; Bernal-Mercado, A.T.; González-Aguilar, G.A.; Ortega-Ramirez, L.A.; Martínez-Téllez, M.A.; Ayala-Zavala, J.F. Antibiofilm action of plant terpenes in Salmonella strains: Potential inhibitors of the synthesis of extracellular polymeric substances. Pathogens 2023, 12, 35. [Google Scholar] [CrossRef]
- Silva, L.N.; Zimmer, K.R.; Macedo, A.J.; Trentin, D.S. Plant natural products targeting bacterial virulence factors. Chem. Rev. 2016, 116, 9162–9236. [Google Scholar] [CrossRef]
- Vazquez-Armenta, F.J.; Bernal-Mercado, A.T.; Lizardi-Mendoza, J.; Silva-Espinoza, B.A.; Cruz-Valenzuela, M.R.; Gonzalez-Aguilar, G.A.; Nazzaro, F.; Fratianni, F.; Ayala-Zavala, J.F. Phenolic extracts from grape stems inhibit Listeria monocytogenes motility and adhesion to food contact surfaces. J. Adhes. Sci. Technol. 2018, 32, 889–907. [Google Scholar] [CrossRef]
- Vazquez-Armenta, F.J.; Bernal-Mercado, A.T.; Tapia-Rodriguez, M.R.; Gonzalez-Aguilar, G.A.; Lopez-Zavala, A.A.; Martinez-Tellez, M.A.; Hernandez-Oñate, M.A.; Ayala-Zavala, J.F. Quercetin reduces adhesion and inhibits biofilm development by Listeria monocytogenes by reducing the amount of extracellular proteins. Food Control 2018, 90, 266–273. [Google Scholar] [CrossRef]
- Vazquez-Armenta, F.J.; Hernandez-Oñate, M.A.; Martinez-Tellez, M.A.; Lopez-Zavala, A.A.; Gonzalez-Aguilar, G.A.; Gutierrez-Pacheco, M.M.; Ayala-Zavala, J.F. Quercetin repressed the stress response factor (sigB) and virulence genes (prfA, actA, inlA, and inlC), lower the adhesion, and biofilm development of L. monocytogenes. Food Microbiol. 2020, 87, 103377. [Google Scholar] [CrossRef]
- Kim, Y.K.; Roy, P.K.; Ashrafudoulla, M.; Nahar, S.; Toushik, S.H.; Hossain, M.I.; Ha, S.D. Antibiofilm effects of quercetin against Salmonella enterica biofilm formation and virulence, stress response, and quorum-sensing gene expression. Food Control 2022, 137, 108964. [Google Scholar] [CrossRef]
- Ortega-Ramirez, L.A.; Gutiérrez-Pacheco, M.M.; Vargas-Arispuro, I.; González-Aguilar, G.A.; Martínez-Téllez, M.A.; Ayala-Zavala, J.F. Inhibition of glucosyltransferase activity and glucan production as an antibiofilm mechanism of lemongrass essential oil against Escherichia coli O157: H7. Antibiotics 2020, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Kostoglou, D.; Protopappas, I.; Giaouris, E. Common plant-derived terpenoids present increased anti-biofilm potential against Staphylococcus bacteria compared to a quaternary ammonium biocide. Foods 2020, 9, 697. [Google Scholar] [CrossRef]
- dos Santos Rodrigues, J.B.; de Carvalho, R.J.; de Souza, N.T.; de Sousa Oliveira, K.; Franco, O.L.; Schaffner, D.; de Souza, E.L.; Magnani, M. Effects of oregano essential oil and carvacrol on biofilms of Staphylococcus aureus from food-contact surfaces. Food Control 2017, 73, 1237–1246. [Google Scholar] [CrossRef]
- Ozma, M.A.; Abbasi, A.; Ahangarzadeh Rezaee, M.; Hosseini, H.; Hosseinzadeh, N.; Sabahi, S.; Kafil, H.S. A critical review on the nutritional and medicinal profiles of garlic’s (Allium sativum L.) bioactive compounds. Food Rev. Int. 2022, 1–38. [Google Scholar] [CrossRef]
- Yang, X.; Sha, K.; Xu, G.; Tian, H.; Wang, X.; Chen, S.; Wang, Y.; Li, J.; Chen, J.; Huang, N. Subinhibitory concentrations of allicin decrease uropathogenic Escherichia coli (UPEC) biofilm formation, adhesion ability, and swimming motility. Int. J. Mol. Sci. 2016, 17, 979. [Google Scholar] [CrossRef]
- Cruz-Valenzuela, M.R.; Ayala-Soto, R.E.; Ayala-Zavala, J.F.; Espinoza-Silva, B.A.; González-Aguilar, G.A.; Martín-Belloso, O.; Soliva-Fortuny, R.; Nazzaro, F.; Fratianni, F.; Tapia-Rodriguez, M.T.; et al. Pomegranate (Punica granatum L.) peel extracts as antimicrobial and antioxidant additives used in alfalfa sprouts. Foods 2022, 11, 2588. [Google Scholar] [CrossRef]
- Bouyahya, A.; Chamkhi, I.; Balahbib, A.; Rebezov, M.; Shariati, M.A.; Wilairatana, P.; El Omari, N. Mechanisms, anti-quorum-sensing actions, and clinical trials of medicinal plant bioactive compounds against bacteria: A comprehensive review. Molecules 2022, 27, 1484. [Google Scholar] [CrossRef] [PubMed]
- Fimbres-García, J.O.; Flores-Sauceda, M.; Othon-Díaz, E.D.; García-Galaz, A.; Tapia-Rodríguez, M.R.; Silva-Espinoza, B.A.; Ayala-Zavala, J.F. Facing resistant bacteria with plant essential oils: Reviewing the oregano case. Antibiotics 2022, 11, 1777. [Google Scholar] [CrossRef] [PubMed]
- Guillín, Y.; Cáceres, M.; Torres, R.; Stashenko, E.; Ortiz, C. Effect of essential oils on the inhibition of biofilm and quorum sensing in Salmonella enteritidis 13076 and Salmonella typhimurium 14028. Antibiotics 2021, 10, 1191. [Google Scholar] [CrossRef] [PubMed]
- Tamfu, A.N.; Ceylan, O.; Kucukaydin, S.; Duru, M.E. HPLC-DAD phenolic profiles, antibiofilm, anti-quorum sensing and enzyme inhibitory potentials of Camellia sinensis (L.) O. Kuntze and Curcuma longa L. LWT 2020, 133, 110150. [Google Scholar] [CrossRef]
- Tapia-Rodriguez, M.R.; Bernal-Mercado, A.T.; Gutierrez-Pacheco, M.M.; Vazquez-Armenta, F.J.; Hernandez-Mendoza, A.; Gonzalez-Aguilar, G.A.; Martinez-Tellez, M.A.; Nazzaro, F.; Ayala-Zavala, J.F. Virulence of Pseudomonas aeruginosa exposed to carvacrol: Alterations of the Quorum sensing at enzymatic and gene levels. J. Cell Commun. Signal. 2019, 13, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.D. Clinically important toxins in bacterial infection: Utility of laboratory detection. Clin. Microbiol. Newsl. 2020, 42, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Nwabor, O.F.; Singh, S.; Syukri, D.M.; Voravuthikunchai, S.P. Bioactive fractions of Eucalyptus camaldulensis inhibit important foodborne pathogens, reduce listeriolysin O-induced haemolysis, and ameliorate hydrogen peroxide-induced oxidative stress on human embryonic colon cells. Food Chem. 2021, 344, 128571. [Google Scholar] [CrossRef]
- Rasooly, R.; Molnar, A.; Choi, H.-Y.; Do, P.; Racicot, K.; Apostolidis, E. In-vitro inhibition of staphylococcal pathogenesis by witch-hazel and green tea extracts. Antibiotics 2019, 8, 244. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhao, X.; Yan, H.; Meng, R.; Zhang, Y.; Li, W.; Liu, Z.; Guo, N. Effect of tea tree oil on Staphylococcus aureus growth and enterotoxin production. Food Control 2016, 62, 257–263. [Google Scholar] [CrossRef]
- Zhang, M.; Li, H.; Agyekumwaa, A.K.; Yu, Y.; Xiao, X. Effects of citronellal on growth and enterotoxins production in Staphylococcus aureus ATCC 29213. Toxicon 2022, 213, 92–98. [Google Scholar] [CrossRef]
- Mooyottu, S.; Kollanoor-Johny, A.; Flock, G.; Bouillaut, L.; Upadhyay, A.; Sonenshein, A.L.; Venkitanarayanan, K. Carvacrol and trans-cinnamaldehyde reduce Clostridium difficile toxin production and cytotoxicity in vitro. Int. J. Mol. Sci. 2014, 15, 4415–4430. [Google Scholar] [CrossRef]
- Tian, F.; Woo, S.Y.; Lee, S.Y.; Park, S.B.; Im, J.H.; Chun, H.S. Plant-based natural flavonoids show strong inhibition of aflatoxin production and related gene expressions correlated with chemical structure. Food Microbiol. 2023, 109, 104141. [Google Scholar] [CrossRef]
- Pok, P.S.; Londoño, V.A.G.; Vicente, S.; Romero, S.M.; Pacín, A.; Tolaba, M.; Alzamora, S.M.; Resnik, S.L. Evaluation of citrus flavonoids against Aspergillus parasiticus in maize: Aflatoxins reduction and ultrastructure alterations. Food Chem. 2020, 318, 126414. [Google Scholar] [CrossRef] [PubMed]
- Hamad, G.M.; Mohdaly, A.A.A.; El-Nogoumy, B.A.; Ramadan, M.F.; Hassan, S.A.; Zeitoun, A.M. Detoxification of aflatoxin B1 and ochratoxin A using Salvia farinacea and Azadirachta indica water extract and application in meat products. Appl. Biochem. Biotechnol. 2021, 193, 3098–3120. [Google Scholar] [CrossRef] [PubMed]
- Ponzilacqua, B.; Rottinghaus, G.E.; Landers, B.R.; Oliveira, C.A.F.D. Effects of medicinal herb and Brazilian traditional plant extracts on in vitro mycotoxin decontamination. Food Control 2019, 100, 24–27. [Google Scholar] [CrossRef]
- Friedman, M.; Jürgens, H.S. Effect of pH on the stability of plant phenolic compounds. J. Agric. Food Chem. 2000, 48, 2101–2110. [Google Scholar] [CrossRef]
- Hatab, S.; Athanasio, R.; Holley, R.; Rodas-Gonzalez, A.; Narvaez-Bravo, C. Survival and reduction of shiga toxin-producing Escherichia coli in a fresh cold-pressed juice treated with antimicrobial plant extracts. J. Food Sci. 2016, 81, M1987–M1995. [Google Scholar] [CrossRef] [PubMed]
- Rattanachaikunsopon, P.; Phumkhachorn, P. Assessment of factors influencing antimicrobial activity of carvacrol and cymene against Vibrio cholerae in food. J. Biosci. Bioeng. 2010, 110, 614–619. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Rojas-Graü, A.; Soliva-Fortuny, R.; Martín-Belloso, O. Physicochemical characterization and antimicrobial activity of food-grade emulsions and nanoemulsions incorporating essential oils. Food Hydrocoll. 2015, 43, 547–556. [Google Scholar] [CrossRef]
- Topuz, O.K.; Özvural, E.B.; Zhao, Q.; Huang, Q.; Chikindas, M.; Gölükçü, M. Physical and antimicrobial properties of anise oil loaded nanoemulsions on the survival of foodborne pathogens. Food Chem. 2016, 203, 117–123. [Google Scholar] [CrossRef]
- Ghazy, O.A.; Fouad, M.T.; Saleh, H.H.; Kholif, A.E.; Morsy, T.A. Ultrasound-assisted preparation of anise extract nanoemulsion and its bioactivity against different pathogenic bacteria. Food Chem. 2021, 341, 128259. [Google Scholar] [CrossRef] [PubMed]
- Ghazy, O.A.; Fouad, M.T.; Morsy, T.A.; Kholif, A.E. Nanoemulsion formulation of Lawsonia inermis extract and its potential antimicrobial and preservative efficacy against foodborne pathogens. Food Control 2023, 145, 109458. [Google Scholar] [CrossRef]
- Bhargava, K.; Conti, D.S.; da Rocha, S.R.; Zhang, Y. Application of an oregano oil nanoemulsion to the control of foodborne bacteria on fresh lettuce. Food Microbiol. 2015, 47, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Paudel, S.K.; Bhargava, K.; Kotturi, H. Antimicrobial activity of cinnamon oil nanoemulsion against Listeria monocytogenes and Salmonella spp. on melons. LWT 2019, 111, 682–687. [Google Scholar] [CrossRef]
- Molet-Rodríguez, A.; Turmo-Ibarz, A.; Salvia-Trujillo, L.; Martín-Belloso, O. Incorporation of antimicrobial nanoemulsions into complex foods: A case study in an apple juice-based beverage. LWT 2021, 141, 110926. [Google Scholar] [CrossRef]
- Luciano, W.A.; Pimentel, T.C.; Bezerril, F.F.; Barão, C.E.; Marcolino, V.A.; Carvalho, R.D.S.F.; dos Santos Lima, M.; Martín-Belloso, O.; Magnani, M. Effect of citral nanoemulsion on the inactivation of Listeria monocytogenes and sensory properties of fresh-cut melon and papaya during storage. Int. J. Food Microbiol. 2023, 384, 109959. [Google Scholar] [CrossRef]
- Ozogul, Y.; Boğa, E.K.; Akyol, I.; Durmus, M.; Ucar, Y.; Regenstein, J.M.; Köşker, A.R. Antimicrobial activity of thyme essential oil nanoemulsions on spoilage bacteria of fish and food-borne pathogens. Food Biosci. 2020, 36, 100635. [Google Scholar] [CrossRef]
- Özogul, Y.; El Abed, N.; Özogul, F. Antimicrobial effect of laurel essential oil nanoemulsion on food-borne pathogens and fish spoilage bacteria. Food Chem. 2022, 368, 130831. [Google Scholar] [CrossRef]
- Özogul, Y.; Özogul, F.; Kulawik, P. The antimicrobial effect of grapefruit peel essential oil and its nanoemulsion on fish spoilage bacteria and food-borne pathogens. LWT 2021, 136, 110362. [Google Scholar] [CrossRef]
- Tang, M.; Liu, F.; Wang, Q.; Wang, D.; Wang, D.; Zhu, Y.; Sun, Z.; Xu, W. Physicochemical characteristics of ginger essential oil nanoemulsion encapsulated by zein/NaCas and antimicrobial control on chilled chicken. Food Chem. 2022, 374, 131624. [Google Scholar] [CrossRef]
- Yang, R.; Miao, J.; Shen, Y.; Cai, N.; Wan, C.; Zou, L.; Chen, C.; Chen, J. Antifungal effect of cinnamaldehyde, eugenol and carvacrol nanoemulsion against Penicillium digitatum and application in postharvest preservation of citrus fruit. LWT 2021, 141, 110924. [Google Scholar] [CrossRef]
- Gundewadi, G.; Sarkar, D.J.; Rudra, S.G.; Singh, D. Preparation of basil oil nanoemulsion using Sapindus mukorossi pericarp extract: Physico-chemical properties and antifungal activity against food spoilage pathogens. Ind. Crops Prod. 2018, 125, 95–104. [Google Scholar] [CrossRef]
- Ribes, S.; Fuentes, A.; Barat, J.M. Effect of oregano (Origanum vulgare L. ssp. hirtum) and clove (Eugenia spp.) nanoemulsions on Zygosaccharomyces bailii survival in salad dressings. Food Chem. 2019, 295, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Zhong, S.; Schwarz, P.; Chen, B.; Rao, J. Physical properties, antifungal and mycotoxin inhibitory activities of five essential oil nanoemulsions: Impact of oil compositions and processing parameters. Food Chem. 2019, 291, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, A.K.; Singh, V.K.; Das, S.; Prasad, J.; Dwivedy, A.K.; Dubey, N.K. Improvement of in vitro and in situ antifungal, AFB1 inhibitory and antioxidant activity of Origanum majorana L. essential oil through nanoemulsion and recommending as novel food preservative. Food Chem. Toxicol. 2020, 143, 111536. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Y.; Zhong, Q. Physical and antimicrobial properties of spray-dried zein–casein nanocapsules with co-encapsulated eugenol and thymol. J. Food Eng. 2015, 144, 93–102. [Google Scholar] [CrossRef]
- Talón, E.; Lampi, A.M.; Vargas, M.; Chiralt, A.; Jouppila, K.; González-Martínez, C. Encapsulation of eugenol by spray-drying using whey protein isolate or lecithin: Release kinetics, antioxidant and antimicrobial properties. Food Chem. 2019, 295, 588–598. [Google Scholar] [CrossRef]
- Sun, X.; Cameron, R.G.; Bai, J. Effect of spray-drying temperature on physicochemical, antioxidant and antimicrobial properties of pectin/sodium alginate microencapsulated carvacrol. Food Hydrocoll. 2020, 100, 105420. [Google Scholar] [CrossRef]
- Plati, F.; Papi, R.; Paraskevopoulou, A. Characterization of oregano essential oil (Origanum vulgare L. subsp. hirtum) particles produced by the novel nano spray drying technique. Foods 2021, 10, 2923. [Google Scholar] [CrossRef]
- Cruz-Molina, A.V.D.L.; Ayala Zavala, J.F.; Bernal Mercado, A.T.; Cruz Valenzuela, M.R.; González-Aguilar, G.A.; Lizardi-Mendoza, J.; Brown-Bojorquez, F.; Silva-Espinoza, B.A. Maltodextrin encapsulation improves thermal and pH stability of green tea extract catechins. J. Food Process. Preserv. 2021, 45, e15729. [Google Scholar] [CrossRef]
- Radünz, M.; dos Santos Hackbart, H.C.; Camargo, T.M.; Nunes, C.F.P.; de Barros, F.A.P.; Dal Magro, J.; Filho, P.J.S.; Gandra, E.A.; Radünz, A.L.; da Rosa Zavareze, E. Antimicrobial potential of spray drying encapsulated thyme (Thymus vulgaris) essential oil on the conservation of hamburger-like meat products. Int. J. Food Microbiol. 2020, 330, 108696. [Google Scholar] [CrossRef] [PubMed]
- do Valle Calomeni, A.; de Souza, V.B.; Tulini, F.L.; Thomazini, M.; Ostroschi, L.C.; de Alencar, S.M.; Massarioli, A.P.; de Carvalho Balieiro, J.C.; de Carvalho, R.A.; Favaro-Trindade, C.S. Characterization of antioxidant and antimicrobial properties of spray-dried extracts from peanut skins. Food Bioprod. Process. 2017, 105, 215–223. [Google Scholar] [CrossRef]
- Vinceković, M.; Viskić, M.; Jurić, S.; Giacometti, J.; Kovačević, D.B.; Putnik, P.; Donsì, F.; Barba, F.J.; Jambrak, A.R. Innovative technologies for encapsulation of Mediterranean plants extracts. Trends Food Sci. Technol. 2017, 69, 1–12. [Google Scholar] [CrossRef]
- de Araújo, J.S.F.; de Souza, E.L.; Oliveira, J.R.; Gomes, A.C.A.; Kotzebue, L.R.V.; da Silva Agostini, D.L.; de Oliveira, D.L.V.; Mazzetto, S.M.; da Silva, A.L.; Cavalcanti, M.T. Microencapsulation of sweet orange essential oil (Citrus aurantium var. dulcis) by liophylization using maltodextrin and maltodextrin/gelatin mixtures: Preparation, characterization, antimicrobial and antioxidant activities. Int. J. Biol. Macromol. 2020, 143, 991–999. [Google Scholar] [CrossRef]
- Viacava, G.E.; Ayala-Zavala, J.F.; González-Aguilar, G.A.; Ansorena, M.R. Effect of free and microencapsulated thyme essential oil on quality attributes of minimally processed lettuce. Postharvest Biol. Technol. 2018, 145, 125–133. [Google Scholar] [CrossRef]
- Garcia-Sotelo, D.; Silva-Espinoza, B.; Perez-Tello, M.; Olivas, I.; Alvarez-Parrilla, E.; González-Aguilar, G.A.; Ayala-Zavala, J.F. Antimicrobial activity and thermal stability of rosemary essential oil: β−cyclodextrin capsules applied in tomato juice. LWT 2019, 111, 837–845. [Google Scholar] [CrossRef]
- Silva, F.; Caldera, F.; Trotta, F.; Nerin, C.; Domingues, F.C. Encapsulation of coriander essential oil in cyclodextrin nanosponges: A new strategy to promote its use in controlled-release active packaging. Innov. Food Sci. Emerg. Technol. 2019, 56, 102177. [Google Scholar] [CrossRef]
- Ozdemir, N.; Pola, C.C.; Teixeira, B.N.; Hill, L.E.; Bayrak, A.; Gomes, C.L. Preparation of black pepper oleoresin inclusion complexes based on beta-cyclodextrin for antioxidant and antimicrobial delivery applications using kneading and freeze drying methods: A comparative study. LWT 2018, 91, 439–445. [Google Scholar] [CrossRef]
- Lin, L.; Gu, Y.; Sun, Y.; Cui, H. Characterization of chrysanthemum essential oil triple-layer liposomes and its application against Campylobacter jejuni on chicken. LWT 2019, 107, 16–24. [Google Scholar] [CrossRef]
- Yang, K.; Liu, A.; Hu, A.; Li, J.; Zen, Z.; Liu, Y.; Tang, S.; Li, C. Preparation and characterization of cinnamon essential oil nanocapsules and comparison of volatile components and antibacterial ability of cinnamon essential oil before and after encapsulation. Food Control 2021, 123, 107783. [Google Scholar] [CrossRef]
- Augustin, M.A.; Sanguansri, P. Challenges and solutions to incorporation of nutraceuticals in foods. Annu. Rev. Food Sci. Technol. 2015, 3, 61–83. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols—A review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Arruda, T.R.; Bernardes, P.C.; e Moraes, A.R.F.; Soares, N.D.F.F. Natural bioactives in perspective: The future of active packaging based on essential oils and plant extracts themselves and those complexed by cyclodextrins. Food Res. Int. 2022, 156, 111160. [Google Scholar] [CrossRef]
- Cui, R.; Zhu, B.; Yan, J.; Qin, Y.; Yuan, M.; Cheng, G.; Yuan, M. Development of a sodium alginate-based active package with controlled release of cinnamaldehyde loaded on halloysite nanotubes. Foods 2021, 10, 1150. [Google Scholar] [CrossRef]
- Cometa, S.; Bonifacio, M.A.; Bellissimo, A.; Pinto, L.; Petrella, A.; De Vietro, N.; Iannaccone, G.; Baruzzi, F.; De Giglio, E. A green approach to develop zeolite-thymol antimicrobial composites: Analytical characterization and antimicrobial activity evaluation. Heliyon 2022, 8, e09551. [Google Scholar] [CrossRef]
- Wang, Y.; Yi, S.; Lu, R.; Sameen, D.E.; Ahmed, S.; Dai, J.; Qin, W.; Li, S.; Liu, Y. Preparation, characterization, and 3D printing verification of chitosan/halloysite nanotubes/tea polyphenol nanocomposite films. Int. J. Biol. Macromol. 2021, 166, 32–44. [Google Scholar] [CrossRef]
- Jiang, Y.; Yin, H.; Zhou, X.; Wang, D.; Zhong, Y.; Xia, Q.; Deng, Y.; Zhao, Y. Antimicrobial, antioxidant and physical properties of chitosan film containing Akebia trifoliata (Thunb.) Koidz. peel extract/montmorillonite and its application. Food Chem. 2021, 361, 130111. [Google Scholar] [CrossRef]
- Riaz, A.; Lei, S.; Akhtar, H.M.S.; Wan, P.; Chen, D.; Jabbar, S.; Abid, M.; Hashim, M.M.; Zeng, X. Preparation and characterization of chitosan-based antimicrobial active food packaging film incorporated with apple peel polyphenols. Int. J. Biol. Macromol. 2018, 114, 547–555. [Google Scholar] [CrossRef]
- Wang, K.; Lim, P.N.; Tong, S.Y.; San Thian, E. Development of grapefruit seed extract-loaded poly (ε-caprolactone)/chitosan films for antimicrobial food packaging. Food Packag. Shelf Life 2019, 22, 100396. [Google Scholar] [CrossRef]
- Surendhiran, D.; Li, C.; Cui, H.; Lin, L. Fabrication of high stability active nanofibers encapsulated with pomegranate peel extract using chitosan/PEO for meat preservation. Food Packag. Shelf Life 2020, 23, 100439. [Google Scholar] [CrossRef]
- Gutiérrez-Pacheco, M.M.; Ortega-Ramírez, L.A.; Silva-Espinoza, B.A.; Cruz-Valenzuela, M.R.; González-Aguilar, G.A.; Lizardi-Mendoza, J.; Miranda, R.; Ayala-Zavala, J.F. Individual and combined coatings of chitosan and carnauba wax with oregano essential oil to avoid water loss and microbial decay of fresh cucumber. Coatings 2020, 10, 614. [Google Scholar] [CrossRef]
- Saberi, B.; Chockchaisawasdee, S.; Golding, J.B.; Scarlett, C.J.; Stathopoulos, C.E. Characterization of pea starch-guar gum biocomposite edible films enriched by natural antimicrobial agents for active food packaging. Food Bioprod. Process. 2017, 105, 51–63. [Google Scholar] [CrossRef]
- Chollakup, R.; Pongburoos, S.; Boonsong, W.; Khanoonkon, N.; Kongsin, K.; Sothornvit, R.; Sukyai, P.; Sukatta, U.; Harnkarnsujarit, N. Antioxidant and antibacterial activities of cassava starch and whey protein blend films containing rambutan peel extract and cinnamon oil for active packaging. LWT 2020, 130, 109573. [Google Scholar] [CrossRef]
- Issa, A.; Ibrahim, S.A.; Tahergorabi, R. Impact of sweet potato starch-based nanocomposite films activated with thyme essential oil on the shelf-life of baby spinach leaves. Foods 2017, 6, 43. [Google Scholar] [CrossRef]
- Aloui, H.; Deshmukh, A.R.; Khomlaem, C.; Kim, B.S. Novel composite films based on sodium alginate and gallnut extract with enhanced antioxidant, antimicrobial, barrier and mechanical properties. Food Hydrocoll. 2021, 113, 106508. [Google Scholar] [CrossRef]
- Kapetanakou, A.E.; Nestora, S.; Evageliou, V.; Skandamis, P.N. Sodium alginate–cinnamon essential oil coated apples and pears: Variability of Aspergillus carbonarius growth and ochratoxin A production. Food Res. Int. 2019, 119, 876–885. [Google Scholar] [CrossRef]
- Nouri, A.; Yaraki, M.T.; Ghorbanpour, M.; Wang, S. Biodegradable κ-carrageenan/nanoclay nanocomposite films containing Rosmarinus officinalis L. extract for improved strength and antibacterial performance. Int. J. Biol. Macromol. 2018, 115, 227–235. [Google Scholar] [CrossRef]
- He, S.; Wang, Y. Antimicrobial and antioxidant effects of kappa-carrageenan coatings enriched with cinnamon essential oil in pork meat. Foods 2022, 11, 2885. [Google Scholar] [CrossRef]
- Göksen, G.; Fabra, M.J.; Ekiz, H.I.; López-Rubio, A. Phytochemical-loaded electrospun nanofibers as novel active edible films: Characterization and antibacterial efficiency in cheese slices. Food Control 2020, 112, 107133. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.W. Preparation of antibacterial poly (lactide)/poly (butylene adipate-co-terephthalate) composite films incorporated with grapefruit seed extract. Int. J. Biol. Macromol. 2018, 120, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Suwanamornlert, P.; Kerddonfag, N.; Sane, A.; Chinsirikul, W.; Zhou, W.; Chonhenchob, V. Poly (lactic acid)/poly (butylene-succinate-co-adipate) (PLA/PBSA) blend films containing thymol as alternative to synthetic preservatives for active packaging of bread. Food Packag. Shelf Life 2020, 25, 100515. [Google Scholar] [CrossRef]
- Requena, R.; Vargas, M.; Chiralt, A. Eugenol and carvacrol migration from PHBV films and antibacterial action in different food matrices. Food Chem. 2019, 277, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Pabast, M.; Shariatifar, N.; Beikzadeh, S.; Jahed, G. Effects of chitosan coatings incorporating with free or nano-encapsulated Satureja plant essential oil on quality characteristics of lamb meat. Food Control 2018, 91, 185–192. [Google Scholar] [CrossRef]
- Amor, G.; Sabbah, M.; Caputo, L.; Idbella, M.; De Feo, V.; Porta, R.; Fechtali, T.; Mauriello, G. Basil essential oil: Composition, antimicrobial properties, and microencapsulation to produce active chitosan films for food packaging. Foods 2021, 10, 121. [Google Scholar] [CrossRef]
- Pan, J.; Ai, F.; Shao, P.; Chen, H.; Gao, H. Development of polyvinyl alcohol/β-cyclodextrin antimicrobial nanofibers for fresh mushroom packaging. Food Chem. 2019, 300, 125249. [Google Scholar] [CrossRef]
- Chen, Z.; Zong, L.; Chen, C.; Xie, J. Development and characterization of PVA-Starch active films incorporated with β-cyclodextrin inclusion complex embedding lemongrass (Cymbopogon citratus) oil. Food Packag. Shelf Life 2020, 26, 100565. [Google Scholar] [CrossRef]
- Lee, J.Y.; Garcia, C.V.; Shin, G.H.; Kim, J.T. Antibacterial and antioxidant properties of hydroxypropyl methylcellulose-based active composite films incorporating oregano essential oil nanoemulsions. LWT 2019, 106, 164–171. [Google Scholar] [CrossRef]
- Shokri, S.; Parastouei, K.; Taghdir, M.; Abbaszadeh, S. Application an edible active coating based on chitosan-Ferulago angulata essential oil nanoemulsion to shelf life extension of Rainbow trout fillets stored at 4 °C. Int. J. Biol. Macromol. 2020, 153, 846–854. [Google Scholar] [CrossRef]
- Abbasi, Z.; Aminzare, M.; Hassanzad Azar, H.; Rostamizadeh, K. Effect of corn starch coating incorporated with nanoemulsion of Zataria multiflora essential oil fortified with cinnamaldehyde on microbial quality of fresh chicken meat and fate of inoculated Listeria monocytogenes. J. Food Sci. Technol. 2021, 58, 2677–2687. [Google Scholar] [CrossRef]
- Corrêa, J.A.F.; dos Santos, J.V.G.; Evangelista, A.G.; Pinto, A.C.S.M.; de Macedo, R.E.F.; Luciano, F.B. Combined application of phenolic acids and essential oil components against Salmonella Enteritidis and Listeria monocytogenes in vitro and in ready-to-eat cooked ham. LWT 2021, 149, 111881. [Google Scholar] [CrossRef]
- Nikkhah, M.; Hashemi, M. Boosting antifungal effect of essential oils using combination approach as an efficient strategy to control postharvest spoilage and preserving the jujube fruit quality. Postharvest Biol. Technol. 2020, 164, 111159. [Google Scholar] [CrossRef]
- Purkait, S.; Bhattacharya, A.; Bag, A.; Chattopadhyay, R.R. Synergistic antibacterial, antifungal and antioxidant efficacy of cinnamon and clove essential oils in combination. Arch. Microbiol. 2020, 202, 1439–1448. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, D.; Cao, Y.; Zhang, Y.; Xiao, X.; Liu, F.; Yu, Y. Synergistic inactivation of Escherichia coli O157: H7 and Staphylococcus aureus by gallic acid and thymol and its potential application on fresh-cut tomatoes. Food Microbiol. 2022, 102, 103925. [Google Scholar] [CrossRef]
- Meira, N.V.; Holley, R.A.; Bordin, K.; de Macedo, R.E.; Luciano, F.B. Combination of essential oil compounds and phenolic acids against Escherichia coli O157: H7 in vitro and in dry-fermented sausage production. Int. J. Food Microbiol. 2017, 260, 59–64. [Google Scholar] [CrossRef]
- Nikkhah, M.; Hashemi, M.; Najafi, M.B.H.; Farhoosh, R. Synergistic effects of some essential oils against fungal spoilage on pear fruit. Int. J. Food Microbiol. 2017, 257, 285–294. [Google Scholar] [CrossRef]
- Aaliya, B.; Sunooj, K.V.; Navaf, M.; Akhila, P.P.; Sudheesh, C.; Mir, S.A.; Sabu, S.; Sasidharan, A.; Theingi Hlaing, M.; George, J. Recent trends in bacterial decontamination of food products by hurdle technology: A synergistic approach using thermal and non-thermal processing techniques. Food Res. Int. 2021, 147, 110514. [Google Scholar] [CrossRef]
- Pinto, L.; Baruzzi, F.; Cocolin, L.; Malfeito-Ferreira, M. Emerging technologies to control Brettanomyces spp. in wine: Recent advances and future trends. Trends Food Sci. Technol. 2020, 99, 88–100. [Google Scholar] [CrossRef]
- Patrignani, F.; Siroli, L.; Braschi, G.; Lanciotti, R. Combined use of natural antimicrobial based nanoemulsions and ultra high pressure homogenization to increase safety and shelf-life of apple juice. Food Control 2020, 111, 107051. [Google Scholar] [CrossRef]
- Chien, S.Y.; Sheen, S.; Sommers, C.; Sheen, L.Y. Combination effect of high-pressure processing and essential oil (Melissa officinalis extracts) or their constituents for the inactivation of Escherichia coli in ground beef. Food Bioproc. Tech. 2019, 12, 359–370. [Google Scholar] [CrossRef]
- Sheen, S.; Huang, C.Y.; Chuang, S. Synergistic effect of high hydrostatic pressure, allyl isothiocyanate, and acetic acid on the inactivation and survival of pathogenic Escherichia coli in ground chicken. J. Food Sci. 2022, 87, 5042–5053. [Google Scholar] [CrossRef] [PubMed]
- González-González, C.R.; Labo-Popoola, O.; Delgado-Pando, G.; Theodoridou, K.; Doran, O.; Stratakos, A.C. The effect of cold atmospheric plasma and linalool nanoemulsions against Escherichia coli O157: H7 and Salmonella on ready-to-eat chicken meat. LWT 2021, 149, 111898. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Benjakul, S.; Vongkamjan, K. Cold plasma combined with liposomal ethanolic coconut husk extract: A potential hurdle technology for shelf-life extension of Asian sea bass slices packaged under modified atmosphere. Innov. Food Sci. Emerg. Technol. 2020, 65, 102448. [Google Scholar] [CrossRef]
- Van Haute, S.; Raes, K.; Devlieghere, F.; Sampers, I. Combined use of cinnamon essential oil and MAP/vacuum packaging to increase the microbial and sensorial shelf life of lean pork and salmon. Food Packag. Shelf Life 2017, 12, 51–58. [Google Scholar] [CrossRef]
- Shiekh, K.A.; Benjakul, S. Melanosis and quality changes during refrigerated storage of Pacific white shrimp treated with Chamuang (Garcinia cowa Roxb.) leaf extract with the aid of pulsed electric field. Food Chem. 2020, 309, 125516. [Google Scholar] [CrossRef]
- Guo, M.; Zhang, L.; He, Q.; Arabi, S.A.; Zhao, H.; Chen, W.; Ye, X.; Liu, D. Synergistic antibacterial effects of ultrasound and thyme essential oils nanoemulsion against Escherichia coli O157: H7. Ultrason. Sonochem. 2020, 66, 104988. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Khang, D. Synergistic effects of 915 MHz microwave heating and essential oils on inactivation of foodborne pathogen in hot-chili sauce. Int. J. Food Microbiol. 2023, 398, 110210. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, S.; Guo, K.; Guo, D.; Li, J.; Wang, M.; Wang, Y.; Zhang, C.; Xia, X.; Shi, C. Efficacy of 405-nm LED illumination and citral used alone and in combination for the inactivation of Cronobacter sakazakii in reconstituted powdered infant formula. Food Res. Int. 2022, 154, 111027. [Google Scholar] [CrossRef]
- Silva-Espinoza, B.A.; Palomares-Navarro, J.J.; Tapia-Rodriguez, M.R.; Cruz-Valenzuela, M.R.; González-Aguilar, G.A.; Silva-Campa, E.; Pedroza-Montero, M.; Almeida-Lopes, M.; Miranda, R.; Ayala-Zavala, J.F. Combination of ultraviolet light-C and clove essential oil to inactivate Salmonella typhimurium biofilms on stainless steel. J. Food Saf. 2020, 40, e12788. [Google Scholar] [CrossRef]
- Chelliah, R.; Jo, K.H.; Yan, P.; Chen, X.; Jo, H.Y.; Madar, I.H.; Sultan, G.; Oh, D.H. Unravelling the sanitization potential of slightly acidic electrolyzed water combined Thymus vulgaris based nanoemulsion against foodborne pathogens and its safety assessment. Food Control 2023, 146, 109527. [Google Scholar] [CrossRef]
- Shankar, S.; Danneels, F.; Lacroix, M. Coating with alginate containing a mixture of essential oils and citrus extract in combination with ozonation or gamma irradiation increased the shelf life of Merluccius sp. fillets. Food Packag. Shelf Life 2019, 22, 100434. [Google Scholar] [CrossRef]
- Reinholds, I.; Pugajeva, I.; Bavrins, K.; Kuckovska, G.; Bartkevics, V. Mycotoxins, pesticides and toxic metals in commercial spices and herbs. Food Addit. Contam. Part B 2017, 10, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, N.; Höferl, M.; Buchbauer, G. Pesticides in essential oils and selected fragrance extracts. Some examples. A review. Flavour Frag. J. 2018, 33, 373–384. [Google Scholar] [CrossRef]
- Corrales, M.; Fernandez, A.; Pinto, M.G.V.; Butz, P.; Franz, C.M.; Schuele, E.; Tauscher, B. Characterization of phenolic content, in vitro biological activity, and pesticide loads of extracts from white grape skins from organic and conventional cultivars. Food Chem. Toxicol. 2010, 48, 3471–3476. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, H.; Ying, G.; Yang, M.; Nian, Y.; Liu, J.; Kong, W. Relationship of mycotoxins accumulation and bioactive components variation in ginger after fungal inoculation. Front. Pharmacol. 2017, 8, 331. [Google Scholar] [CrossRef]
- Hassan, H.F.; Koaik, L.; Khoury, A.E.; Atoui, A.; El Obeid, T.; Karam, L. Dietary exposure and risk assessment of mycotoxins in thyme and thyme-based products marketed in Lebanon. Toxins 2022, 14, 331. [Google Scholar] [CrossRef]



| Plant Antimicrobial | Spray Drying Inlet Temperature (°C) | Protective Matrix | Microbial Targets | Advantages | Disadvantages | Data from Ref. |
|---|---|---|---|---|---|---|
| Eugenol and thymol | 105 | Zein/casein | E. coli O157:H7, L. monocytogenes Scott A | Good dispersion in water and good stability during storage | not reported | [223] |
| Eugenol | 180 | Whey protein/maltodextrin/chitosan | E. coli, L. innocua | High encapsulation efficiency and thermal stability | Chitosan inclusion negatively affects thermal stability, releasing and antimicrobial properties of the powder | [224] |
| Carvacrol | 100–190 | Pectin/sodium alginate | E. coli K12 | Better thermal stability | High inlet temperature affects dissolution time and hygroscopicity | [225] |
| Oregano EO | 100 | Whey protein/maltodextrin | E. coli, S. aureus | Low residence time, high yield, low inlet temperature | Low throughput, extended processing hours, high production cost | [226] |
| Green tea extract | 150 | Maltodextrin | - | High thermal stability and reduced weight loss | not reported | [227] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, L.; Tapia-Rodríguez, M.R.; Baruzzi, F.; Ayala-Zavala, J.F. Plant Antimicrobials for Food Quality and Safety: Recent Views and Future Challenges. Foods 2023, 12, 2315. https://doi.org/10.3390/foods12122315
Pinto L, Tapia-Rodríguez MR, Baruzzi F, Ayala-Zavala JF. Plant Antimicrobials for Food Quality and Safety: Recent Views and Future Challenges. Foods. 2023; 12(12):2315. https://doi.org/10.3390/foods12122315
Chicago/Turabian StylePinto, Loris, Melvin R. Tapia-Rodríguez, Federico Baruzzi, and Jesús Fernando Ayala-Zavala. 2023. "Plant Antimicrobials for Food Quality and Safety: Recent Views and Future Challenges" Foods 12, no. 12: 2315. https://doi.org/10.3390/foods12122315