The Effect of Sodium Bicarbonate Injection on the Physico-Chemical Quality of Post-Harvest Trout
Abstract
:1. Introduction
2. Materials and Methods
2.1. SBC Applications
2.2. Proximate Analysis
2.3. Physicochemical Analysis
2.3.1. pH Analysis
2.3.2. Determination of Rigor Index
2.3.3. Water Holding Capacity
2.4. Physical Properties
2.4.1. Texture Profile Analysis
2.4.2. Color Assessment
2.5. Statistical Analysis
3. Results and Discussion
3.1. Proximate Composition
3.2. Physicochemical Analysis
3.2.1. pH Changes
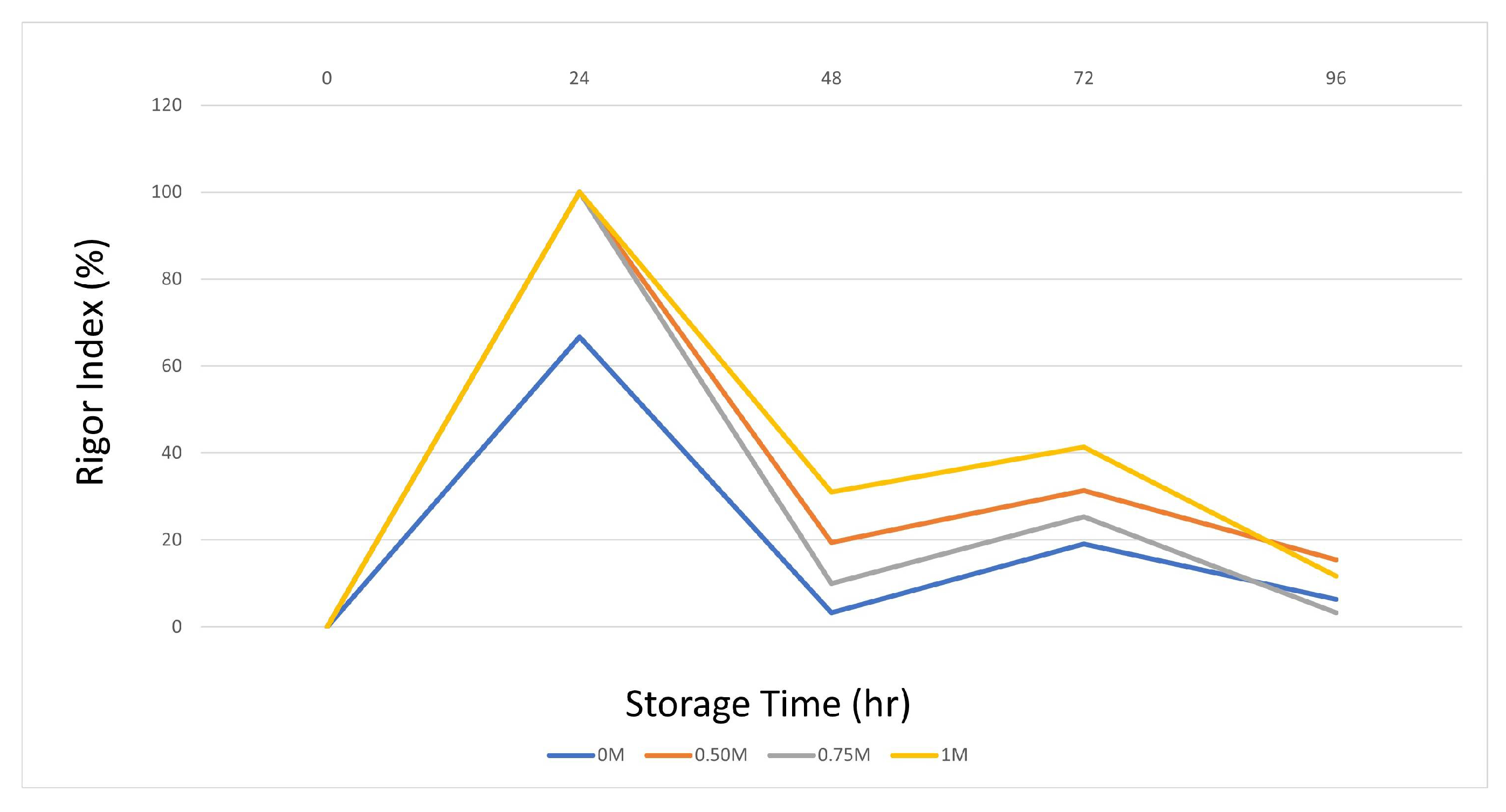
3.2.2. Determination of Rigor Index
3.2.3. Water Holding Capacity
3.3. Physical Properties
3.3.1. Texture Profile Analysis
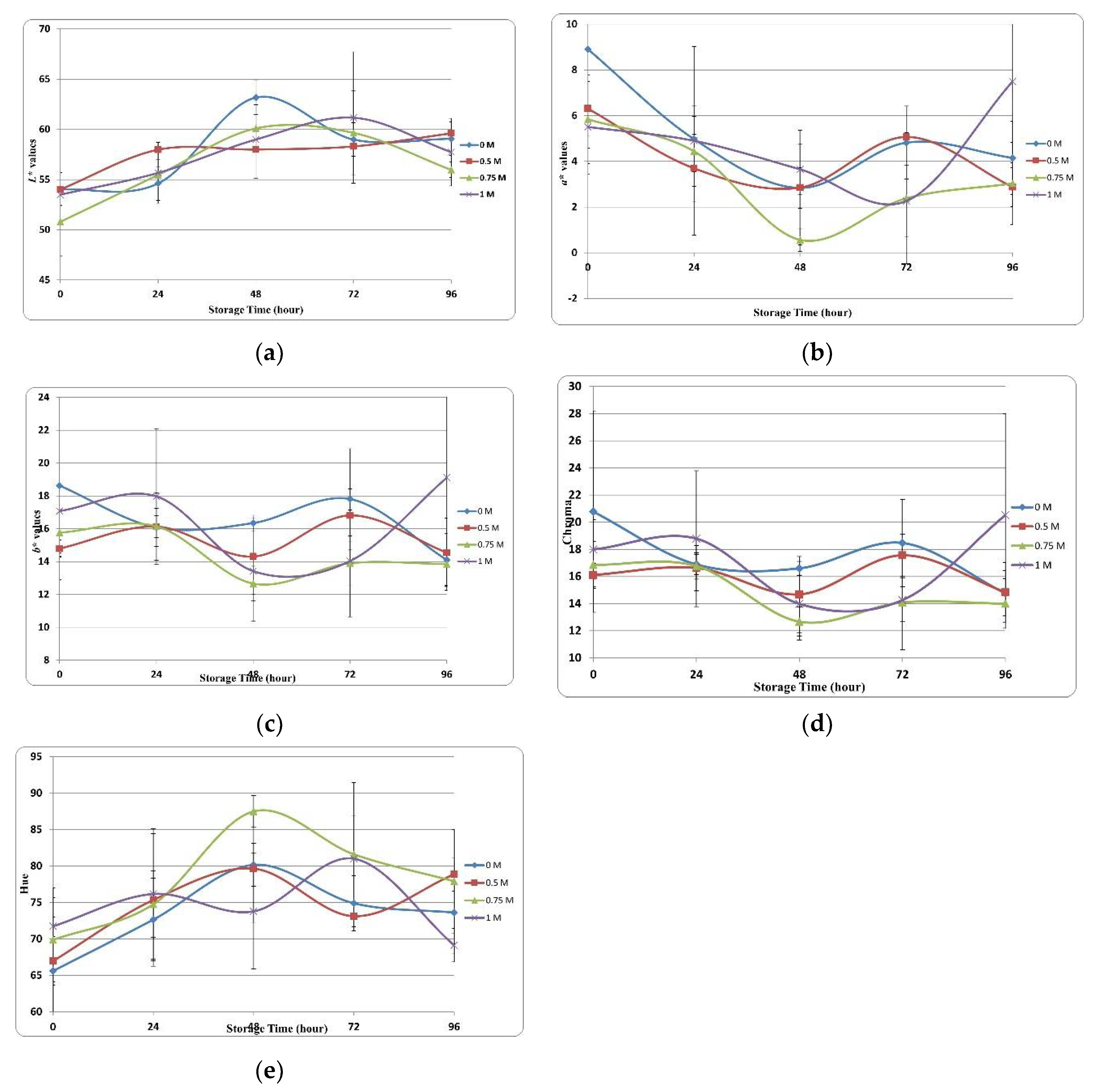
3.3.2. Color Assessment
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Kaneko, G.; Li, Y.; Xie, J.; Wang, G.; Li, Z.; Tian, J.; Zhang, K.; Gong, W.; Xia, Y.; et al. Reactive oxygen species (ROS)-mediated regulation of muscle texture in grass carp fed with dietary oxidants. Aquaculture 2021, 544, 737150. [Google Scholar] [CrossRef]
- Kang, Z.L.; Zhang, X.H.; Li, X.; Song, Z.J.; Ma, H.J.; Lu, F.; Zhu, M.-M.; Zhao, S.-M.; Wang, Z.R. The effects of sodium chloride on proteins aggregation, conformation and gel properties of pork myofibrillar protein Running Head: Relationship aggregation, conformation and gel properties. J. Food Sci. Technol. 2021, 58, 2258–2264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Q.; Hong, H.; Luo, Y.; Lametsch, R. Search for proteomic markers for stunning stress and stress-induced textural tenderization in silver carp (Hypophthalmichthys molitrix) fillets using label-free strategy. Food Res. Int. 2020, 137, 109678. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Benjakul, S. Proteolysis and its control using protease inhibitors in fish and fish products: A review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 496–509. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, B.L.; da Costa, M.P.; da Silva Frasão, B.; da Silva, F.A.; Mársico, E.T.; da Silveira Alvares, T.; Conte-Junior, C.A. Instrumental texture parameters as freshness indicators in five farmed Brazilian freshwater fish species. Food Anal. Methods 2017, 10, 3589–3599. [Google Scholar] [CrossRef]
- Monteiro, M.L.; Mársico, E.T.; Rosenthal, A.; Conte-Junior, C.A. Synergistic effect of ultraviolet radiation and high hydrostatic pressure on texture, colour, and oxidative stability of refrigerated tilapia fillets. J. Sci. Food Agric. 2019, 99, 4474–4481. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shan, J.; Han, S.; Zhao, J.; Zhang, Y. Optimization of fish quality by evaluation of total volatile basic nitrogen (TVB-N) and texture profile analysis (TPA) by near-infrared (NIR) hyperspectral imaging. Anal. Lett. 2019, 52, 1845–1859. [Google Scholar] [CrossRef]
- Boughattas, F.; Vilkova, D.; Kondratenko, E.; Karoui, R. Targeted and untargeted techniques coupled with chemometric tools for the evaluation of sturgeon (Acipenser gueldenstaedtii) freshness during storage at 4 °C. Food Chem. 2020, 312, 126000. [Google Scholar] [CrossRef]
- Astruc, T.; Vénien, A.; Clerjon, S.; Favier, R.; Loison, O.; Mirade, P.S.; Portanguen, S.; Rouel, J.; Lethiec, M.; Germond, A. Effect of dry salt versus brine injection plus dry salt on the physicochemical characteristics of smoked salmon after filleting. Heliyon 2022, 8, e11245. [Google Scholar] [CrossRef] [PubMed]
- Rotabakk, B.T.; Stien, L.H.; Skåra, T. Thaw rigor in Atlantic salmon (Salmo salar) fillets, as affected by thawing rate and frozen storage time. LWT 2022, 167, 113793. [Google Scholar] [CrossRef]
- Rosenthal, A.J. Texture profile analysis–how important are the parameters? J. Texture Stud. 2010, 41, 672–684. [Google Scholar] [CrossRef]
- Cropotova, J.; Mozuraityte, R.; Standal, I.B.; Grøvlen, M.S.; Rustad, T. Superchilled, chilled and frozen storage of Atlantic mackerel (Scomber scombrus) fillets–changes in texture, drip loss, protein solubility and oxidation. Int. J. Food Sci. Technol. 2019, 54, 2228–2235. [Google Scholar] [CrossRef] [Green Version]
- Bonfim, B.D.C.; Monteiro, M.L.G.; Santos, A.F.G.N.D.; Vilar, J.D.S.; Conte-Junior, C.A. Nutritional improvement and consumer perspective of fish nuggets with partial substitution of wheat flour coating by fish (Priacanthus arenatus, Cuvier, 1829) waste flour. J. Aquat. Food Prod. 2020, 29, 28–42. [Google Scholar] [CrossRef]
- Wang, X.; Dong, Y.; Wu, R.; Liu, D.; Hu, F.; Wang, C.; Wu, D. A method to improve water-holding capacity of beef during freezing-thawing process using ultrasound treatment. J. Food Process. Preserv. 2021, 45, e15004. [Google Scholar] [CrossRef]
- Li, Y.P.; Zou, X.L.; Kang, Z.L.; Ma, H.J. Effect of sodium bicarbonate on techno-functional and rheological properties of pale, soft, and exudative (PSE) meat batters. Meat Sci. 2022, 194, 108990. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.C. The Evaluation of Muscle Quality. In Quality and Grading of Carcasses of Meat Animals; CRC Press: Boca Raton, FL, USA, 2020; pp. 83–107. [Google Scholar]
- Singh, A.; Buamard, N.; Zhou, A.; Benjakul, S. Effect of sodium bicarbonate on textural properties and acceptability of gel from unwashed Asian sea bass mince. J. Food Sci. Technol. 2022, 59, 3109–3119. [Google Scholar] [CrossRef] [PubMed]
- Socaciu, M.I.; Semeniuc, C.A.; Vodnar, D.C. Edible films and coatings for fresh fish packaging: Focus on quality changes and shelf-life extension. Coatings 2018, 8, 366. [Google Scholar] [CrossRef] [Green Version]
- Matos, E.; Gonçalves, A.; Nunes, M.L.; Dinis, M.T.; Dias, J. Effect of harvesting stress and slaughter conditions on selected flesh quality criteria of gilthead seabream (Sparus aurata). Aquaculture 2010, 305, 66–72. [Google Scholar] [CrossRef]
- Balestra, F.; Petracci, M. Techno Functional Ingredients for Meat Products: Current Challenges. In Sustainable Meat Production And Processing; Academic Press: Cambridge, MA, USA, 2019; pp. 45–68. [Google Scholar]
- Kang, Z.L.; Zhang, X.H.; Li, K.; Li, Y.P.; Lu, F.; Ma, H.J.; Song, Z.-J.; Zhao, S.-M.; Zhu, M.M. Effects of sodium bicarbonate on the gel properties, water distribution and mobility of low-salt pork batters. LWT 2021, 139, 110567. [Google Scholar] [CrossRef]
- Zou, Y.; Shi, H.; Xu, P.; Jiang, D.; Zhang, X.; Xu, W.; Wang, D. Combined effect of ultrasound and sodium bicarbonate marination on chicken breast tenderness and its molecular mechanism. Ultrason. Sonochem. 2019, 59, 104. [Google Scholar] [CrossRef]
- Xiong, G.; Fu, X.; Pan, D.; Qi, J.; Xu, X.; Jiang, X. Influence of ultrasound-assisted sodium bicarbonate marination on the curing efficiency of chicken breast meat. Ultrason. Sonochem. 2020, 60, 104808. [Google Scholar] [CrossRef] [PubMed]
- Lopkulkiaert, W.; Prapatsornwattana, K.; Rungsardthong, V. Effects of sodium bicarbonate containing traces of citric acid in combination with sodium chloride on yield and some properties of white shrimp (Penaeus vannamei) frozen by shelf freezing, air-blast and cryogenic freezing. LWT 2009, 42, 768–776. [Google Scholar] [CrossRef]
- Martínez-Alvarez, O.; Gómez-Guillén, M.D.C. Effect of soaking with hydrogen peroxide and carbonate/bicarbonate buffer solutions on chemical composition and protein extractability of desalted cod. Eur. Food Res. Technol. 2008, 226, 661–669. [Google Scholar] [CrossRef]
- Aksun, E.T.; Karakaya Tokur, B. Effects of sodium bicarbonate injection on sensory and chemical qualities of rainbow trout during iced storage. Ege J. Fish Aqua. Sci. 2014, 31, 97–104. [Google Scholar]
- AOAC. Official Method 955.04. Nitrogen (Total) in Seafood. Fish and Other Marine Products. In Official Methods of Analysis of AOAC International (Chapter 35); Hungerford, J.M., Cunniff, P., Eds.; Association of Official Analytical Chemists: Arlington, VA, USA, 1998; p. 6. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- AOAC. Official Method 920.153. Ash Content. In Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Gaithersburg, MA, USA, 2002. [Google Scholar]
- AOAC. Official Method 950.46. Moisture Content in Meat. In Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Gaithersburg, MA, USA, 2002. [Google Scholar]
- Korkmaz, K.; Tokur, B. Proximate composition of three different fish (trout, anchovy and whiting) waste during catching season. Turk. J. Marit. Mari. Sci. 2019, 5, 133–140. [Google Scholar]
- Guvenın, D.; Tokur, B.; Korkmaz, K.; Ucar, Y. Proximate composition of traditional Turkish stuffed meatballs produced with rainbow trout (Oncorhynchus mykiss Walbaum, 1792) mince and determination of its colour and sensory quality during frozen storage (−18 ºC). Acta Aquat. Turc. 2021, 17, 361–375. [Google Scholar] [CrossRef]
- Lima dos Santos, C.A.M.; James, D.; Teutscher, F. Guidelines for Chilled Fish Storage Experiments; FAO: Rome, Italy, 1981. [Google Scholar]
- Bito, M. Studies on rigor-mortis of fish-1. Difference in the mode of rigor-mortis among some varieties of fish by modified cutting’s method. Bull. Tokai Reg. Rab. 1983, 109, 89–96. [Google Scholar]
- Borresen, T. New methods for determination of liquid holding capacity, saltwater holding capacity and cook loss in fish muscle. FTFI Rep. 1980, 663, 1–7. [Google Scholar]
- Buamard, N.; Benjakul, S. Combination effect of high-pressure treatment and ethanolic extract from coconut husk on gel properties of sardine surimi. LWT 2018, 91, 361–367. [Google Scholar] [CrossRef]
- Bourne, M. Food Texture and Viscosity: Concept and Measurement; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Calder, B.L. The Use of Polyphosphates to Maintain Yield and Quality of Whole Cooked, Cryogenically Frozen Lobster (Homarus americanus) and the Use of Sorbitol and Tocopherol to Maintain Quality of Whole Cooked, Cryogenically Frozen Crab (Cancer irroratus); The University of Maine: Orono, ME, USA, 2003. [Google Scholar]
- Çelik, M.; Kızak, V. Meat yield and proximate composition of rainbow trout (Oncorhynchus mykiss) harvesting from different aquaculture systems. Int. J. Pure Appl. Sci. Technol. 2018, 4, 117–123. [Google Scholar]
- Çelik, M.; Gökçe, M.A.; Başusta, N.; Küçükgülmez, A.; Taşbozan, O.; Tabakoğlu, Ş.S. Nutritional quality of rainbow trout (Oncorhynchus mykiss) caught from the Atatürk dam lake in Turkey. J. Muscle Foods 2008, 19, 50–61. [Google Scholar] [CrossRef]
- Tarricone, S.; Jambrenghi, A.C.; Cagnetta, P.; Ragni, M. Wild and farmed sea bass (Dicentrarchus Labrax): Comparison of biometry traits, chemical and fatty acid composition of fillets. Fishes 2022, 7, 45. [Google Scholar] [CrossRef]
- Hamm, R. Functional Properties of the Myofibrillar System and Their Measurements, in Muscle as Food; Bechtel, P.J., Ed.; Academic Press: New York, NY, USA, 1986; pp. 135–139. [Google Scholar]
- Åsli, M.; Mørkøre, T. Quality assessment of lightly salted Atlantic salmon fillets injected with brine solutions containing sodium bicarbonate. J. Food Res. 2013, 3, 110–121. [Google Scholar] [CrossRef]
- Petracci, M.; Laghi, L.; Rocculi, P.; Rimini, S.; Panarese, V.; Cremonini, M.A. The use of sodium bicarbonate for marination of broiler breast meat. Poult. Sci. 2012, 91, 526–534. [Google Scholar] [CrossRef]
- González-Rodríguez, M.N.; Sanz, J.J.; Santos, J.A.; Otero, A.; Garcia-Lopez, M.L. Bacteriological quality of aquacultured freshwater fish portions in prepackaged trays stored at 3 C. J. Food Prot. 2001, 64, 1399–1404. [Google Scholar] [CrossRef]
- Gimenez, B.; Roncales, P.; Beltran, J.A. Modified atmosphere packaging of filleted rainbow trout. J. Sci. Food Agric. 2002, 82, 1154–1159. [Google Scholar] [CrossRef]
- Åsli, M.; Mørkøre, T. Brines added sodium bicarbonate improve liquid retention and sensory attributes of lightly salted Atlantic cod. LWT 2012, 46, 196–202. [Google Scholar] [CrossRef]
- Zhu, D.Y.; Kang, Z.L.; Ma, H.J.; Xu, X.L.; Zhou, G.H. Effect of sodium chloride or sodium bicarbonate in the chicken batters: A physico-chemical and Raman spectroscopy study. Food Hydrocoll. 2018, 83, 222–228. [Google Scholar] [CrossRef]
- Ageeva, T.N.; Olsen, R.L.; Joensen, S.; Esaiassen, M. Effects of Long-Term Feed Deprivation on the Development of Rigor Mortis and Aspects of Muscle Quality in Live-Stored Mature Atlantic Cod (Gadus Morhua L.). J. Aquat. Food Prod. Technol. 2018, 27, 477–485. [Google Scholar] [CrossRef]
- Bjørlykke, G.A.; Roth, B.; Sørheim, O.; Kvamme, B.O.; Slinde, E. The effects of carbon monoxide on Atlantic salmon (Salmo salar L.). Food Chem. 2011, 127, 1706–1711. [Google Scholar] [CrossRef]
- Matarneh, S.K.; Scheffler, T.L.; Gerrard, D.E. The Conversion of Muscle to Meat. In Lawrie’s Meat Science; Woodhead Publishing: Cambridge, UK, 2023; pp. 159–194. [Google Scholar]
- Concollato, A.; Parisi, G.; Olsen, R.E.; Kvamme, B.O.; Slinde, E.; Dalle Zotte, A. Effect of carbon monoxide for Atlantic salmon (Salmo salar L.) slaughtering on stress response and fillet shelf life. Aquaculture 2014, 433, 13–18. [Google Scholar] [CrossRef]
- Moriya, K.; Nakazawa, N.; Osako, K.; Okazaki, E. Effect of subzero temperature treatment at −2 °C before thawing on prevention of thaw rigor, biochemical changes and rate of ATP consumption in frozen chub mackerel (Scomber japonicus). LWT 2019, 114, 108396. [Google Scholar] [CrossRef]
- Bahuaud, D.; Mørkøre, T.; Østbye, T.K.; Veiseth-Kent, E.; Thomassen, M.S.; Ofstad, R. Muscle structure responses and lysosomal cathepsins B and L in farmed Atlantic salmon (Salmo salar L.) pre-and post-rigor fillets exposed to short and long-term crowding stress. Food Chem. 2010, 118, 602–615. [Google Scholar] [CrossRef]
- Li, H.; Bai, X.; Li, Y.; Du, X.; Wang, B.; Li, F.; Shi, S.; Pan, N.; Zhang, Q.; Xia, X.; et al. The positive contribution of ultrasound technology in muscle food key processing and its mechanism-a review. Crit. Rev. Food Sci. Nutr. 2022, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Zhao, M.; Sun, W. Effect of pH on the interaction of porcine myofibrillar proteins with pyrazine compounds. Food Chem. 2019, 287, 93–99. [Google Scholar] [CrossRef]
- Alvarado, C.Z.; Sams, A.R. Injection marination strategies for remediation of pale, exudative broiler breast meat. Poult. Sci. 2003, 82, 1332–1336. [Google Scholar] [CrossRef] [PubMed]
- Warner, R.D. The Eating Quality of Meat: IV—Water Holding Capacity and Juiciness. In Lawrie’s Meat Science; Woodhead Publishing: Cambridge, UK, 2023; pp. 457–508. [Google Scholar]
- Tulli, F.; Fabbro, A.; D’Agaro, E.; Messina, M.; Bongiorno, T.; Venir, E.; Lippe, G.; Tibaldi, E.; Stecchini, M.L. The effect of slaughtering methods on actin degradation and on muscle quality attributes of farmed European sea bass (Dicentrarchus labrax). J. Food Sci. Technol. 2015, 52, 7182–7190. [Google Scholar] [CrossRef]
- Barroso, M.; Careche, M.; Borderıas, A.J. Quality control of frozen fish using rheological techniques. Trends Food Sci. Technol. 1998, 9, 223–229. [Google Scholar] [CrossRef] [Green Version]
- Mishra, G.; Sahni, P.; Pandiselvam, R.; Panda, B.K.; Bhati, D.; Mahanti, N.K.; Kothakota, A.; Kothakota, M.; Cozzolino, D. Emerging Non-destructive Techniques to Quantify the Textural Properties of Food: A State-of-Art Review. J. Texture Stud. 2023, 54, 173–205. [Google Scholar] [CrossRef]
- Gao, M.; Feng, L.; Jiang, T.; Zhu, J.; Fu, L.; Yuan, D.; Li, J. The use of rosemary extract in combination with nisin to extend the shelf life of pompano (Trachinotus ovatus) fillet during chilled storage. Food Control 2014, 37, 1–8. [Google Scholar] [CrossRef]
- Liu, M.; Wei, Y.; Li, X.; Quek, S.Y.; Zhao, J.; Zhong, H.; Zhang, D.; Liu, Y. Quantitative phosphoproteomic analysis of caprine muscle with high and low meat quality. Meat Sci. 2018, 141, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, J.; Wang, J.; Lv, W. Applying different methods to evaluate the freshness of large yellow croacker (Pseudosciaena crocea) fillets during chilled storage. J. Agric. Food Chem. 2012, 60, 11387–11394. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.L.; Zeng, Q.X.; Zhu, Z.W.; Song, G.S. Relation between protein characteristics and TPA texture characteristics of crisp grass carp (Ctenopharyngodon idellus). J. Texture Stud. 2012, 43, 1–11. [Google Scholar] [CrossRef]
- Iaconisi, V.; Marono, S.; Parisi, G.; Gasco, L.; Genovese, L.; Maricchiolo, G.; Bovera, F.; Piccolo, G. Dietary inclusion of Tenebrio molitor larvae meal: Effects on growth performance and final quality treats of blackspot sea bream (Pagellus bogaraveo). Aquaculture 2017, 476, 49–58. [Google Scholar] [CrossRef]
- Wu, F.; Wen, H.; Tian, J.; Jiang, M.; Liu, W.; Yang, C.; Yu, L.; Lu, X. Effect of stocking density on growth performance, serum biochemical parameters, and muscle texture properties of genetically improved farm tilapia, Oreochromis niloticus. Aquac. Int. 2018, 26, 1247–1259. [Google Scholar] [CrossRef]
- Tang, M.; Dai, H.; Ma, L.; Yu, Y.; Liu, T.; Feng, X.; Hu, W.; Li, Y.; Zhang, Y. Degradation of structural proteins and their relationship with the quality of Mandarin fish (Siniperca chuatsi) during post-mortem storage and cooking. Int. J. Food Sci. Technol. 2020, 55, 1617–1628. [Google Scholar] [CrossRef]
- Hassoun, A.; Karoui, R. Quality evaluation of fish and other seafood by traditional and nondestructive instrumental methods: Advantages and limitations. Crit. Rev. Food Sci. Nutr. 2017, 57, 1976–1998. [Google Scholar] [CrossRef]
- Jiang, Q.; Yang, F.; Jia, S.; Yu, D.; Gao, P.; Xu, Y.; Xia, W. The role of endogenous proteases in degrading grass carp (Ctenopharyngodon idella) myofibrillar structural proteins during ice storage. LWT 2022, 154, 112743. [Google Scholar] [CrossRef]
- Zhang, Y.; Chang, S.K. Protein extraction from catfish byproducts and physicochemical properties of the protein isolates. J. Food Sci. 2021, 86, 3061–3074. [Google Scholar] [CrossRef]
- Bao, Y.; Wang, K.; Yang, H.; Regenstein, J.M.; Ertbjerg, P.; Zhou, P. Protein degradation of black carp (Mylopharyngodon piceus) muscle during cold storage. Food Chem. 2020, 308, 125576. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, L.; Lu, H.; Song, S.; Luo, Y. Comparison of postmortem changes in ATP-related compounds, protein degradation and endogenous enzyme activity of white muscle and dark muscle from common carp (Cyprinus carpio) stored at 4 °C. LWT 2017, 78, 317–324. [Google Scholar] [CrossRef]
- Lougovois, V.; Kyrana, V.R. Freshness quality and spoilage of chill-stored fish. Food Policy 2005, 1, 35–86. [Google Scholar]
- Adebisi, O.E. Effect of Rigor State, When Processed, on Rainbow Trout (Oncorhynchus mykiss) Fillet Quality; West Virginia University: Morgantown, WV, USA, 2012. [Google Scholar]
- Dong, X.P.; Wu, Q.; Li, D.Y.; Wang, T.; Pan, J.F.; Zheng, J.J.; Fu, X.-X.; Qi, L.-B.; Chen, G.B. Physicochemical, micro-structural, and textural properties of different parts from farmed common carp (Cyprinus carpio). Int. J. Food Prop. 2017, 20, 946–955. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.D.; Jeong, J.Y.; Jung, E.Y.; Yang, H.S.; Lim, H.T.; Joo, S.T. The influence of fiber size distribution of type IIB on carcass traits and meat quality in pigs. Meat Sci. 2013, 94, 267–273. [Google Scholar] [CrossRef]
- Shabanpour, B.; Etemadian, Y. Chemical changes and shelf-life of conventional surimi and proteins recovered using pH change method from common carp (Cyprinus carpio) muscle during 5 months storage at-18 °C. Iran. J. Fish. Sci. 2016, 15, 311–332. [Google Scholar]
- Xu, S. Developing Healthy Snack Chips by Continuous Vacuum Belt Drying. Ph.D. Thesis, Department of Food Science and Technology, University of Georgia, Athens, GA, USA, 2012. [Google Scholar]
- Cuttle, C.K.; Bierman, A.; Gotti, M.B. Effects of light exposure on the appearance of meat displays. LEUKOS 2001, 30, 116–123. [Google Scholar] [CrossRef]

| Hour | Molarite (M) | |||
|---|---|---|---|---|
| 0 M | 0.50 M | 0.75 M | 1 M | |
| 0 | 6.789 ± 0.273 az | 6.817 ± 0.440 ay | 7.051 ± 0.500 by | 6.869 ± 0.195 ax |
| 24 | 6.590 ± 0.700 axy | 6.754 ± 0.196 bxy | 6.818 ± 0.295 bx | 6.757 ± 0.228 bx |
| 48 | 6.624 ± 0.848 axy | 6.675 ± 0.211 ax | 6.949 ± 0.265 cxy | 6.775 ± 0.154 bx |
| 72 | 6.549 ± 0.048 ax | 6.673 ± 0.179 bx | 6.856 ± 0.211 cx | 6.796 ± 0.143 cx |
| 96 | 6.646 ± 0.101 ay | 6.681 ± 0.114 abxy | 6.856 ± 0.207 cx | 6.747 ± 0.219 bx |
| Hour | Region | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| 0 | 6.738 ± 0.266 ax | 7.005 ± 0.488 by | 6.865 ± 0.387 abx | 6.870 ± 0.354 abx | 6.868 ± 0.374 aby |
| 24 | 6.665 ± 0.230 ax | 6.709 ± 0.256 ax | 6.712 ± 0.201 ax | 6.795 ± 0.243 ax | 6.768 ± 0.196 axy |
| 48 | 6.674 ± 0.149 ax | 6.703 ± 0.171 ax | 6.736 ± 0.185 abx | 6.857 ± 0.291 bx | 6.810 ± 0.264 abxy |
| 72 | 6.649 ± 0.142 ax | 6.653 ± 0.152 ax | 6.760 ± 0.210 abx | 6.788 ± 0.245 bx | 6.744 ± 0.184 abxy |
| 96 | 6.711 ± 0.148 abx | 6.678 ± 0.124 ax | 6.778 ± 0.196 abx | 6.812 ± 0.245 bx | 6.683 ± 0.162 ax |
| Region | |||||
|---|---|---|---|---|---|
| Molarite (M) | 1 | 2 | 3 | 4 | 5 |
| 0 | 6.643 ± 0.229 ax | 6.650 ± 0.196 ax | 6.619 ± 0.104 ax | 6.635 ± 0.145 ax | 6.651 ± 0.100 ax |
| 0.5 | 6.643 ± 0.137 ax | 6.756 ± 0.429 axy | 6.745 ± 0.215 ay | 6.739 ± 0.196 ax | 6.719 ± 0.207 axy |
| 0.75 | 6.741 ± 0.195 ax | 6.686 ± 0.271 abxy | 6.957 ± 0.340 bz | 7.029 ± 0.344 bz | 6.940 ± 0.370 bz |
| 1 | 6.722 ± 0.189 ax | 6.729 ± 0.208 axy | 6.759 ± 0.148 ay | 6.895 ± 0.204 by | 6.789 ± 0.151 ay |
| Hour | Molarite (M) | |||
|---|---|---|---|---|
| 0 | 0.50 | 0.75 | 1 | |
| 0 | 89.837 ± 2.164 ax | 89.766 ± 1.318 ax | 90.476 ± 1.2446 bx | 90.078 ± 0.748 abx |
| 24 | 92.990 ± 3.781 axy | 93.581 ± 1.639 abxy | 93.901 ± 1.176 abxy | 94.360 ± 1.970 by |
| 48 | 91.443 ± 3.482 ax | 94.706 ± 1.985 aby | 94.812 ± 3.485 aby | 94.967 ± 5.582 by |
| 72 | 90.754 ± 0.324 ax | 91.855 ± 4.914 abx | 92.690 ± 2.949 bxy | 91.209 ± 5.175 abx |
| 96 | 90.652 ± 2.323 ax | 93.268 ± 6.628 bxy | 93.790 ± 4.379 bxy | 92.453 ± 4.325 abxy |
| Concentration | Hardness (Newton) (N) | Springiness (Ratio) (%) | Cohesiveness (Ratio) (%) | Gumminess (Newton) (N) | Chewiness (Newton) (N) | Resilience (Ratio) (%) | Storage Hours |
|---|---|---|---|---|---|---|---|
| 1. Region | |||||||
| 0 | 8.665 ± 2.121 a | 1.577 ± 0.518 a | 0.819 ± 0.043 b | 7.144 ± 1.553 a | 12.527 ± 1.673 b | 0.621 ± 0.019 c | 0 |
| 11.978 ± 0.926 b | 1.231 ± 0.419 a | 0.710 ± 0.037 a | 12.871 ± 0.798 b | 14.808 ± 1.737 b | 0.552 ± 0.051 b | 24 | |
| 9.843 ± 1.052 a | 1.279 ± 0.256 a | 0.731 ± 0.019 a | 7.205 ± 0.881 a | 9.068 ± 0.821 a | 0.497 ± 0.003 ab | 48 | |
| 9.780 ± 0.784 a | 0.993 ± 0.008 a | 0.721 ± 0.024 a | 7.587 ± 1.235 a | 7.530 ± 1.164 a | 0.474 ± 0.006 a | 72 | |
| 6.224 ± 1.057 a | 1.289 ± 0.519 a | 0.678 ± 0.061 a | 7.095 ± 0.151 a | 7.705 ± 1.289 a | 0.443 ± 0.041 a | 96 | |
| 0.5 | 12.221 ± 1.220 c | 1.780 ± 0.323 b | 0.807 ± 0.019 b | 9.831 ± 1.007 c | 19.003 ± 2.569 b | 0.615 ± 0.031 c | 0 |
| 11.044 ± 1.531 bc | 1.021 ± 0.046 a | 0.746 ± 0.037 a | 8.364 ± 1.066 bc | 8.599 ± 0.818 a | 0.525 ± 0.026 b | 24 | |
| 10.733 ± 0.966 bc | 0.964 ± 0.043 a | 0.705 ± 0.043 a | 8.120 ± 1.075 bc | 7.798 ± 0.678 a | 0.470 ± 0.023 a | 48 | |
| 9.152 ± 1.195 ab | 1.117 ± 0.306 a | 0.733 ± 0.024 a | 6.690 ± 0.702 ab | 6.687 ± 0.965 a | 0.471 ± 0.018 a | 72 | |
| 6.294 ± 1.478 a | 1.150 ± 0.290 a | 0.696 ± 0.031 a | 5.396 ± 0.829 a | 6.043 ± 0.488 a | 0.434 ± 0.021 a | 96 | |
| 0.75 | 7.769 ± 0.959 a | 1.766 ± 0.343 b | 0.793 ± 0.020 c | 6.149 ± 0.607 a | 11.816 ± 1.302 c | 0.582 ± 0.036 c | 0 |
| 12.477 ± 1.084 b | 1.059 ± 0.139 a | 0.743 ± 0.034 b | 8.903 ± 0.487 b | 9.384 ± 0.752 b | 0.524 ± 0.036 bc | 24 | |
| 7.272 ± 1.831 a | 0.982 ± 0.017 a | 0.710 ± 0.017 ab | 5.128 ± 1.270 a | 5.033 ± 1.305 a | 0.448 ± 0.036 a | 48 | |
| 9.194 ± 1.285 a | 1.001 ± 0.013 a | 0.741 ± 0.018 b | 6.830 ± 1.096 a | 6.846 ± 1.172 a | 0.478 ± 0.028 ab | 72 | |
| 9.744 ± 1.124 ab | 0.988 ± 0.007 a | 0.699 ± 0.015 a | 6.338 ± 1.537 a | 6.269 ± 1.557 a | 0.439 ± 0.024 a | 96 | |
| 1 | 9.900 ± 0.670 b | 1.800 ± 0.184 b | 0.754 ± 0.023 ab | 7.494 ± 0.474 b | 14.297 ± 1.261 b | 0.572 ± 0.025 b | 0 |
| 11.693 ± 2.121 b | 1.069 ± 0.132 a | 0.749 ± 0.024 ab | 8.141 ± 0.445 b | 7.694 ± 0.659 a | 0.539 ± 0.012 b | 24 | |
| 6.607 ± 1.804 a | 1.210 ± 0.285 ab | 0.715 ± 0.055 ab | 4.779 ± 1.126 a | 7.580 ± 0.803 a | 0.457 ± 0.009 a | 48 | |
| 10.564 ± 1.201 b | 1.197 ± 0.384 ab | 0.691 ± 0.029 a | 6.713 ± 1.474 b | 7.671 ± 0.610 a | 0.450 ± 0.020 a | 72 | |
| 6.628 ± 0.267 a | 1.292 ± 0.517 ab | 0.759 ± 0.025 b | 5.029 ± 1.474 a | 6.530 ± 2.765 a | 0.477 ± 0.049 a | 96 | |
| 2. Region | |||||||
| 0 | 10.893 ± 1.382 a | 1.650 ± 0.569 a | 0.827 ± 0.048 c | 8.428 ± 1.612 a | 15.064 ± 0.90 b | 0.655 ± 0.057 b | 0 |
| 16.522 ± 0.624 b | 1.254 ± 0.456 a | 0.776 ± 0.042 bc | 13.588 ± 1.139 b | 18.893 ± 0.906 c | 0.606 ± 0.043 b | 24 | |
| 11.637 ± 0.796 a | 1.483 ± 0.444 a | 0.734 ± 0.010 ab | 8.538 ± 0.480 a | 13.904 ± 1.154 b | 0.518 ± 0.024 a | 48 | |
| 10.788 ± 0.696 a | 1.268 ± 0.480 a | 0.750 ± 0.032 ab | 8.198 ± 0.390 a | 10.060 ± 0.657 a | 0.508 ± 0.021 a | 72 | |
| 11.737 ± 0.814 a | 1.308 ± 0.518 a | 0.684 ± 0.046 a | 8.021 ± 0.620 a | 9.312 ± 1.003 a | 0.457 ± 0.041 a | 96 | |
| 0.5 | 10.559 ± 0.782 b | 1.976 ± 0.025 b | 0.828 ± 0.030 b | 9.437 ± 1.574 b | 17.422 ± 1.070 d | 0.644 ± 0.031 c | 0 |
| 15.277 ± 1.029 d | 1.265 ± 0.492 a | 0.740 ± 0.020 a | 10.182 ± 0.962 b | 11.850 ± 1.329 c | 0.535 ± 0.012 b | 24 | |
| 12.587 ± 1.061 c | 0.984 ± 0.021 a | 0.756 ± 0.031 a | 9.159 ± 0.755 b | 9.003 ± 0.578 b | 0.529 ± 0.022 b | 48 | |
| 9.253 ± 0.678 ab | 1.009 ± 0.050 a | 0.756 ± 0.018 a | 7.040 ± 0.500 a | 7.917 ± 0.694 ab | 0.500 ± 0.021 ab | 72 | |
| 8.626 ± 0.748 a | 1.259 ± 0.465 a | 0.723 ± 0.009 a | 6.233 ± 0.472 a | 6.832 ± 0.842 a | 0.463 ± 0.009 a | 96 | |
| 0.75 | 9.797 ± 1.465 a | 1.612 ± 0.361 b | 0.808 ± 0.010 b | 7.916 ± 1.188 ab | 13.584 ± 0.906 c | 0.613 ± 0.031 b | 0 |
| 15.298 ± 1.006 b | 1.267 ± 0.455 ab | 0.768 ± 0.030 ab | 12.843 ± 0.710 b | 14.170 ± 1.289 c | 0.570 ± 0.037 b | 24 | |
| 10.298 ± 0.777 a | 0.976 ± 0.051 a | 0.719 ± 0.051 a | 6.294 ± 1.066 a | 6.670 ± 0.428 a | 0.471 ± 0.024 a | 48 | |
| 10.724 ± 0.496 a | 1.069 ± 0.129 a | 0.757 ± 0.008 ab | 8.123 ± 0.380 b | 8.458 ± 0.379 b | 0.505 ± 0.024 a | 72 | |
| 10.710 ± 1.076 a | 1.071 ± 0.125 a | 0.723 ± 0.013 a | 7.738 ± 0.782 ab | 8.224 ± 0.095 b | 0.473 ± 0.007 a | 96 | |
| 1 | 10.864 ± 0.854 bc | 1.910 ± 0.107 b | 0.784 ± 0.002 a | 8.513 ± 0.654 b | 16.556 ± 0.936 c | 0.610 ± 0.008 c | 0 |
| 17.324 ± 1.087 d | 1.059 ± 0.119 a | 0.744 ± 0.043 a | 12.643 ± 0.825 c | 12.429 ± 0.973 b | 0.555 ± 0.034 b | 24 | |
| 9.852 ± 0.737 ab | 1.251 ± 0.335 ab | 0.728 ± 0.070 a | 7.267 ± 0.402 a | 10.096 ± 0.688 ab | 0.490 ± 0.034 a | 48 | |
| 12.246 ± 1.159 c | 1.279 ± 0.539 ab | 0.719 ± 0.022 a | 8.790 ± 0.702 b | 9.528 ± 1.154 ab | 0.488 ± 0.016 a | 72 | |
| 8.353 ± 0.930 a | 1.319 ± 0.531 ab | 0.753 ± 0.007 a | 6.286 ± 0.652 a | 8.305 ± 3.482 a | 0.494 ± 0.022 a | 96 | |
| 3. Region | |||||||
| 0 | 9.978 ± 0.702 a | 1.659 ± 0.299 a | 0.870 ± 0.062 c | 8.644 ± 0.536 ab | 11.386 ± 0.826 b | 0.693 ± 0.071 c | 0 |
| 21.530 ± 1.488 c | 1.101 ± 0.194 a | 0.760 ± 0.016 b | 16.532 ± 1.222 c | 18.103 ± 1.790 d | 0.581 ± 0.050 b | 24 | |
| 12.432 ± 0.559 b | 1.482 ± 0.476 a | 0.732 ± 0.028 ab | 9.543 ± 0.864 b | 14.648 ± 0.687 c | 0.520 ± 0.048 ab | 48 | |
| 12.402 ± 0.679 b | 1.151 ± 0.262 a | 0.726 ± 0.009 ab | 8.662 ± 0.855 ab | 9.349 ± 0.595 a | 0.481 ± 0.007 b | 72 | |
| 11.161 ± 0.345 ab | 1.294 ± 0.525 a | 0.665 ± 0.065 a | 7.420 ± 0.714 a | 8.416 ± 1.152 a | 0.436 ± 0.053 b | 96 | |
| 0.5 | 12.636 ± 0.427 c | 1.946 ± 0.030 b | 0.857 ± 0.055 b | 11.505 ± 0.569 c | 22.028 ± 0.544 d | 0.686 ± 0.064 b | 0 |
| 21.201 ± 1.064 e | 1.264 ± 0.328 a | 0.720 ± 0.010 a | 15.227 ± 0.714 d | 18.392 ± 0.960 c | 0.529 ± 0.008 a | 24 | |
| 15.189 ± 0.721 d | 0.969 ± 0.019 a | 0.730 ± 0.060 a | 10.408 ± 1.000 c | 10.078 ± 0.859 b | 0.508 ± 0.056 a | 48 | |
| 10.016 ± 0.747 b | 1.135 ± 0.307 a | 0.753 ± 0.026 a | 7.929 ± 0.906 b | 8.241 ± 0.610 a | 0.493 ± 0.027 a | 72 | |
| 8.032 ± 0.629 a | 1.291 ± 0.536 a | 0.727 ± 0.033 a | 6.332 ± 1.076 a | 7.489 ± 0.528 a | 0.464 ± 0.030 a | 96 | |
| 0.75 | 10.772 ± 0.743 a | 1.954 ± 0.036 b | 0.836 ± 0.044 b | 8.527 ± 0.816 ab | 17.158 ± 0.648 d | 0.645 ± 0.057 c | 0 |
| 18.841 ± 0.239 c | 1.276 ± 0.496 a | 0.748 ± 0.046 a | 14.100 ± 0.886 c | 14.016 ± 0.955 c | 0.558 ± 0.060 b | 24 | |
| 13.301 ± 0.670 b | 1.034 ± 0.054 a | 0.697 ± 0.016 a | 9.319 ± 0.418 b | 9.110 ± 0.883 b | 0.472 ± 0.025 a | 48 | |
| 11.524 ± 0.749 a | 1.072 ± 0.148 a | 0.725 ± 0.005 a | 8.350 ± 0.483 ab | 8.903 ± 0.686 ab | 0.480 ± 0.017 a | 72 | |
| 10.292 ± 0.879 a | 1.059 ± 0.109 a | 0.703 ± 0.010 a | 7.235 ± 0.693 a | 7.626 ± 0.560 a | 0.457 ± 0.011 a | 96 | |
| 1 | 11.500 ± 0.817 bc | 1.507 ± 0.402 a | 0.779 ± 0.011 b | 8.919 ± 0.683 b | 15.680 ± 1.019 c | 0.608 ± 0.015 c | 0 |
| 21.399 ± 1.423 d | 0.994 ± 0.014 a | 0.710 ± 0.031 a | 15.017 ± 0.769 c | 14.849 ± 0.730 c | 0.525 ± 0.015 b | 24 | |
| 12.383 ± 0.494 c | 1.421 ± 0.392 a | 0.708 ± 0.043 a | 8.960 ± 0.622 b | 12.401 ± 0.986 ab | 0.485 ± 0.026 ab | 48 | |
| 10.247 ± 0.739 ab | 1.268 ± 0.495 a | 0.715 ± 0.025 a | 7.353 ± 0.574 a | 9.333 ± 0.474 ab | 0.478 ± 0.007 a | 72 | |
| 8.857 ± 0.965 a | 1.306 ± 0.541 a | 0.732 ± 0.036 ab | 6.471 ± 0.536 a | 8.589 ± 4.147 a | 0.472 ± 0.038 a | 96 | |
| 4. Region | |||||||
| 0 | 18.106 ± 1.862 c | 1.864 ± 0.104 b | 0.841 ± 0.073 c | 16.188 ± 1.601 d | 28.044 ± 1.231 e | 0.671 ± 0.077 c | 0 |
| 25.006 ± 1.528 d | 0.990 ± 0.002 a | 0.761 ± 0.040 bc | 20.779 ± 1.309 e | 19.088 ± 1.306 d | 0.574 ± 0.051 b | 24 | |
| 15.446 ± 0.630 b | 1.212 ± 0.417 a | 0.723 ± 0.021 ab | 11.567 ± 1.119 c | 13.332 ± 1.241 c | 0.507 ± 0.040 ab | 48 | |
| 13.986 ± 0.592 b | 0.999 ± 0.018 a | 0.678 ± 0.007 ab | 9.450 ± 0.444 b | 9.384 ± 0.516 b | 0.440 ± 0.004 a | 72 | |
| 10.977 ± 0.797 a | 1.033 ± 0.092 a | 0.647 ± 0.071 a | 7.091 ± 0.778 a | 7.324 ± 0.978 a | 0.412 ± 0.056 a | 96 | |
| 0.5 | 18.421 ± 0.939 c | 1.646 ± 0.587 a | 0.805 ± 0.052 c | 14.453 ± 0.699 c | 18.906 ± 1.656 c | 0.629 ± 0.067 b | 0 |
| 28.637 ± 0.892 e | 0.960 ± 0.021 a | 0.628 ± 0.050 a | 18.239 ± 0.729 d | 17.537 ± 0.744 c | 0.443 ± 0.050 a | 24 | |
| 22.168 ± 0.727 d | 0.949 ± 0.034 a | 0.665 ± 0.050 ab | 14.647 ± 0.590 c | 13.896 ± 0.372 b | 0.464 ± 0.055 a | 48 | |
| 12.111 ± 0.728 b | 1.167 ± 0.386 a | 0.720 ± 0.032 b | 8.353 ± 0.642 a | 8.651 ± 0.823 a | 0.462 ± 0.034 a | 72 | |
| 8.115 ± 0.674 a | 1.164 ± 0.365 a | 0.702 ± 0.019 ab | 5.706 ± 0.447 a | 7.776 ± 0.821 a | 0.435 ± 0.024 a | 96 | |
| 0.75 | 11.854 ± 0.805 b | 1.555 ± 0.490 b | 0.814 ± 0.040 c | 9.471 ± 0.745 b | 14.630 ± 1.171 c | 0.622 ± 0.052 c | 0 |
| 28.785 ± 1.917 d | 1.118 ± 0.260 ab | 0.717 ± 0.045 b | 20.101 ± 1.537 d | 22.866 ± 0.751 d | 0.530 ± 0.056 b | 24 | |
| 17.217 ± 0.761 c | 0.976 ± 0.014 a | 0.649 ± 0.029 a | 11.210 ± 0.349 c | 10.895 ± 0.315 b | 0.430 ± 0.010 a | 48 | |
| 10.442 ± 0.921 ab | 0.988 ± 0.006 a | 0.726 ± 0.020 b | 7.578 ± 0.725 a | 7.483 ± 0.689 a | 0.466 ± 0.011 a | 72 | |
| 10.175 ± 0.431 a | 0.963 ± 0.047 a | 0.690 ± 0.006 ab | 6.503 ± 0.939 a | 6.243 ± 0.279 a | 0.436 ± 0.009 a | 96 | |
| 1 | 16.019 ± 1.588 c | 1.589 ± 0.246 a | 0.761 ± 0.008 b | 12.209 ± 1.276 d | 19.210 ± 1.387 d | 0.594 ± 0.019 b | 0 |
| 25.632 ± 1.048 d | 0.973 ± 0.008 a | 0.663 ± 0.034 a | 16.984 ± 0.404 e | 16.524 ± 0.270 c | 0.478 ± 0.037 a | 24 | |
| 17.149 ± 0.815 c | 1.109 ± 0.211 a | 0.652 ± 0.050 a | 10.740 ± 0.239 c | 11.498 ± 0.930 b | 0.440 ± 0.039 a | 48 | |
| 11.499 ± 0.951 b | 1.245 ± 0.549 a | 0.676 ± 0.017 a | 7.772 ± 0.539 b | 10.452 ± 0.913 b | 0.445 ± 0.017 a | 72 | |
| 8.486 ± 0.667 a | 1.322 ± 0.559 a | 0.736 ± 0.031 b | 6.024 ± 0.748 a | 5.916 ± 0.604 a | 0.460 ± 0.024 a | 96 | |
| 5. Region | |||||||
| 0 | 26.786 ± 1.071 c | 1.510 ± 0.494 a | 0.817 ± 0.081 c | 21.046 ± 0.752 d | 30.795 ± 1.742 d | 0.668 ± 0.087 c | 0 |
| 19.460 ± 0.853 b | 1.553 ± 0.503 a | 0.775 ± 0.076 bc | 15.117 ± 0.848 c | 26.950 ± 0.730 c | 0.593 ± 0.079 bc | 24 | |
| 20.890 ± 1.124 b | 0.978 ± 0.049 a | 0.690 ± 0.041 abc | 14.381 ± 0.241 c | 14.053 ± 0.523 b | 0.487 ± 0.053 ab | 48 | |
| 18.922 ± 1.480 b | 1.170 ± 0.349 a | 0.660 ± 0.036 ab | 12.185 ± 0.812 b | 12.764 ± 1.002 b | 0.431 ± 0.029 a | 72 | |
| 11.916 ± 0.683 a | 1.255 ± 0.452 a | 0.627 ± 0.098 a | 7.092 ± 1.004 a | 8.925 ± 0.893 a | 0.398 ± 0.084 a | 96 | |
| 0.5 | 17.025 ± 1.995 c | 1.726 ± 0.276 b | 0.830 ± 0.050 b | 13.628 ± 1.487 c | 20.440 ± 0.885 d | 0.662 ± 0.067 b | 0 |
| 28.628 ± 0.983 e | 0.950 ± 0.069 a | 0.655 ± 0.055 a | 16.832 ± 0.910 d | 14.474 ± 0.832 c | 0.455 ± 0.046 a | 24 | |
| 26.192 ± 0.614 d | 0.920 ± 0.061 a | 0.650 ± 0.063 a | 16.580 ± 0.974 d | 15.226 ± 0.868 c | 0.447 ± 0.069 a | 48 | |
| 13.813 ± 0.991 b | 1.056 ± 0.158 a | 0.705 ± 0.021 a | 9.331 ± 0.905 b | 9.758 ± 0.519 b | 0.441 ± 0.030 a | 72 | |
| 8.022 ± 0.969 a | 1.009 ± 0.027 a | 0.705 ± 0.006 a | 6.380 ± 0.719 a | 5.670 ± 0.519 a | 0.430 ± 0.014 a | 96 | |
| 0.75 | 17.347 ± 1.272 b | 1.554 ± 0.166 b | 0.787 ± 0.014 b | 13.734 ± 1.033 b | 21.254 ± 1.486 c | 0.603 ± 0.038 b | 0 |
| 22.898 ± 2.470 c | 0.918 ± 0.026 a | 0.678 ± 0.087 a | 14.423 ± 1.353 b | 13.049 ± 1.202 b | 0.465 ± 0.059 a | 24 | |
| 20.825 ± 2.106 c | 0.943 ± 0.067 a | 0.630 ± 0.011 a | 13.010 ± 1.277 b | 12.112 ± 1.776 b | 0.419 ± 0.009 a | 48 | |
| 11.815 ± 0.897 a | 0.986 ± 0.022 a | 0.705 ± 0.011 a | 8.277 ± 0.587 a | 8.101 ± 0.527 a | 0.454 ± 0.013 a | 72 | |
| 8.975 ± 0.501 a | 0.993 ± 0.002 a | 0.692 ± 0.005 a | 6.614 ± 1.079 a | 6.564 ± 1.055 a | 0.427 ± 0.007 a | 96 | |
| 1 | 21.452 ± 2.089 c | 1.105 ± 0.209 a | 0.740 ± 0.011 b | 14.103 ± 1.284 cd | 16.903 ± 0.598 e | 0.560 ± 0.041 c | 0 |
| 29.314 ± 0.857 d | 0.975 ± 0.022 a | 0.685 ± 0.026 ab | 15.242 ± 0.569 d | 14.884 ± 0.584 d | 0.498 ± 0.020 bc | 24 | |
| 20.562 ± 1.834 c | 0.973 ± 0.043 a | 0.630 ± 0.056 a | 12.712 ± 0.448 c | 11.860 ± 1.126 c | 0.425 ± 0.045 a | 48 | |
| 13.187 ± 0.684 b | 1.145 ± 0.408 a | 0.670 ± 0.022 a | 9.189 ± 1.015 b | 9.105 ± 0.853 b | 0.431 ± 0.041 ab | 72 | |
| 8.237 ± 0.875 a | 1.318 ± 0.557 a | 0.692 ± 0.033 ab | 6.038 ± 1.038 a | 6.842 ± 1.321 a | 0.436 ± 0.032 ab | 96 | |
| 6. Region | |||||||
| 0 | 31.552 ± 1.256 d | 1.394 ± 0.297 b | 0.811 ± 0.086 c | 24.102 ± 0.712 d | 28.529 ± 2.722 d | 0.667 ± 0.093 c | 0 |
| 32.398 ± 1.995 d | 1.008 ± 0.033 a | 0.756 ± 0.049 bc | 24.853 ± 1.309 d | 25.290 ± 1.504 c | 0.580 ± 0.066 bc | 24 | |
| 20.890 ± 1.124 c | 0.962 ± 0.055 a | 0.675 ± 0.029 ab | 17.652 ± 0.595 c | 17.236 ± 0.472 b | 0.488 ± 0.045 ab | 48 | |
| 18.922 ± 1.480 b | 0.941 ± 0.076 a | 0.609 ± 0.053 a | 13.836 ± 0.951 b | 11.727 ± 0.588 a | 0.398 ± 0.047 a | 72 | |
| 11.916 ± 0.683 a | 1.062 ± 0.134 a | 0.600 ± 0.100 a | 8.991 ± 0.628 a | 9.823 ± 1.269 a | 0.384 ± 0.096 a | 96 | |
| 0.5 | 17.897 ± 0.906 b | 1.667 ± 0.498 b | 0.811 ± 0.019 b | 14.350 ± 0.708 c | 23.073 ± 1.188 e | 0.645 ± 0.044 b | 0 |
| 25.016 ± 1.900 c | 1.130 ± 0.313 a | 0.699 ± 0.024 a | 17.245 ± 1.247 d | 16.932 ± 0.764 d | 0.495 ± 0.000 a | 24 | |
| 23.552 ± 1.623 c | 0.931 ± 0.117 a | 0.663 ± 0.117 a | 16.640 ± 0.477 d | 14.300 ± 0.823 c | 0.455 ± 0.099 a | 48 | |
| 16.266 ± 0.854 b | 0.979 ± 0.040 a | 0.705 ± 0.027 a | 11.321 ± 0.770 b | 11.230 ± 0.573 b | 0.445 ± 0.007 a | 72 | |
| 12.122 ± 0.686 a | 1.073 ± 0.166 a | 0.661 ± 0.024 a | 7.893 ± 0.449 a | 8.079 ± 0.631 a | 0.412 ± 0.018 a | 96 | |
| 0.75 | 26.629 ± 0.915 b | 1.497 ± 0.334 b | 0.788 ± 0.015 b | 20.892 ± 0.813 c | 29.100 ± 2.340 d | 0.597 ± 0.027 c | 0 |
| 30.731 ± 1.063 c | 0.959 ± 0.156 a | 0.704 ± 0.124 ab | 20.789 ± 1.662 c | 18.530 ± 1.741 c | 0.479 ± 0.068 b | 24 | |
| 25.643 ± 1.058 b | 0.883 ± 0.065 a | 0.609 ± 0.027 a | 15.897 ± 0.973 b | 13.230 ± 0.528 b | 0.406 ± 0.029 a | 48 | |
| 15.285 ± 0.790 a | 0.984 ± 0.008 a | 0.696 ± 0.010 ab | 10.583 ± 0.607 a | 10.390 ± 0.643 a | 0.458 ± 0.011 ab | 72 | |
| 15.525 ± 0.819 a | 0.982 ± 0.009 a | 0.670 ± 0.005 a | 10.462 ± 0.411 a | 10.277 ± 0.397 a | 0.431 ± 0.004 ab | 96 | |
| 1 | 23.786 ± 0.856 c | 0.997 ± 0.018 a | 0.726 ± 0.023 a | 17.384 ± 0.653 d | 17.217 ± 0.614 b | 0.533 ± 0.034 b | 0 |
| 18.275 ± 1.658 b | 1.223 ± 0.399 a | 0.723 ± 0.062 a | 13.058 ± 0.993 c | 15.409 ± 0.723 b | 0.528 ± 0.049 b | 24 | |
| 35.652 ± 0.964 d | 0.940 ± 0.045 a | 0.625 ± 0.040 a | 16.437 ± 0.720 d | 15.623 ± 1.174 b | 0.422 ± 0.042 a | 48 | |
| 17.824 ± 1.044 b | 0.905 ± 0.088 a | 0.484 ± 0.329 a | 9.280 ± 1.121 b | 8.699 ± 1.190 a | 0.432 ± 0.031 a | 72 | |
| 10.597 ± 0.630 a | 1.311 ± 0.575 a | 0.698 ± 0.019 a | 7.456 ± 0.412 a | 8.191 ± 1.041 a | 0.433 ± 0.043 a | 96 | |
| 7. Region | |||||||
| 0 | 41.474 ± 1.885 d | 1.557 ± 0.519 b | 0.724 ± 0.073 b | 31.715 ± 0.599 d | 45.698 ± 2.644 d | 0.589 ± 0.080 c | 0 |
| 41.990 ± 3.933 d | 0.965 ± 0.037 a | 0.684 ± 0.036 ab | 28.051 ± 2.441 c | 27.600 ± 2.630 c | 0.522 ± 0.032 bc | 24 | |
| 35.763 ± 1.846 c | 0.947 ± 0.056 a | 0.661 ± 0.047 ab | 22.919 ± 0.779 b | 21.171 ± 1.179 b | 0.487 ± 0.063 abc | 48 | |
| 23.420 ± 1.446 b | 0.945 ± 0.068 a | 0.627 ± 0.032 ab | 14.305 ± 0.900 a | 13.772 ± 0.615 a | 0.411 ± 0.026 ab | 72 | |
| 17.802 ± 0.868 a | 1.113 ± 0.309 ab | 0.597 ± 0.101 a | 11.961 ± 0.825 a | 12.681 ± 1.393 a | 0.382 ± 0.093 a | 96 | |
| 0.5 | 16.158 ± 0.748 b | 1.650 ± 0.526 b | 0.795 ± 0.045 b | 6.322 ± 1.057 a | 11.617 ± 0.961 b | 0.642 ± 0.036 b | 0 |
| 34.062 ± 2.017 d | 0.931 ± 0.089 a | 0.630 ± 0.085 a | 24.375 ± 1.626 e | 23.777 ± 2.063 d | 0.456 ± 0.072 a | 24 | |
| 33.502 ± 3.215 d | 0.906 ± 0.076 a | 0.634 ± 0.089 a | 22.159 ± 0.822 d | 22.224 ± 1.542 d | 0.435 ± 0.090 a | 48 | |
| 25.024 ± 1.045 c | 0.927 ± 0.078 a | 0.643 ± 0.022 ab | 15.803 ± 0.562 c | 14.733 ± 0.649 c | 0.419 ± 0.010 a | 72 | |
| 8.097 ± 1.426 a | 0.964 ± 0.050 a | 0.618 ± 0.049 a | 9.387 ± 0.765 b | 9.047 ± 0.798 a | 0.389 ± 0.034 a | 96 | |
| 0.75 | 34.207 ± 2.849 d | 1.204 ± 0.344 a | 0.769 ± 0.012 a | 26.122 ± 2.020 d | 31.324 ± 2.750 c | 0.615 ± 0.015 b | 0 |
| 29.716 ± 2.062 c | 1.023 ± 0.409 a | 0.649 ± 0.138 a | 17.183 ± 0.720 c | 16.424 ± 0.925 b | 0.452 ± 0.120 a | 24 | |
| 28.535 ± 0.524 c | 0.850 ± 0.052 a | 0.551 ± 0.078 a | 17.324 ± 1.109 c | 14.457 ± 0.893 b | 0.360 ± 0.057 a | 48 | |
| 21.287 ± 1.878 b | 0.963 ± 0.013 a | 0.658 ± 0.023 a | 14.642 ± 0.541 b | 14.032 ± 0.536 b | 0.443 ± 0.022 a | 72 | |
| 16.691 ± 0.679 a | 0.966 ± 0.010 a | 0.661 ± 0.025 a | 9.456 ± 1.062 a | 9.162 ± 0.983 a | 0.419 ± 0.022 a | 96 | |
| 1 | 26.858 ± 3.199 c | 1.036 ± 0.039 a | 0.717 ± 0.042 b | 18.582 ± 1.965 c | 19.402 ± 2.250 d | 0.520 ± 0.032 a | 0 |
| 42.222 ± 1.697 d | 0.962 ± 0.028 a | 0.669 ± 0.030 b | 15.836 ± 0.529 b | 15.410 ± 0.660 c | 0.454 ± 0.062 ab | 24 | |
| 22.724 ± 1.385 b | 0.873 ± 0.051 a | 0.560 ± 0.026 a | 19.872 ± 0.981 c | 17.409 ± 0.870 cd | 0.380 ± 0.023 a | 48 | |
| 25.411 ± 0.982 bc | 0.874 ± 0.096 a | 0.643 ± 0.076 ab | 16.116 ± 0.588 b | 12.875 ± 0.759 b | 0.442 ± 0.075 ab | 72 | |
| 11.522 ± 1.169 a | 1.303 ± 0.587 a | 0.685 ± 0.073 b | 7.197 ± 0.905 a | 9.555 ± 0.552 a | 0.435 ± 0.069 ab | 96 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korkmaz, K. The Effect of Sodium Bicarbonate Injection on the Physico-Chemical Quality of Post-Harvest Trout. Foods 2023, 12, 2437. https://doi.org/10.3390/foods12132437
Korkmaz K. The Effect of Sodium Bicarbonate Injection on the Physico-Chemical Quality of Post-Harvest Trout. Foods. 2023; 12(13):2437. https://doi.org/10.3390/foods12132437
Chicago/Turabian StyleKorkmaz, Koray. 2023. "The Effect of Sodium Bicarbonate Injection on the Physico-Chemical Quality of Post-Harvest Trout" Foods 12, no. 13: 2437. https://doi.org/10.3390/foods12132437





