Chemical Fingerprinting, Aorta Endothelium Relaxation Effect, and Enzymatic Inhibition of Canelo (Drimys winteri J. R. Forst. & G. Forst, (D.C) A. Gray, Family Winteraceae) Fruits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Determination of Proximal Composition and Mineral Content
2.4. Ultrahigh Liquid Chromatography Orbitrap MS Analysis (UHPLC OT-MS)
2.5. Antioxidant Activity and Flavonoid and Phenolics Contents
2.6. Determination of Inhibitory Enzymatic Activity
2.7. Docking Simulations
2.8. Isolation of Rat Aorta and Vascular Reactivity Assays
2.9. Statistical Analysis
3. Results and Discussion
3.1. Proximal Composition and Minerals of D. winteri Fruits
3.2. HPLC-PDA-MS Identification of the Ethanolic Extract from Canelo Fruits
3.2.1. Phenolic Acids
3.2.2. C-glycosyl Flavonoids
3.2.3. O-glycosyl Flavonoids and Aglycones
3.2.4. Isoflavones
3.2.5. Sesquiterpenes
3.2.6. Fatty Acids
3.2.7. Other Compounds
3.3. Total Phenolic and Flavonoid Contents and Antioxidant Activity
3.4. Enzymatic Inhibitory Activity
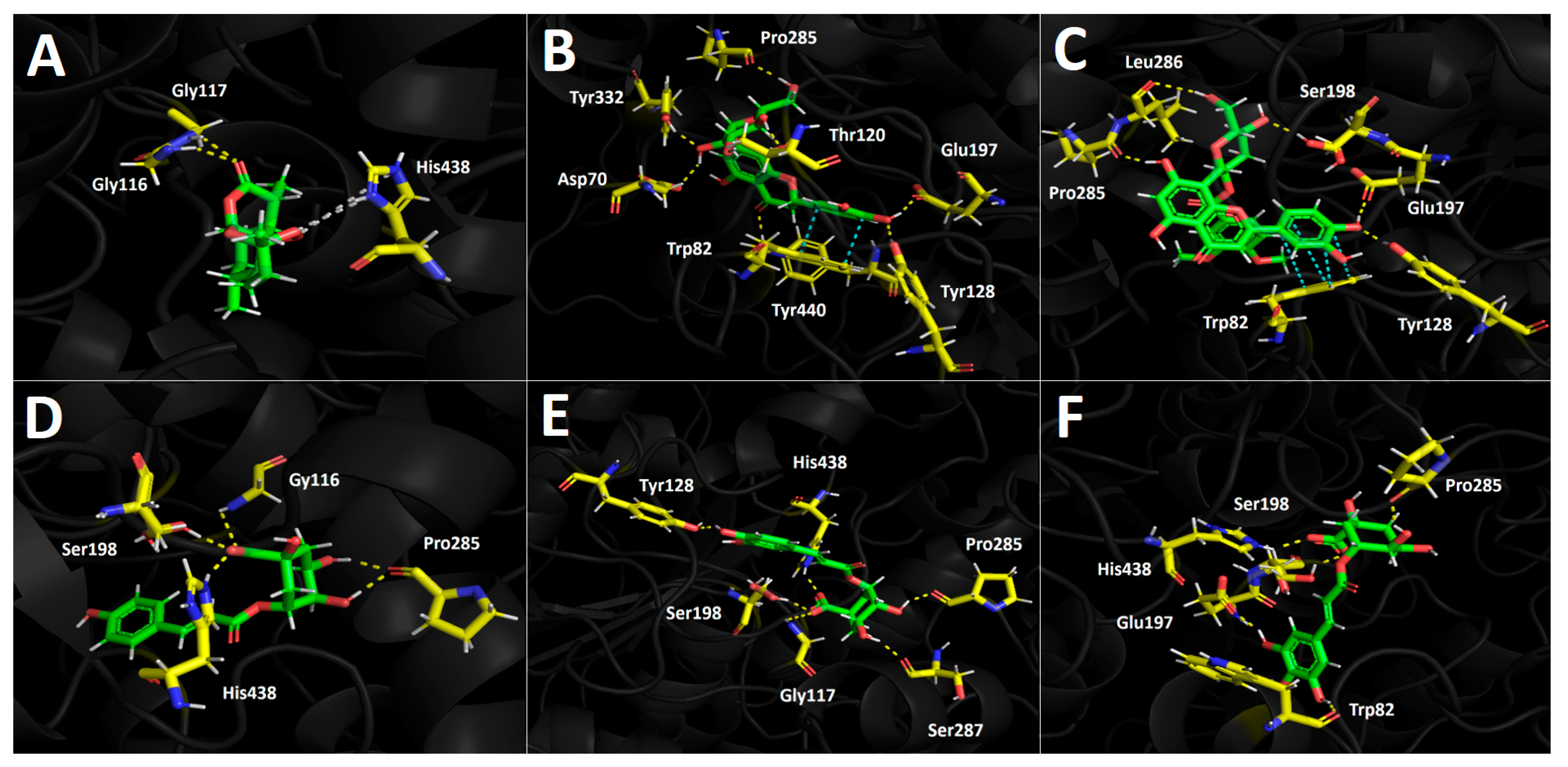
3.5. Docking Simulations
3.5.1. Acetylcholinesterase (TcAChE) Docking Results
3.5.2. Butyrylcholinesterase (hBChE) Docking Results
3.5.3. Tyrosinase Docking Results
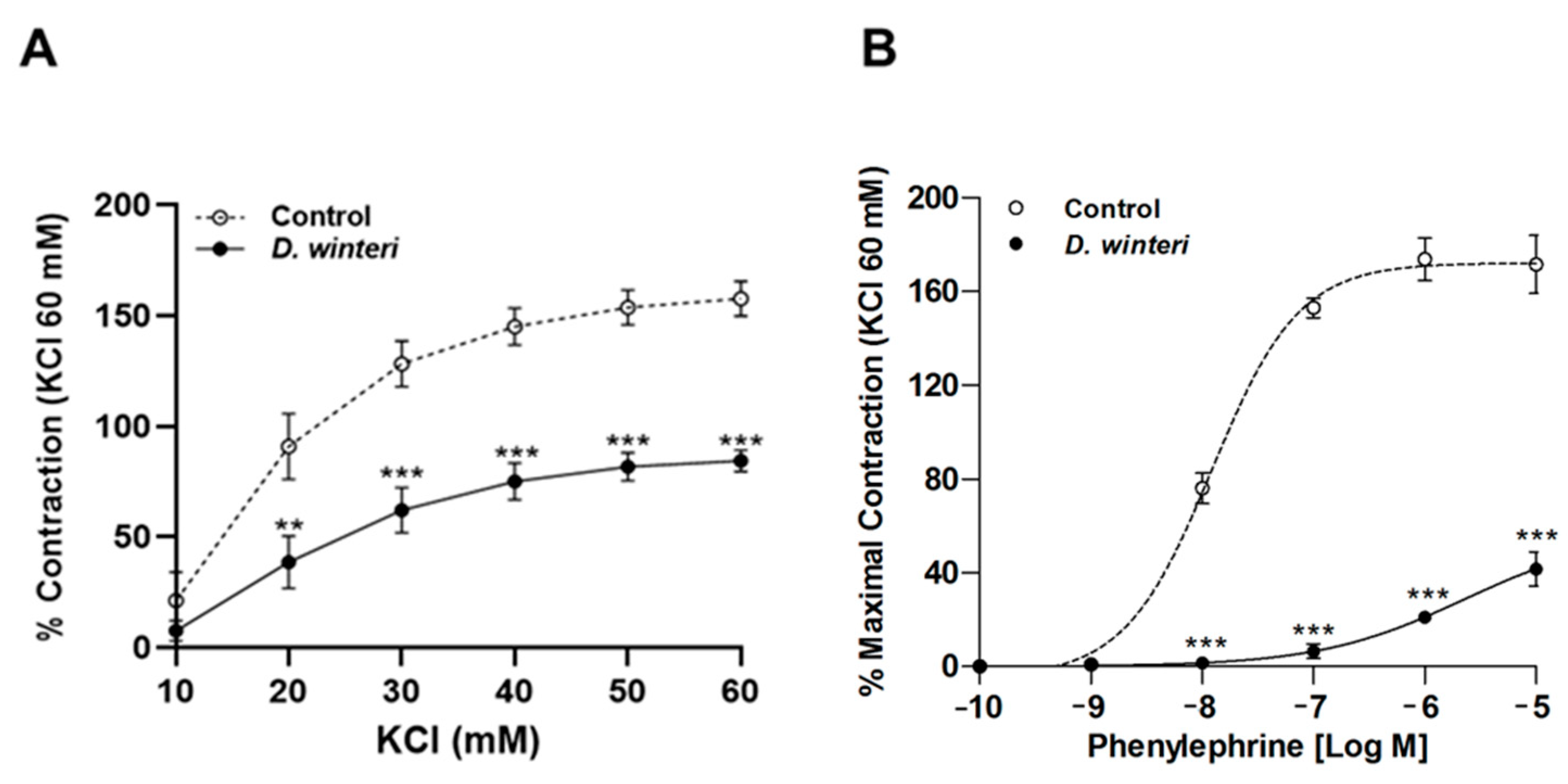
3.6. Effect of D. winteri on Vascular Relaxation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodríguez, R.; Marticorena, C.; Alarcón, D.; Baeza, C.; Cavieres, L.A.; Finot, V.; Fuentes, N.; Kiessling, A.; Mihoc, M.; Pauchard, A.; et al. Catálogo de Las Plantas Vasculares de Chile. Gayana Bot. 2018, 75, 1–430. [Google Scholar] [CrossRef] [Green Version]
- Novoa, P.; An-Der Fuhren, F.; Gonzales, C. Drimys winteri J.R. Forst. & G. Forst. var. chilensis (DC.) A. Gray; 2015. Available online: https://clasificacionespecies.mma.gob.cl/wp-content/uploads/2019/10/Drimys_winteri_FIN_13RCE.pdf (accessed on 27 June 2023).
- Houghton, P.; Manby, J. Medicinal Plants of the Mapuche. J. Ethnopharmacol. 1985, 13, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Concha, D.; Vogel, H.; Razmilic, I. Variation of Chemical Compounds in Leaves of Drimys spp. (Magnoliophyta: Winteraceae) Populations in Chile. Rev. Chil. Hist. Nat. 2004, 77, 43–50. [Google Scholar] [CrossRef]
- Muñoz, O.; Tapia-Merino, J.; Nevermann, W.; San-Martín, A. Phytochemistry and Biological Properties of Drimys winteri JR et G. Forster Var Chilensis (DC) A. Bol. Latinoam. Caribe Plantas Med. Aromat. 2021, 20, 443–462. [Google Scholar] [CrossRef]
- Barrientos, R.E.; Ahmed, S.; Cortés, C.; Fernández-Galleguillos, C.; Romero-Parra, J.; Simirgiotis, M.J.; Echeverría, J. Chemical Fingerprinting and Biological Evaluation of the Endemic Chilean Fruit Greigia sphacelata (Ruiz and Pav.) Regel (Bromeliaceae) by UHPLC-PDA-Orbitrap-Mass Spectrometry. Molecules 2020, 25, 3750. [Google Scholar] [CrossRef]
- Cuesta, L.; Palacios, J.; Barrientos, R.E.; Gómez, J.; Castagnini, J.M.; Barba, F.J.; Tapia, A.; Paredes, A.; Cifuentes, F.; Simirgiotis, M.J. UHPLC-MS Phenolic Fingerprinting, Aorta Endothelium Relaxation Effect, Antioxidant, and Enzyme Inhibition Activities of Azara Dentata Ruiz & Pav Berries. Foods 2023, 12, 643. [Google Scholar]
- Official Methods of Analysis of AOAC International, 18th ed.; Revision 3; AOAC International: Rockville, MD, USA, 2006.
- Vargas-Arana, G.; Merino-Zegarra, C.; del-Castillo, Á.M.R.; Quispe, C.; Viveros-Valdez, E.; Simirgiotis, M.J. Antioxidant, Antiproliferative and Anti-Enzymatic Capacities, Nutritional Analysis and UHPLC-PDA-MS Characterization of Ungurahui Palm Fruits (Oenocarpus bataua Mart) from the Peruvian Amazon. Antioxidants 2022, 11, 1598. [Google Scholar] [CrossRef]
- Petersson, G.A.; Bennett, A.; Tensfeldt, T.G.; Al-Laham, M.A.; Shirley, W.A.; Mantzaris, J. A Complete Basis Set Model Chemistry. I. The Total Energies of Closed-Shell Atoms and Hydrides of the First-Row Elements. J. Chem. Phys. 1988, 89, 2193–2218. [Google Scholar] [CrossRef]
- Tapia, A.; Rodriguez, J.; Theoduloz, C.; Lopez, S.; Egly, G.; Schmeda-Hirschmann, G.; Feresin, G.E.; Schmeda-Hirschmann, G. Free Radical Scavengers and Antioxidants from Baccharis grisebachii. J. Ethnopharmacol. 2004, 95, 155–161. [Google Scholar] [CrossRef]
- Re, R.; Pellegrinia, N.; Proteggente, A.; Pannalaa, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-Throughput Assay of Oxygen Radical Absorbance Capacity (ORAC) Using a Multichannel Liquid Handling System Coupled with a Microplate Fluorescence Reader in 96-Well Format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef] [PubMed]
- Adamo, C.; Barone, V. Toward Reliable Density Functional Methods without Adjustable Parameters: The PBE0 Model Seeking for Parameter-Free Double-Hybrid Functionals: The PBE0-DH Model Accurate Excitation Energies from Time-Dependent Density Functional Theory: Assessing the PBE0. Cit. J. Chem. Phys. 1999, 110, 2889. [Google Scholar] [CrossRef]
- Frisch, A. Gaussian 09W Reference 2009; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Release, S. Maestro, Version 11.8. Schrodinger, LLC, New York; Scientific Research Publishing: Wuhan, China, 2018. [Google Scholar]
- Greenblatt, H.M.; Kryger, G.; Lewis, T.; Silman, I.; Sussman, J.L. Structure of Acetylcholinesterase Complexed with (-)-Galanthamine at 2.3 Å Resolution. FEBS Lett. 1999, 463, 321–326. [Google Scholar] [CrossRef] [Green Version]
- Nachon, F.; Carletti, E.; Ronco, C.; Trovaslet, M.; Nicolet, Y.; Jean, L.; Renard, P.Y. Crystal Structures of Human Cholinesterases in Complex with Huprine W and Tacrine: Elements of Specificity for Anti-Alzheimer’s Drugs Targeting Acetyl- and Butyryl-Cholinesterase. Biochem. J. 2013, 453, 393–399. [Google Scholar] [CrossRef] [Green Version]
- Ismaya, W.T.; Rozeboom, H.J.; Weijn, A.; Mes, J.J.; Fusetti, F.; Wichers, H.J.; Dijkstra, B.W. Crystal Structure of Agaricus bisporus Mushroom Tyrosinase: Identity of the Tetramer Subunits and Interaction with Ropolone. Biochemistry 2011, 50, 5477–5486. [Google Scholar] [CrossRef] [Green Version]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Sussman, J.L.; Harel, M.; Frolow, F.; Oefner, C.; Goldman, A.; Toker, L.; Silman, I. Atomic Structure of Acetylcholinesterase from Torpedo Californica: A Prototypic Acetylcholine-Binding Protein. Science 1991, 253, 872–879. [Google Scholar] [CrossRef]
- Silman, I.; Harel, M.; Axelsen, P.; Raves, M.; Sussman, J. Three-Dimensional Structures of Acetylcholinesterase and of Its Complexes with Anticholinesterase Agents. In Proceedings of the Structure, Mechanism and Inhibition of Neuroactive Enzymes; Portland Press Limited.: London, UK, 1994; Volume 22, pp. 745–749. [Google Scholar]
- Nicolet, Y.; Lockridge, O.; Masson, P.; Fontecilla-Camps, J.C.; Nachon, F. Crystal Structure of Human Butyrylcholinesterase and of Its Complexes with Substrate and Products. J. Biol. Chem. 2003, 278, 41141–41147. [Google Scholar] [CrossRef] [Green Version]
- Tallini, L.R.; Bastida, J.; Cortes, N.; Osorio, E.H.; Theoduloz, C.; Schmeda-Hirschmann, G. Cholinesterase Inhibition Activity, Alkaloid Profiling and Molecular Docking of Chilean Rhodophiala (Amaryllidaceae). Molecules 2018, 23, 1532. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, A.P.; Silva, N.d.F.; Andrade, E.H.A.; Gratieri, T.; Setzer, W.N.; Maia, J.G.S.; da Silva, J.K.R. Tyrosinase Inhibitory Activity, Molecular Docking Studies and Antioxidant Potential of Chemotypes of Lippia origanoides (Verbenaceae) Essential Oils. PLoS ONE 2017, 12, e0175598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Ye, Y.; Ran, M.; Li, Q.; Ruan, Z.; Jin, N. Inhibition of Tyrosinase by Mercury Chloride: Spectroscopic and Docking Studies. Front. Pharmacol. 2020, 11, 81. [Google Scholar] [CrossRef] [PubMed]
- Sherman, W.; Day, T.; Jacobson, M.P.; Friesner, R.A.; Farid, R. Novel Procedure for Modeling Ligand/Receptor Induced Fit Effects. J. Med. Chem. 2006, 49, 534–553. [Google Scholar] [CrossRef] [PubMed]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra Precision Glide: Docking and Scoring Incorporating a Model of Hydrophobic Enclosure for Protein-Ligand Complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef] [Green Version]
- DeLano, W.L. The PyMOL Molecular Graphics System. Available online: http://www.pymol.org (accessed on 27 June 2023).
- Özcan, M.M.; Akbulut, M. Estimation of Minerals, Nitrate and Nitrite Contents of Medicinal and Aromatic Plants Used as Spices, Condiments and Herbal Tea. Food Chem. 2008, 106, 852–858. [Google Scholar] [CrossRef]
- Lee, J.G.; Chae, Y.; Shin, Y.; Kim, Y.J. Chemical Composition and Antioxidant Capacity of Black Pepper Pericarp. Appl. Biol. Chem. 2020, 63, 35. [Google Scholar] [CrossRef]
- Alcázar Magaña, A.; Kamimura, N.; Soumyanath, A.; Stevens, J.F.; Maier, C.S. Caffeoylquinic Acids: Chemistry, Biosynthesis, Occurrence, Analytical Challenges, and Bioactivity. Plant J. 2021, 107, 1299–1319. [Google Scholar] [CrossRef]
- Courts, F.L.; Williamson, G. The Occurrence, Fate and Biological Activities of c-Glycosyl Flavonoids in the Human Diet. Crit. Rev. Food Sci. Nutr. 2015, 55, 1352–1367. [Google Scholar] [CrossRef] [Green Version]
- Roy, A.; Khan, A.; Ahmad, I.; Alghamdi, S.; Rajab, B.S.; Babalghith, A.O.; Alshahrani, M.Y.; Islam, S.; Islam, M.R. Flavonoids a Bioactive Compound from Medicinal Plants and Its Therapeutic Applications. Biomed Res. Int. 2022, 2022, 5445291. [Google Scholar] [CrossRef]
- Krishna, S.; Bustamante, L.; Haynes, R.K.; Staines, H.M. Artemisinins: Their Growing Importance in Medicine. Trends Pharmacol. Sci. 2008, 29, 520–527. [Google Scholar] [CrossRef] [Green Version]
- Claudino, V.D.; da Silva, K.C.; Filho, V.C.; Yunes, R.A.; Monache, F.D.; Giménez, A.; Salamanca, E.; Gutierrez-Yapu, D.; Malheiros, A. Drimanes from Drimys brasiliensis with Leishmanicidal and Antimalarial Activity. Mem. Inst. Oswaldo Cruz 2013, 108, 140–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, T.M.; Na, M.K.; Thuong, P.T.; Su, N.D.; Sok, D.E.; Song, K.S.; Seong, Y.H.; Bae, K.H. Antioxidant Activity of Caffeoyl Quinic Acid Derivatives from the Roots of Dipsacus asper Wall. J. Ethnopharmacol. 2006, 108, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, R.E.; Ibáñez, E.; Puerta, A.; Padrón, J.M.; Paredes, A.; Cifuentes, F.; Romero-Parra, J.; Palacios, J.; Bórquez, J.; Simirgiotis, M.J. Phenolic Fingerprinting and Bioactivity Profiling of Extracts and Isolated Compounds from Gypothamnium pinifolium Phil. Antioxidants 2022, 11, 2313. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, R.; Fernández-Galleguillos, C.; Pastene, E.; Simirgiotis, M.; Romero-Parra, J.; Ahmed, S.; Echeverría, J. Metabolomic Analysis, Fast Isolation of Phenolic Compounds, and Evaluation of Biological Activities of the Bark From Weinmannia trichosperma Cav. (Cunoniaceae). Front. Pharmacol. 2020, 11, 780. [Google Scholar] [CrossRef]
- Trendafilova, A.; Ivanova, V.; Rangelov, M.; Todorova, M.; Ozek, G.; Yur, S.; Ozek, T.; Aneva, I.; Veleva, R.; Moskova-Doumanova, V.; et al. Caffeoylquinic Acids, Cytotoxic, Antioxidant, Acetylcholinesterase and Tyrosinase Enzyme Inhibitory Activities of Six Inula Species from Bulgaria. Chem. Biodivers. 2020, 17, e2000051. [Google Scholar] [CrossRef]





| Composition | D. winteri |
|---|---|
| Moisture | 15.2 ± 0.10 |
| Ash | 6.42 ± 0.26 |
| Fatty matter | 0.42 ± 0.02 |
| Crude protein | 12.74 ± 0.01 |
| Crude fiber | 7.26 ± 0.17 |
| Carbohydrates | 57.96 ± 0.01 |
| Minerals | |
| Ca | 1.45 ± 0.03 |
| Mg | 7.72 ± 0.03 |
| Fe | 4.54 ± 0.21 |
| Zn | 2.99 ± 0.02 |
| Mn | 1.08 ± 0.03 |
| Cu | 0.82 ± 0.02 |
| K | 53.03 ± 0.20 |
| Na | 0.087 ± 0.00 |
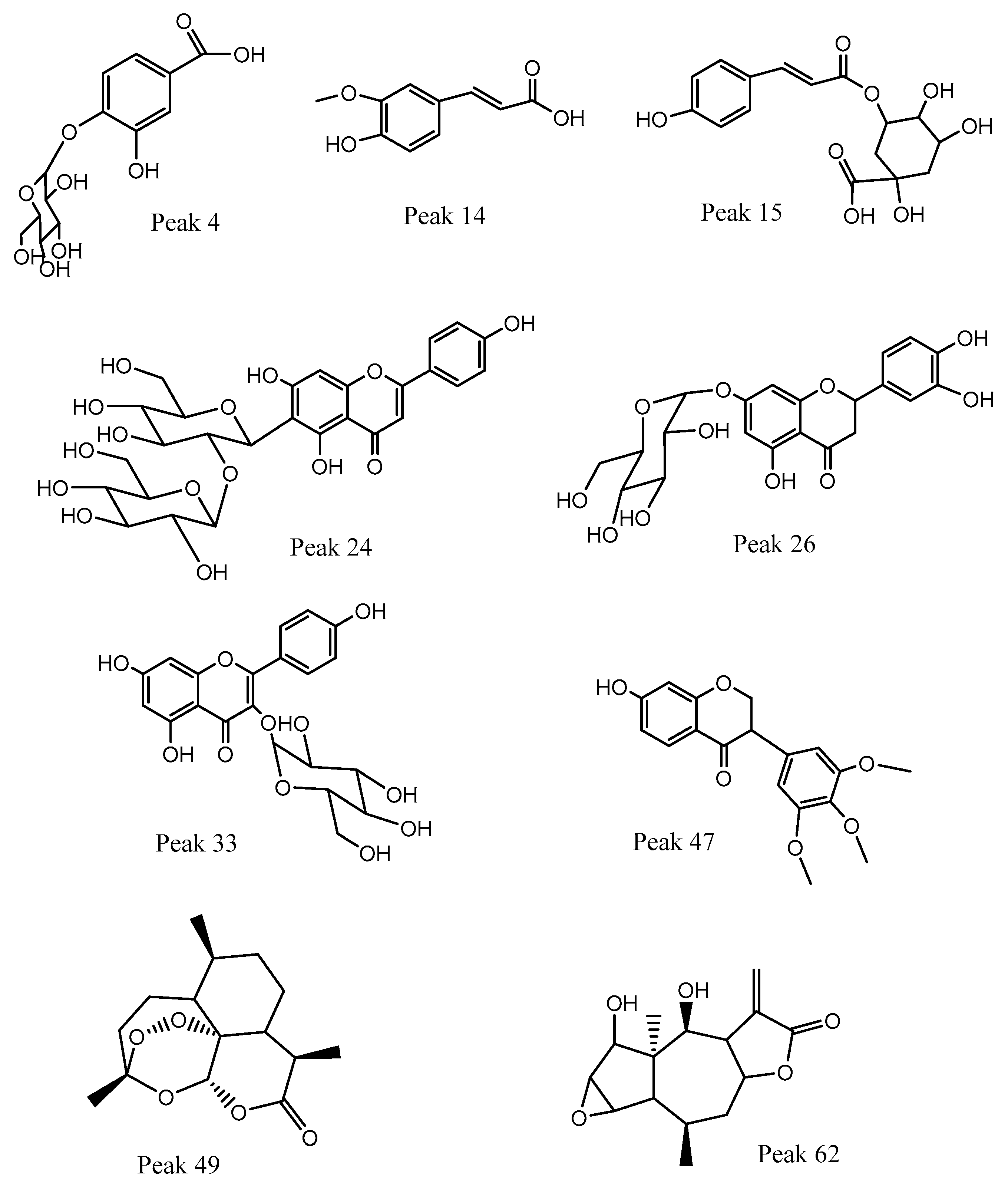
| Peak Number | Retention Time | UV Max | Tentative Identification | Molecular Formula [M-H] | Measured Mass (m/z) | Theoretical Mass (m/z) | Accuracy (ppm) | Ions MSn |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.38 | 227–272 | Gluconic acid | C6H11O7− | 195.05058 | 195.04993 | 3.351 | - |
| 2 | 1.43 | 229–274 | Malic acid | C4H5O5− | 133.01355 | 133.01315 | 3.008 | - |
| 3 | 1.80 | 227 | Citric acid | C6H7O7− | 191.01933 | 191.01863 | 3.684 | - |
| 4 | 2.75 | 273 | Dihydroxybenzoic acid glucoside (Protocatechuic acid 4-O-glucoside) | C13H15O9− | 315.07248 | 315.07106 | 4.509 | 153.05591, 109.2866, 108.60805 |
| 5 | 2.85 | 273 | Hydroxybenzoic acid hexoside (Salicylic acid 4-O-glucoside) | C13H15O8− | 299.07718 | 299.07614 | 3.461 | 239.37044, 179.71397, 137.47914, 136.52159, 119.98631 |
| 6 | 3.21 | 196–227 | Unknown | C18H31O13− | 455.17709 | 455.17592 | 2.584 | 430.82227, 255.95665, 215.00977, 144.00832 |
| 7 | 3.92 | 196–204–269 | Unknown | C15H21O7− | 313.12918 | 313.12818 | 3.198 | - |
| 8 | 5.07 | 227–265 | 3,4-Dihydroxybenzoic acid (protocatechuic acid) | C7H5O4− | 153.01869 | 153.18242 | 2.987 | - |
| 9 | 7.15 | 227–257–312 | 3-O-Caffeoylquinic acid * | C16H17O9− | 353.08774 | 353.08671 | 2.916 | - |
| 10 | 7.72 | 227–257–310 | Hydroxycaffeoyl quinic acid | C16H19O10− | 371.09866 | 371.09727 | 3.746 | 354.11142, 341.99585, 191.15846, 173.71552, 135.04477 |
| 11 | 7.86 | 227–257–312 | Caffeoyl acid hexoside | C15H17O9− | 341.08786 | 341.08671 | 3.377 | 179.78584, 160.84163, 135.04446 |
| 12 | 8.24 | 227–280–309 | 3,4-Dihydroxybenzaldehyde | C7H5O3− | 137.02373 | 137.02332 | 2.969 | |
| 13 | 8.47 | Unknown | C19H25O13− | 461.12979 | 461.12897 | 1.787 | - | |
| 14 | 8.97 | 214–280–311 | Ferulic acid * | C10H9O4− | 193.05020 | 193.04954 | 3.450 | - |
| 15 | 9.06 | 214–280–312 | 3-O-p-Coumaroyl quinic acid | C16H17O8− | 337.09283 | 337.09179 | 3.087 | 191.14720, 172.27388, 162.83879, 119.16192 |
| 16 | 9.29 | - | Quinic acid | C7H11O6− | 191.05563 | 191.05501 | 3.240 | |
| 17 | 9.30 | 227–257–310 | 5-O-Caffeoyl quinic acid | C16H17O9− | 353.08783 | 353.08671 | 3.175 | 191.05560, 135.04462, 133.99362 |
| 18 | 9.30 | 227–257–311 | Caffeoyl quinic acid dimer | C32H35O18− | 707.18042 | 707.18234 | 2.721 | 533.58563, 515.55426, 461.33383, 353.01492, 323.06729, 242.81560, 191.05563 |
| 19 | 9.43 | 214–280–325 | p-Coumaroyl acid hexoside | C15H17O8− | 325.09293 | 325.09179 | 3.482 | 163.03905, 162.83839, 119.04916 |
| 20 | 9.63 | 247–315–337 | 2″-O-(3″′,4″′-Dimethoxybenzoyl) vitexin | C27H31O15− | 595.16620 | 595.16575 | 0.760 | 475.91458, 412.85297, 292.31461 |
| 21 | 9.66 | 265 | Eudesmin | C22H25O6− | 385.16354 | 385.16456 | −2.651 | - |
| 22 | 9.81 | 247–267–324 | Caffeic acid * | C9H7O4− | 179.03442 | 179.03389 | 3.008 | - |
| 23 | 9.85 | 247–267 | Ferulic acid 3-O-glucoside | C16H19O9− | 355.10352 | 355.10236 | 3.258 | 193.81488, 178.68074 |
| 24 | 9.93 | 248–270-332 | Isovitexin 2″-O-beta-D-glucoside | C27H29O15− | 593.15063 | 593.15010 | 0.906 | 413.05600 |
| 25 | 10.15 | 214–280-323 | 5-O-p-Coumaroyl quinic acid | C16H17O8− | 337.09299 | 337.09179 | 3.540 | 173.67874, 163.36201, 119.62347 |
| 26 | 10.26 | 280 | Eriodictyol 7-O-hexoside | C21H21O11− | 449.10904 | 449.10784 | 2.675 | 423.92548, 301.42798, 287.62851, 259.05487, 174.98462, 151.24983 |
| 27 | 10.49 | 250–325 | 3-O-Feruloylquinic acid | C17H19O9− | 367.10355 | 367.10236 | 3.235 | 191.03110, 178.99828 |
| 28 | 10.50 | 250 | Myricetin * | C15H9O8− | 317.03009 | 317.02919 | 2.828 | 315.07285, 288.19870, 178.08804 |
| 29 | 10.70 | 249–283 | 4-Hydroxycinnamic acid | C9H7O3− | 163.03955 | 163.03897 | 3.558 | - |
| 30 | 10.71 | 206–249–300 | 5-O-Feruloylquinic acid | C17H19O9− | 367.10376 | 367.10236 | 3.817 | - |
| 31 | 10.89 | 251–304–329 | Sophoraflavonoloside | C27H29O16− | 609.14545 | 609.14557 | 0.189 | 255.02881, 151.06294 |
| 32 | 10.98 | 249–264–334 | Isovitexin * | C21H19O10− | 431.09727 | 431.09727 | 2.941 | 341.30762, 323.05405, 311.62509, 283.10669, |
| 33 | 11.12 | 255–300–351 | Isoquercitrin (Quercetin 3-O-glucoside) | C21H19O12− | 463.08832 | 463.08710 | 2.888 | 300.02777, 151.00316 |
| 34 | 11.32 | 249–281–322 | Kaempferol-3-O-rutinoside | C27H29O15− | 593.15057 | 593.15010 | 0.805 | 287.44540 |
| 35 | 11.33 | 249–283–324 | Unknown | C19H25O10− | 413.14551 | 413.14422 | 3.109 | - |
| 36 | 11.36 | 248–267–334 | Vitexin * | C21H19O10− | 431.09836 | 431.09727 | 2.517 | 341.05933, 311.35144, 283.06186 |
| 37 | 11.45 | 250–285–304 | Kaempferol 3-O-galactose | C21H19O11− | 447.09341 | 447.09219 | 2.743 | 327.28265, 284.03296, 255.02979, 226.90129 |
| 38 | 11.53 | 255–300–351 | Avicularin | C20H17O11− | 433.07779 | 433.07654 | 2.890 | 385.14127, 300.02771, 301.05417, 302.11899, 271.15466, 255.48294, 227.09221, 151.03943 |
| 39 | 11.55 | 253–288–311 | Taxifolin | C15H11O7− | 303.05078 | 303.04993 | 2.811 | 284.30374, 125.87235 |
| 40 | 11.62 | 254–354 | Isorhamnetin 3-O-glucoside | C22H21O12− | 477.10400 | 477.10275 | 2.623 | 357.95270, 315.36295, 313.99323 |
| 41 | 11.63 | - | Unknown | C17H29O10− | 393.17676 | 393.17552 | 3.139 | |
| 42 | 11.69 | 256–348 | Luteolin 5-O-glucoside | C21H19O11− | 447.09351 | 447.09219 | 2.948 | 447.09274, 301.03470, 300.02780, 271.02429, 151.00296 |
| 43 | 11.87 | 265–365 | Kaempferol 3-O-pentoside | C20H17O10− | 417.08279 | 417.08162 | 2.808 | 284.03210, 255.0317, 227.07626 |
| 44 | 11.91 | 251–288–332 | Isoorientin (Luteolin-6-C-glucoside) * | C21H19O11− | 447.09335 | 447.09219 | 2.607 | 357.40594, 327.08704, 285.13358 |
| 45 | 12.05 | 250–281–311 | Astilbin * | C21H21O11− | 449.10934 | 449.10784 | 3.355 | 284.37854, 151.61490 |
| 46 | 12.08 | 251–281 | Diosmetin 7-O-glucose | C22H21O11− | 461.10898 | 461.10784 | 2.473 | 301.03540, 299.37292 |
| 47 | 12.20 | - | Hexenyl-3-hydroxy-3-methyl-glutaryl hexoside | C18H29O10− | 405.17676 | 405.17552 | 3.0464 | 342.00220, 261.29782, 178.37297, 160.84148, 125.87243, 101.02345 |
| 48 | 12.35 | 250–281-311 | 3′-O-Methylviolanone | C18H17O6− | 329.10651 | 329.10251 | −12.139 | 314.09686, 299.15054, 285.11633, 162.83897, 161.40355 |
| 49 | 13.05 | 251–267–280 | Lonchocarpenin | C27H27O6− | 447.17923 | 447.18021 | −2.203 | 215.00941 |
| 50 | 13.20 | 251–280–285 | Artemisinin * | C15H21O5− | 281.13947 | 281.13835 | 3.966 | - |
| 51 | 13.78 | 252–274–281 | 2-Hydroxyenterodiol | C18H21O5− | 317.14285 | 317.13890 | −12,455 | 298.55157, 287.13138, 267.64462, 258.56961 |
| 52 | 13.83 | 283 | Eriodictyol * | C15H11O6− | 287.05597 | 287.05501 | 3.326 | 151.46794, 134.95647 |
| 53 | 14.01 | 253–274–283 | Fisetin | C15H9O6− | 285.04028 | 285.03936 | 3.223 | - |
| 54 | 14.38 | 251–311 | Quercetin * | C15H9O7− | 301.03549 | 301.03428 | 4.029 | 284.31473, 151.00281 |
| 55 | 14.74 | 252–288–304 | Isorhamnetin * | C16H11O7− | 315.05096 | 315.04993 | 3.286 | 270.46667, 151.20018, 108.47343, 107.73669 |
| 56 | 16.40 | 251–277–283 | 9,12,13-Trihydroxy-10,15-octadecadienoic acid | C18H31O5− | 327.21762 | 327.21660 | 3.134 | 211.13336, 229.13232 |
| 57 | 17.35 | 249–288 | Kaempferol * | C15H9O6− | 285.04028 | 285.03936 | 3.223 | 133.25186, 117.21797 |
| 58 | 18.10 | 245–285–311 | Leptospermone | C15H21O4− | 265.14441 | 265.14344 | 3.672 | 251.12869, 249.92970, 196.82272 |
| 59 | 18.28 | 206–251–286 | Pinellic acid | C18H33O5− | 329.23334 | 329.23225 | 3.301 | 228.57948, 215.00943, 171.10269 |
| 60 | 19.34 | 254–288–324 | Diosmetin | C16H11O6− | 299.05621 | 299.05501 | 4.009 | 284.03271 |
| 61 | 19.40 | 253–279 | Cirsimaritin * | C17H13O6− | 313.07178 | 313.07066 | 3.554 | 297.04083, 296.52487, 283.02441 |
| 62 | 19.93 | 252–286–311 | Autumnolide | C15H19O5− | 279.12387 | 279.12455 | 4.194 | - |
| 63 | 19.96 | 254–290–311 | Unknown | C14H19O3− | 235.13377 | 235.13287 | 3.840 | 191.14365, 163.11220, 144.00845, 119.04957 |
| 64 | 20.15 | 253–292–311 | Zinniol | C15H21O4− | 265.14450 | 265.14344 | 4.017 | 266.14767, 158.84607 |
| 65 | 20.51 | 206–255–271 | Apigenin 7-O-methyl ether | C16H11O5− | 283.06116 | 283.06010 | 3.735 | 268.03784 |
| 66 | 21.39 | 255–292 | Octadecanedioic acid | C18H33O4− | 313.23865 | 313.23734 | 4.187 | 312.97437, 291.35034, 270.84320, 215.00909 |
| 67 | 22.29 | 255–292 | Hydroxyoctadecatrienoic acid | C18H29O3− | 293.21228 | 293.21112 | 3.953 | 274.50204, 221.15472, 183.12144, 171.08131 |
| Assay | TPC a | TFC b | DPPH c | ABTS c | ORAC d | FRAP d |
|---|---|---|---|---|---|---|
| Canelo pepper | 57.33 ± 0.82 | 38.42 ± 1.32 | 6.65 ± 0.5 | 9.5 ± 0.05 | 25.33 ± 1.2 | 45.56 ± 1.32 |
| Gallic acid | - | - | 14.32 ± 0.5 | 1.67 ± 0.25 | - | - |
| Assay | AChE | BChE | Tyrosinase |
|---|---|---|---|
| Canelo | 1.94 ± 0.07 | 2.73 ± 0.05 | 9.92 ± 0.05 |
| Galantamine | 0.26 ± 0.02 | 3.82 ± 0.02 | - |
| Kojic acid | - | - | 3.51 ± 0.02 |
| Compound | Binding Energy (kcal/mol) Acetylcholinesterase | Binding Energy (kcal/mol) Butyrylcholinesterase | Binding Energy (kcal/mol) Tyrosinase |
|---|---|---|---|
| Artemisinin | −8.030 | −6.585 | −4.203 |
| Eriodictyol 7-O-hexoside | −18.206 | −14.486 | −8.423 |
| 2″-O-(3″′,4″′-Dimethoxybenzoyl) vitexin | −16.406 | −14.778 | −9.218 |
| 5-O-p-Coumaroyl quinic acid | −10.369 | −9.509 | −10.074 |
| 3-O-Caffeoylquinic acid | −12.566 | −10.910 | −9.898 |
| Hydroxy caffeoylquinic acid | −12.964 | −11.425 | −10.429 |
| Galantamine | −12.989 | −7.125 | - |
| Kojic acid | - | - | −6.050 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barrientos, R.E.; Romero-Parra, J.; Cifuentes, F.; Palacios, J.; Romero-Jola, N.J.; Paredes, A.; Vargas-Arana, G.; Simirgiotis, M.J. Chemical Fingerprinting, Aorta Endothelium Relaxation Effect, and Enzymatic Inhibition of Canelo (Drimys winteri J. R. Forst. & G. Forst, (D.C) A. Gray, Family Winteraceae) Fruits. Foods 2023, 12, 2580. https://doi.org/10.3390/foods12132580
Barrientos RE, Romero-Parra J, Cifuentes F, Palacios J, Romero-Jola NJ, Paredes A, Vargas-Arana G, Simirgiotis MJ. Chemical Fingerprinting, Aorta Endothelium Relaxation Effect, and Enzymatic Inhibition of Canelo (Drimys winteri J. R. Forst. & G. Forst, (D.C) A. Gray, Family Winteraceae) Fruits. Foods. 2023; 12(13):2580. https://doi.org/10.3390/foods12132580
Chicago/Turabian StyleBarrientos, Ruth E., Javier Romero-Parra, Fredi Cifuentes, Javier Palacios, Néstor Jaime Romero-Jola, Adrián Paredes, Gabriel Vargas-Arana, and Mario J. Simirgiotis. 2023. "Chemical Fingerprinting, Aorta Endothelium Relaxation Effect, and Enzymatic Inhibition of Canelo (Drimys winteri J. R. Forst. & G. Forst, (D.C) A. Gray, Family Winteraceae) Fruits" Foods 12, no. 13: 2580. https://doi.org/10.3390/foods12132580









