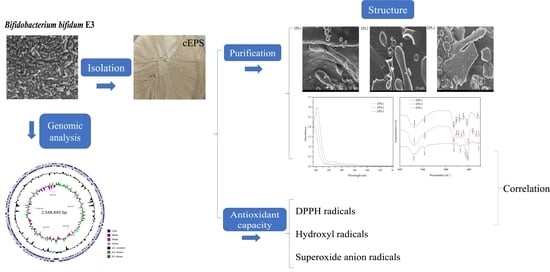
Genome Analysis of Bifidobacterium Bifidum E3, Structural Characteristics, and Antioxidant Properties of Exopolysaccharides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culture of Strain
2.2. Whole Genome Sequencing
2.3. Isolation and Purification of cEPS
2.4. Chemical Composition, Monosaccharide Composition, and Molecular Weight
2.5. Ultraviolet-Visible (UV-Vis) Spectrophotometry and Fourier-Transform Infrared (FT-IR) Spectroscopy
2.6. Congo Red Test
2.7. Field Emission Scanning Electron Microscopes (FE-SEM)
2.8. DPPH Radical Scavenging Assay
2.9. Hydroxyl Radical Scavenging Assay
2.10. Superoxide Anion Radical Scavenging Assay
2.11. Statistical Analysis
3. Results and Discussion
3.1. Antioxidant Capacity of IC, CFE, and CFS
3.2. Analysis of the Genome
3.2.1. Genome Composition
3.2.2. Analysis of COG and KEGG
3.3. Sugar Transport System
3.4. Synthesis of Sugar Nucleotides and Gene Cluster
3.5. Antioxidant Capacity of cEPS
3.6. Yield, Chemical Compositions, Monosaccharide Compositions, and Molecular Weight
3.7. UV-Vis Spectrophotometry and FT-IR Spectroscopy
3.8. Field Emission Scanning Electron Microscope
3.9. Colorimetric Determination of Triple Helix Structures
3.10. Antioxidant Capacity of EPS-1, EPS-2, and EPS-3
3.11. Correlation Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abdelhamid, A.G.; El-Dougdoug, N.K. Comparative genomics of the gut commensal Bifidobacterium bifidum reveals adaptation to carbohydrate utilization. Biochem. Biophys. Res. Commun. 2021, 547, 155–161. [Google Scholar] [CrossRef]
- Sharma, M.; Wasan, A.; Sharma, R.K. Recent developments in probiotics: An emphasis on Bifidobacterium. Food Biosci. 2021, 41, 100993. [Google Scholar] [CrossRef]
- Ventura, M.; O’Flaherty, S.; Claesson, M.J.; Turroni, F.; Klaenhammer, T.R.; Sinderen, D.V.; O’Toole, P.W. Genome-scale analyses of health-promoting bacteria: Probiogenomics. Nat. Rev. Microbiol. 2009, 7, 61–71. [Google Scholar] [CrossRef] [Green Version]
- Milani, C.; Turroni, F.; Duranti, S.; Lugli, G.A.; Mancabelli, L.; Ferrario, C.; van Sinderen, D.; Ventura, M. Genomics of the Genus Bifidobacterium Reveals Species-Specific Adaptation to the Glycan-Rich Gut Environment. Appl. Environ. Microbiol. 2015, 82, 980–991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrario, C.; Milani, C.; Mancabelli, L.; Lugli, G.A.; Duranti, S.; Mangifesta, M.; Viappiani, A.; Turroni, F.; Margolles, A.; Ruas-Madiedo, P.; et al. Modulation of the eps-ome transcription of bifidobacteria through simulation of human intestinal environment. FEMS Microbiol. Ecol. 2016, 92, fiw056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vurmaz, M.; Sahin, E.; Dertli, E. Potential Health Promoting Functions of Exopolysaccharides (EPS) from Lactic Acid Bacteria (LAB). In Proceedings of the 3rd International Conference on Advanced Engineering Technologies, Bayburt, Turkey, 19–21 September 2019; pp. 1379–1382. Available online: https://www.researchgate.net/publication/337027171 (accessed on 26 June 2023).
- Nguyen, P.T.; Nguyen, T.T.; Bui, D.C.; Hong, P.T.; Hoang, Q.-K.; Nguyen, H.-T. Exopolysaccharide production by lactic acid bacteria: The manipulation of environmental stresses for industrial applications. Aims Microbiol. 2020, 6, 451–469. [Google Scholar] [CrossRef]
- Zhou, Y.; Cui, Y.; Qu, X. Exopolysaccharides of lactic acid bacteria: Structure, bioactivity and associations: A review. Carbohydr. Polym. 2019, 207, 317–332. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxid Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Shao, C.; Liu, L.; Guo, X.; Xu, Y.; Lü, X. Optimization, partial characterization and antioxidant activity of an exopolysaccharide from Lactobacillus plantarum KX041. Int. J. Biol. Macromol. 2017, 103, 1173–1184. [Google Scholar] [CrossRef]
- Inturri, R.; Molinaro, A.; Di Lorenzo, F.; Blandino, G.; Tomasello, B.; Hidalgo-Cantabrana, C.; De Castro, C.; Ruas-Madiedo, P. Chemical and biological properties of the novel exopolysaccharide produced by a probiotic strain of Bifidobacterium longum. Carbohydr. Polym. 2017, 174, 1172–1180. [Google Scholar] [CrossRef]
- Granato, D.; Branco, G.F.; Cruz, A.G.; Faria, J.D.A.F.; Shah, N.P. Probiotic dairy products as functional foods. Compr. Rev. Food Sci. Food Safe 2010, 9, 455–470. [Google Scholar] [CrossRef]
- Maryam, A.; Zaiton, H.; Mohamed, I.; Sharifah, N.R.S.A. Antioxidant activity of lactic acid bacteria (LAB) fermented skim milk as determined by 1,1-diphenyl-2-picrylhydrazyl (DPPH) and ferrous chelating activity (FCA). Afr. J. Microbiol. Res. 2012, 6, 6358–6364. [Google Scholar] [CrossRef]
- Liu, T.; Zhou, K.; Yin, S.; Liu, S.; Zhu, Y.; Yang, Y.; Wang, C. Purification and characterization of an exopolysaccharide produced by Lactobacillus plantarum HY isolated from home-made Sichuan Pickle. Int. J. Biol. Macromol. 2019, 134, 516–526. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 22–25. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye-binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.Z.; Meng, F.L.; Liu, Z.Q.; Chen, R.P.; Zhang, M. Antitumor activities of different fractions of polysaccharide purified from Ornithogalum caudatum Ait. Carbohydr. Polym. 2010, 80, 845–851. [Google Scholar] [CrossRef]
- Annadurai, V.; Ganesan, S.; Perumalsamy, B.; Krishnamurthy, M.; Arivalagan, P.; Yongkun, M.; Soundarapandian, S.; Ramasamy, T. Structural characterization, functional and biological activities of an exopolysaccharide produced by probiotic Bacillus licheniformis AG-06 from Indian polyherbal fermented traditional medicine. Int. J. Biol. Macromol. 2021, 174, 144–152. [Google Scholar] [CrossRef]
- Mao, Y.H.; Song, A.X.; Li, L.Q.; Yang, Y.; Yao, Z.P.; Wu, J.Y. A high-molecular weight exopolysaccharide from the Cs-HK1 fungus: Ultrasonic degradation, characterization and in vitro fecal fermentation. Carbohydr. Polym. 2020, 246, 116636. [Google Scholar] [CrossRef]
- Yang, X.; Ren, Y.; Zhang, L.; Wang, Z.; Li, L. Structural characteristics and antioxidant properties of exopolysaccharides isolated from soybean protein gel induced by lactic acid bacteria. LWT 2021, 150, 111811. [Google Scholar] [CrossRef]
- Li, W.; Xia, X.; Tang, W.; Ji, J.; Rui, X.; Chen, X.H.; Jiang, M.; Zhou, J.Z.; Zhang, Q.Q.; Dong, M.S. Structural Characterization and Anticancer Activity of Cell-Bound Exopolysaccharide from Lactobacillus helveticus MB2-1. J. Agric. Food Chem. 2015, 63, 3454–3463. [Google Scholar] [CrossRef]
- Wang, K.N.; Song, M.M.; Song, D.W.; Zhao, X.J.; Wu, J.; Lu, Y.; Niu, B.X.; Cai, G. Preparation, partial characterization and biological activity of exopolysaccharidesproduced from Lactobacillus fermentum S1. J. Biosci. Bioeng. 2020, 129, 206–214. [Google Scholar] [CrossRef]
- Du, R.; Qiao, X.; Zhao, F.; Song, Q.; Zhou, Q.; Wang, Y.; Pan, L.; Han, Y.; Zhou, Z. Purification, characterization and antioxidant activity of dextran produced by Leuconostoc pseudomesenteroides from homemade wine. Carbohydr. Polym. 2019, 198, 529–536. [Google Scholar] [CrossRef]
- Ma, X.; Pan, Y.; Zhao, W.; Sun, P.; Zhao, J.; Yan, S.; Wang, R.; Han, Y.; Liu, W.; Tan, S.; et al. Bifidobacterium infantis strain YLGB-1496 possesses excellent antioxidant and skin barrier-enhancing efficacy in vitro. Exp. Dermatol. 2022, 31, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, S.; Dong, J.; Shi, J.; Guan, J.; Liu, D.Y.; Liu, F.; Li, B.L.; Huo, G.C. Identification, Characterization, and Antioxidant Potential of Bifidobacterium longum subsp. longum Strains Isolated From Feces of Healthy Infants. Front. Microbiol. 2021, 12, 756519. [Google Scholar] [CrossRef] [PubMed]
- Jolly, L.; Stingele, F. Molecular organization and functionality of exopolysaccharide gene clusters in lactic acid bacteria. Int. Dairy J. 2001, 11, 733–745. [Google Scholar] [CrossRef]
- Li, M.; Li, W.; Li, D.; Tian, J.; Xiao, L.; Kwok, L.Y.; Li, W.; Sun, Z. Structure characterization, antioxidant capacity, rheological characteristics and expression of biosynthetic genes of exopolysaccharides produced by Lactococcus lactis subsp. lactis IMAU11823. Food Chem. 2022, 384, 132566. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huang, R.; Shah, N.P.; Tao, X.; Xiong, Y.; Wei, H. Antioxidant and antibacterial activities of exopolysaccharides from Bifidobacterium bifidum WBIN03 and Lactobacillus plantarum R315. J. Dairy Sci. 2014, 97, 7334–7343. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.M.; Zhou, J.M.; Zhou, Q.Q.; Li, P.; Xie, Y.Y.; Zhou, T.; Gu, Q. Purification, characterization and biological activities of exopolysaccharides from Lactobacillus rhamnosus ZFM231 isolated from milk. LWT 2021, 147, 111561. [Google Scholar] [CrossRef]
- Ge, Z.W.; Bao, X.; Li, Z.Y.; Chen, X.H.; Li, W.; Rui, X.; Wu, J.J.; Zhang, Q.Q.; Dong, M.S. In situ exopolysaccharides produced by Lactobacillus helveticus MB2-1 and its effect on gel properties of Sayram ketteki yoghurt. Int. J. Biol. Macromol. 2022, 208, 314–323. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, G.; Liu, R.; Wei, M.; Sun, C. EPS364, a Novel Deep-Sea Bacterial Exopolysaccharide, Inhibits Liver Cancer Cell Growth and Adhesion. J. Appl. Phycol. 2021, 33, 2983–2994. [Google Scholar] [CrossRef]
- Tukenmez, U.; Aktas, B.; Aslim, B.; Yavuz, S. The relationship between the structural characteristics of lactobacilli-EPS and its ability to induce apoptosis in colon cancer cells in vitro. Sci. Rep. 2019, 9, 8268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynch, K.M.; Zannini, E.; Coffey, A.; Arendt, E.K. Lactic Acid Bacteria Exopolysaccharides in Foods and Beverages: Isolation, Properties, Characterization, and Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 155–176. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, L.; Zeng, F.; Kennedy, J.F. Structure and antitumor activities of the water-soluble polysaccharides from Ganoderma tsugae mycelium. Carbohydr. Polym. 2005, 59, 385–392. [Google Scholar] [CrossRef]
- Sasikumar, K.; Vaikkath, D.K.; Devendra, L.; Nampoothiri, K.M. An exopolysaccharide (EPS) from a Lactobacillus plantarum BR2 with potential benefits for making functional foods. Bioresour. Technol. 2017, 241, 1152–1156. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, X.; Tian, Z.; Yang, Y.; Yang, Z. Characterization of an exopolysaccharide produced by Lactobacillus plantarum YW11 isolated from Tibet Kefir. Carbohydr. Polym. 2015, 125, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Amiri, S.; Mokarram, R.R.; Khiabani, M.S.; Bari, M.R.; Khaledabad, M.A. Exopolysaccharides production by Lactobacillus acidophilus LA5 and Bifidobacterium animalis subsp. lactis BB12: Optimization of fermentation variables and characterization of structure and bioactivities. Int. J. Biol. Macromol. 2019, 123, 752–765. [Google Scholar] [CrossRef]
- Ayyash, M.; Abu-Jdayil, B.; Itsaranuwat, P.; Almazrouei, N.; Galiwango, E.; Esposito, G.; Hunashal, Y.; Hamed, F.; Najjar, Z. Exopolysaccharide produced by the potential probiotic Lactococcus garvieae C47: Structural characteristics, rheological properties, bioactivities and impact on fermented camel milk. Food Chem. 2020, 333, 127418. [Google Scholar] [CrossRef]
- Di, W.; Zhang, L.; Wang, S.; Yi, H.; Han, X.; Fan, R.; Zhang, Y. Physicochemical characterization and antitumour activity of exopolysaccharides produced by Lactobacillus casei SB27 from yak milk. Carbohydr. Polym. 2017, 171, 307–315. [Google Scholar] [CrossRef]
- Rajoka, M.S.R.; Mehwish, M.H.; Fang, H.; Padhiar, A.A.; Zeng, X.; Khurshid, M.; He, Z.D.; Zhao, L.Q. Characterization and anti-tumor activity of exopolysaccharide produced by Lactobacillus kefiri isolated from Chinese kefir grains. J. Funct. Foods 2019, 63, 103588. [Google Scholar] [CrossRef]
- Chen, Z.; Shi, J.; Yang, X.; Nan, B.; Liu, Y.; Wang, Z. Chemical and physical characteristics and antioxidant activities of the exopolysaccharide produced by Tibetan kefir grains during milk fermentation. Int. Dairy J. 2015, 43, 15–21. [Google Scholar] [CrossRef]
- Xing, H.; Du, R.; Zhao, F.; Han, Y.; Xiao, H.; Zhou, Z. Optimization, chain conformation and characterization of exopolysaccharide isolated from Leuconostoc mesenteroides DRP105. Int. J. Biol. Macromol. 2018, 112, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Li, X.; Tian, X.; Yang, S.; Li, Y.; Li, Z.G.; Guo, T.T.; Kong, J. Characterization of the antioxidant activities of the exopolysaccharides produced by Streptococcus thermophilus CGMCC 7.179. LWT 2023, 173, 114256. [Google Scholar] [CrossRef]
- Xu, R.H.; Shang, N.; Li, P.L. In vitro and in vivo antioxidant activity of exopolysaccharide fractions from Bifidobacterium animalis RH. Anaerobe 2011, 17, 226–231. [Google Scholar] [CrossRef]
- Liu, L.; Xu, J.; Du, R.; Ping, W.; Ge, J.; Zhao, D. The response surface optimization of exopolysaccharide produced by Saccharomyces cerevisiae Y3 and its partial characterization. Prep. Biochem. Biotechnol. 2021, 52, 566–577. [Google Scholar] [CrossRef]
- Jiang, C.; Xiong, Q.; Gan, D.; Jiao, Y.P.; Liu, J.; Ma, L.P.; Zeng, X.X. Antioxidant activity and potential hepatoprotective effect of polysaccharides from Cyclina sinensis. Carbohydr. Polym. 2013, 91, 262–268. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, X.; Yang, Y.; Zhao, A.M.; Yang, Z.N. Characterization and bioactivities of an exopolysaccharide produced by Lactobacillus plantarum YW32. Int. J. Biol. Macromol. 2014, 74, 119–126. [Google Scholar] [CrossRef]
- Choudhuri, I.; Khanra, K.; Maity, P.; Patra, A.; Maity, G.N.; Pati, B.R.; Nag, A.; Mondal, S.; Bhattacharyya, N. Structure and biological properties of exopolysaccharide isolated from Citrobacter freundii. Int. J. Biol. Macromol. 2021, 168, 537–549. [Google Scholar] [CrossRef]
- Zheng, J.Q.; Mao, X.J.; Geng, L.J.; Yang, G.M.; Xu, C.P. Production optimization, preliminary characterization and bioactivity of exopolysaccharides from Incutis tamaricis (Pat.) Fiasson & Niemela. J. Taiwan Inst. Chem. Eng. 2014, 45, 725–733. [Google Scholar] [CrossRef]
- Xiang, Y.L.; Xu, X.Q.; Li, J. Chemical properties and antioxidant activity of exopolysaccharides fractions from mycelial culture of Inonotus obliquus in a ground corn stover medium. Food Chem. 2012, 134, 1899–1905. [Google Scholar] [CrossRef]
- Xu, R.B.; Yang, X.; Wang, J.; Zhao, H.T.; Lu, W.H.; Cui, J.; Cheng, C.L.; Zou, P.; Huang, W.W.; Wang, P.; et al. Chemical composition and antioxidant activities of three polysaccharide fractions from pine cones. Int. J. Mol. Sci. 2012, 13, 14262–14277. [Google Scholar] [CrossRef] [PubMed]







| Specificity | Locus_Tag | Product |
|---|---|---|
| Glucose | E3_00280 | PTS system glucose-specific EIIA component |
| N-acetylglucosamine | E3_00281 | PTS N-acetylglucosamine transporter subunit IIABC |
| Galactose | E3_01493 | Galactose permease |
| Fructose | E3_01538 | PTS system, fructose/glucose-specific IIABC |
| Fucose | E3_00598 | Fucose permease |
| Lactose | E3_01046 | Lactose permease Lactose transport system permease protein LacF |
| E3_01493 | ||
| Sugar | E3_01045 | multiple-sugar transport system permease protein |
| E3_01046 | ABC-type sugar transport system, permease component | |
| E3_01047, E3_01393 | multiple-sugar transport system substrate-binding protein | |
| E3_01751 | PTS sugar transporter subunit IIB |
| Sample | EPS-1 | EPS-2 | EPS-3 |
|---|---|---|---|
| Yield (%) | 29.33% | 23.00% | 10.67% |
| Neutral sugar (%) | 90.53 ± 0.52 a | 89.16 ± 1.34 a | 72.47 ± 2.81 b |
| Protein (%) | 0.25 ± 0.09 b | 1.04 ± 0.10 b | 2.61 ± 0.63 a |
| Uronic acid (%) | 0.24 ± 0.08 c | 0.49 ± 0.06 b | 0.94 ± 0.07 a |
| Sulphate (%) | 1.65 ± 0.31 b | 2.26 ± 0.34 a | 2.33 ± 0.11 a |
| Molecular weight (Da) | 4.15 × 104 | 3.67 × 104 | 5.89 × 104 |
| Molar ratio | Man: Glu = 1:5.04 | Rha: Man: Glu = 0.32: 1: 1.56 | Rha: Man: GlcN: Gal: glu: GluA = 1.10: 1: 0.82: 1.05: 1.39: 0.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, Y.; Wang, Y.; Han, Y.; Zhang, Y.; Cao, T.; Huo, G.; Li, B. Genome Analysis of Bifidobacterium Bifidum E3, Structural Characteristics, and Antioxidant Properties of Exopolysaccharides. Foods 2023, 12, 2988. https://doi.org/10.3390/foods12162988
Yue Y, Wang Y, Han Y, Zhang Y, Cao T, Huo G, Li B. Genome Analysis of Bifidobacterium Bifidum E3, Structural Characteristics, and Antioxidant Properties of Exopolysaccharides. Foods. 2023; 12(16):2988. https://doi.org/10.3390/foods12162988
Chicago/Turabian StyleYue, Yingxue, Yuqi Wang, Yu Han, Yifan Zhang, Ting Cao, Guicheng Huo, and Bailiang Li. 2023. "Genome Analysis of Bifidobacterium Bifidum E3, Structural Characteristics, and Antioxidant Properties of Exopolysaccharides" Foods 12, no. 16: 2988. https://doi.org/10.3390/foods12162988
APA StyleYue, Y., Wang, Y., Han, Y., Zhang, Y., Cao, T., Huo, G., & Li, B. (2023). Genome Analysis of Bifidobacterium Bifidum E3, Structural Characteristics, and Antioxidant Properties of Exopolysaccharides. Foods, 12(16), 2988. https://doi.org/10.3390/foods12162988