Effect of Extraction Methods on the Physicochemical Properties, Chemical Composition, and Antioxidant Activities of Samara Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Elaeagnus mollis Diels Kernels Preparation
2.3. Solvent Extraction
2.4. Mechanical Extraction
2.5. Color Determination
2.6. Acid Value (AV) and Peroxide Value (PV)
2.7. Fatty Acid Composition
2.8. Polyphenols
2.9. Carotenoids
2.10. Tocopherols
2.11. Antioxidant Activities
2.12. Oxidative Stability Index
2.13. Statistical Analysis
3. Results and Discussion
3.1. Color
3.2. Acid Value and Peroxide Value
3.3. Fatty Acid Composition
3.4. Polyphenols
3.5. Carotenoids
3.6. Tocopherols
3.7. Antioxidant Activities
3.8. Oxidative Stability Index
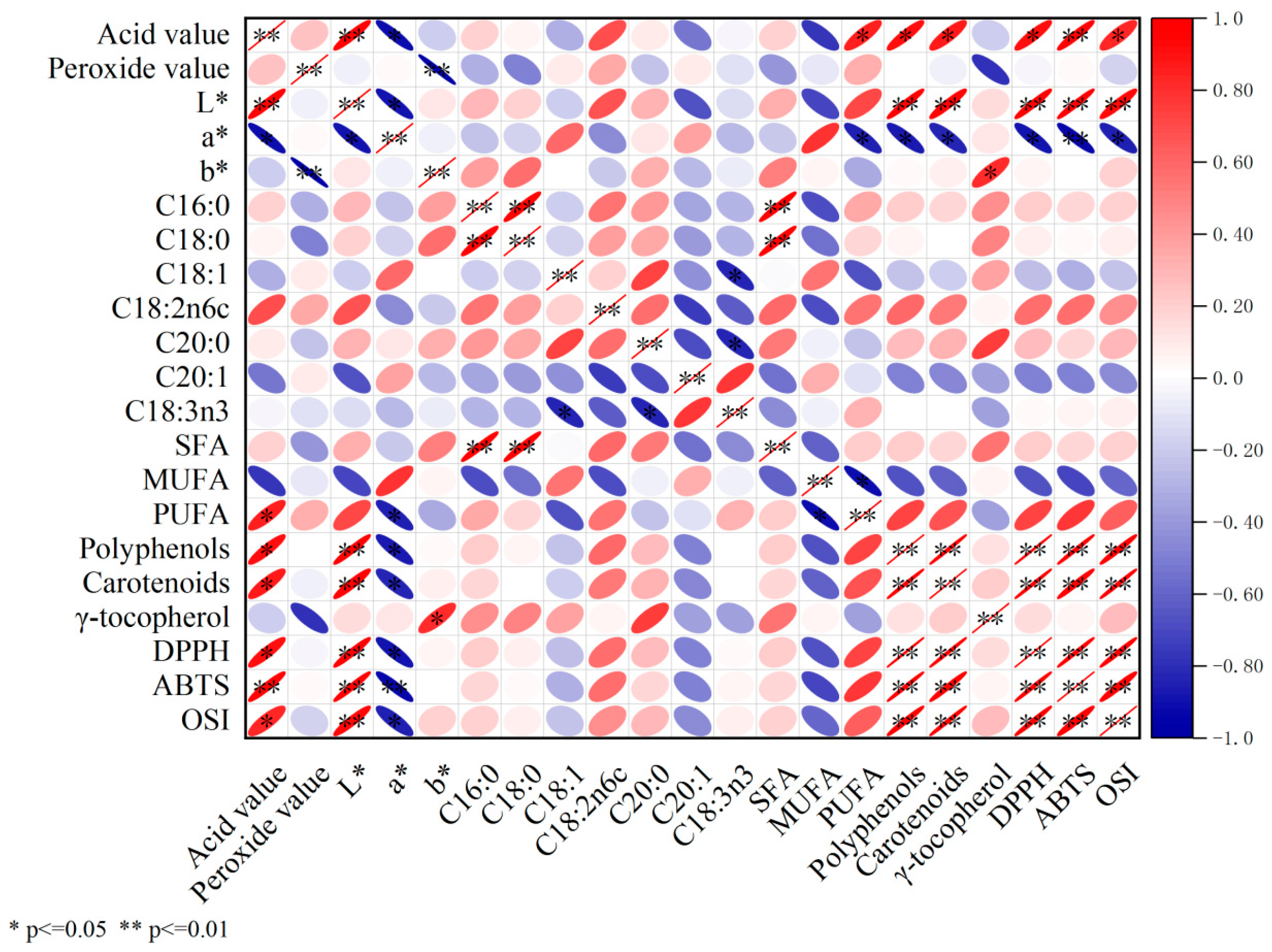
3.9. Correlation Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mu, J.; Wu, G.; Chen, Z.; Brennan, C.S.; Tran, K.; Dilrukshi, H.N.N.; Shi, C.; Zhen, H.; Hui, X. Identification of the fatty acids profiles in supercritical CO2 fluid and Soxhlet extraction of Samara oil from different cultivars of Elaeagnus mollis Diels seeds. J. Food Compos. Anal. 2021, 101, 103982. [Google Scholar] [CrossRef]
- Hu, B.; Xi, X.; Li, H.; Qin, Y.; Li, C.; Zhang, Z.; Liu, Y.; Zhang, Q.; Liu, A.; Liu, S.; et al. A comparison of extraction yield, quality and thermal properties from Sapindus mukorossi seed oil between microwave assisted extraction and Soxhlet extraction. Ind. Crop. Prod. 2021, 161, 113185. [Google Scholar] [CrossRef]
- Dun, Q.; Yao, L.; Deng, Z.; Li, H.; Li, J.; Fan, Y.; Zhang, B. Effects of hot and coldpressed processes on volatile compounds of peanut oil and corresponding analysis of characteristic flavor components. LWT 2019, 112, 107648. [Google Scholar] [CrossRef]
- Hama, J.R. Comparison of fatty acid profile changes between unroasted and roasted brown sesame (Sesamum indicum L.) seeds oil. Int. J. Food Prop. 2016, 20, 957–967. [Google Scholar] [CrossRef]
- Tian, Y.; Xu, Z.; Zheng, B.; Lo, Y.M. Optimization of ultrasonic-assisted extraction of pomegranate (Punica granatum L.) seed oil. Ultrason. Sonochem. 2013, 20, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Zhou, X.; Shi, Q.; Pan, J.; Zhan, H.; Ge, F. High-pressure supercritical carbon dioxide extraction of Idesia polycarpa oil: Evaluation the influence of process parameters on the extraction yield and oil quality. Ind. Crop. Prod. 2022, 188, 115586. [Google Scholar] [CrossRef]
- Barbi, R.C.T.; de Souza, A.R.C.; Hamerski, F.; Teixeira, G.L.; Corazza, M.L.; Ribani, R.H. Subcritical propane extraction of high-quality inajá (Maximiliana maripa) pulp oil. J. Supercrit. Fluids 2019, 153, 104576. [Google Scholar] [CrossRef]
- Wu, Y.; Yuan, W.; Han, X.; Hu, J.; Yin, L.; Lv, Z. Integrated analysis of fatty acid, sterol and tocopherol components of seed oils obtained from four varieties of industrial and environmental protection crops. Ind. Crop. Prod. 2020, 154, 112655. [Google Scholar] [CrossRef]
- Zhang, D.; Li, X.; Zhang, Z.; Zhang, J.; Sun, Q.; Duan, X.; Sun, H.; Cao, Y. Influence of roasting on the physicochemical properties, chemical composition and antioxidant activities of peanut oil. LWT 2022, 154, 112613. [Google Scholar] [CrossRef]
- AOCS. Official Methods and Recommended Practices of the American oil Chemist’s Society; American Oil Chemists’ Society: Urbana, IL, USA, 2009. [Google Scholar]
- Zhang, D.; Duan, X.; Wang, Y.; Shang, B.; Liu, H.; Sun, H.; Wang, Y. A comparative investigation on physicochemical properties, chemical composition, and in vitro antioxidant activities of rice bran oils from different japonica rice (Oryza Sativa L.) varieties. J. Food Meas. Charact. 2021, 15, 2064–2077. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Chiou, A.; Mylona, A.; Ioannou, M.S.; Andrikopoulos, N.K. Recovery and distribution of natural antioxidants (polyphenols, hydroxy pentacyclic terpenic acids and α-tocopherol) during the pan-frying of Mediterranean finfish in virgin olive oil. Food Chem. 2007, 100, 509–517. [Google Scholar] [CrossRef]
- Chouaibi, M.; Rezig, L.; Hamdi, S.; Ferrari, G. Chemical characteristics and compositions of red pepper seed oils extracted by different methods. Ind. Crop. Prod. 2019, 128, 363–370. [Google Scholar] [CrossRef]
- Zhang, D.; Li, X.; Cao, Y.; Wang, C.; Xue, Y. Effect of roasting on the chemical components of peanut oil. LWT 2020, 125, 109249. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.; Hu, Q.; Sun, S.; Wan, Z. Multifactorial revealing the association between components and lipid oxidation of edible vegetable oils in bulk and emulsion systems. LWT 2023, 183, 114909. [Google Scholar] [CrossRef]
- Suri, K.; Singh, B.; Kaur, A.; Yadav, M.P.; Singh, N. Impact of infrared and dry air roasting on the oxidative stability, fatty acid composition, Maillard reaction products and other chemical properties of black cumin (Nigella sativa L.) seed oil. Food Chem. 2019, 295, 537–547. [Google Scholar] [CrossRef]
- FAO; WHO. Report of the 21st Session of the Codex Alimentarius Committed on Fats and Oils. Kolakinabala, Malaysia; WHO: Geneva, Switzerland, 2009. [Google Scholar]
- Anjum, F.; Anwar, F.; Jamil, A.; Iqbal, M. Microwave roasting effects on the physico-chemical composition and oxidative stability of sunflower seed oil. J. Am. Oil Chem. Soc. 2006, 83, 777–784. [Google Scholar] [CrossRef]
- Gao, P.; Liu, R.; Jin, Q.; Wang, X. Effects of processing methods on the chemical composition and antioxidant capacity of walnut (Juglans regia L.) oil. LWT 2021, 135, 109958. [Google Scholar] [CrossRef]
- Singh, A.; Kumar, V. Phyto-chemical and bioactive compounds of pumpkin seed oil as affected by different extraction methods. Food Chem. Adv. 2023, 2, 100211. [Google Scholar] [CrossRef]
- Liu, N.; Ren, G.; Faiza, M.; Li, D.; Cui, J.; Zhang, K.; Yao, X.; Zhao, M. Comparison of conventional and green extraction methods on oil yield, physicochemical properties, and lipid compositions of pomegranate seed oil. J. Food Compos. Anal. 2022, 114, 104747. [Google Scholar] [CrossRef]
- Gai, Z.; Hu, S.; Gong, G.; Zhao, J. Recent advances in understanding dietary polyphenols protecting against hypertension. Trends Food Sci. Technol. 2023, 138, 685–696. [Google Scholar] [CrossRef]
- Kopec, R.E.; Failla, M.L. Recent advances in the bioaccessibility and bioavailability of carotenoids and effects of other dietary lipophiles. J. Food Compos. Anal. 2018, 68, 16–30. [Google Scholar] [CrossRef]
- Azzi, A. Tocopherols, tocotrienols and tocomonoenols: Many similar molecules but only one vitamin E. Redox Biol. 2019, 26, 101259. [Google Scholar] [CrossRef]
- Kallio, H.; Yang, B.R.; Peippo, P.; Tahvonen, R.; Pan, R. Triacylglycerols, glycerophospholipids, tocopherols, and tocotrienols in berries and seeds of two subspecies (ssp. sinensis and mongolica) of sea buckthorn (hippophae rhamnoides). J. Agric. Food Chem. 2002, 50, 3004–3009. [Google Scholar] [CrossRef] [PubMed]
- Rumpf, J.; Burger, R.; Schulze, M. Statistical evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu assays to assess the antioxidant capacity of lignins. Int. J. Biol. Macromol. 2023, 233, 123470. [Google Scholar] [CrossRef] [PubMed]
- Suri, K.; Singh, B.; Kaur, A.; Yadav, M.P.; Singh, N. Influence of microwave roasting on chemical composition, oxidative stability and fatty acid composition of flaxseed (Linum usitatissimum L.) oil. Food Chem. 2020, 326, 126974. [Google Scholar] [CrossRef] [PubMed]
- Kedir, W.M.; Geletu, A.K.; Weldegirum, G.S.; Sima, M.F. Antioxidant activity of selected plants extract for palm oil stability via accelerated and deep frying study. Heliyon 2023, 9, e17980. [Google Scholar] [CrossRef]
| Parameters | Solvent Extraction | Mechanical Extraction | ||||
|---|---|---|---|---|---|---|
| Ethyl Acetate | Acetone | n-Hexane | Petroleum Ether | Hot-Pressing | Cold-Pressing | |
| L* | 27.82 ± 0.01 d | 31.03 ± 0.01 f | 27.24 ± 0.01 b | 28.17 ± 0.02 e | 27.50 ± 0.00 c | 26.72 ± 0.01 a |
| a* | 0.00 ± 0.00 c | −0.48 ± 0.01 a | −0.08 ± 0.05 b | 0.00 ± 0.00 c | 0.23 ± 0.01 d | 0.28 ± 0.06 d |
| b* | 6.42 ± 0.07 a | 9.18 ± 0.04 c | 9.17 ± 0.01 c | 10.41 ± 0.01 d | 9.04 ± 0.02 b | 9.01 ± 0.00 b |
| Acid value (mg KOH/g) | 1.02 ± 0.02 e | 1.33 ± 0.07 f | 0.81 ± 0.01 c | 0.86 ± 0.01 d | 0.77 ± 0.01 b | 0.66 ± 0.02 a |
| Peroxide value (mmol/kg) | 1.11 ± 0.03 e | 0.55 ± 0.01 c | 0.47 ± 0.00 b | 0.36 ± 0.00 a | 0.60 ± 0.00 d | 0.52 ± 0.02 c |
| Fatty Acid | Solvent Extraction | Mechanical Extraction | ||||
|---|---|---|---|---|---|---|
| Ethyl Acetate | Acetone | n-Hexane | Petroleum Ether | Hot-Pressing | Cold-Pressing | |
| C16:0 | 4.11 ± 0.01 a | 4.12 ± 0.02 a | 4.10 ± 0.01 a | 4.14 ± 0.06 a | 4.09 ± 0.00 a | 4.12 ± 0.00 a |
| C18:0 | 2.61 ± 0.01 a | 2.62 ± 0.01 a | 2.61 ± 0.03 a | 2.65 ± 0.07 a | 2.60 ± 0.01 a | 2.62 ± 0.02 a |
| C18:1 | 36.94 ± 0.13 a,b | 36.90 ± 0.06 a,b | 36.78 ± 0.14 a | 36.96 ± 0.11 a,b | 37.14 ± 0.06 b | 37.00 ± 0.05 a,b |
| C18:2 n6 | 48.28 ± 0.12 a | 48.30 ± 0.02 a | 48.07 ± 0.26 a | 48.26 ± 0.08 a | 48.17 ± 0.04 a | 48.17 ± 0.02 a |
| C18:3 n3 | 7.42 ± 0.03 a,b | 7.44 ± 0.01 a,b | 7.57 ± 0.15 b | 7.37 ± 0.02 a | 7.37 ± 0.02 a | 7.44 ± 0.01 a,b |
| C20:0 | 0.19 ± 0.01 a | 0.20 ± 0.00 a | 0.18 ± 0.02 a | 0.20 ± 0.01 a | 0.20 ± 0.00 a | 0.20 ± 0.01 a |
| C20:1 | 0.45 ± 0.03 a | 0.42 ± 0.00 a | 0.48 ± 0.00 a | 0.42 ± 0.00 a | 0.43 ± 0.00 a | 0.47 ± 0.03 b |
| SFA | 6.91 ± 0.02 a,b | 6.94 ± 0.03 b | 6.89 ± 0.02 a | 6.99 ± 0.00 c | 6.89 ± 0.00 a | 6.93 ± 0.00 a,b |
| MUFA | 37.39 ± 0.17 a | 37.32 ± 0.06 a | 37.46 ± 0.13 a | 37.38 ± 0.11 a | 37.57 ± 0.06 c | 37.47 ± 0.02 b |
| PUFA | 55.70 ± 0.15 a | 55.74 ± 0.03 a | 55.65 ± 0.11 a | 55.63 ± 0.11 a | 55.54 ± 0.06 a | 55.60 ± 0.03 a |
| Bioactive Constituents | Solvent Extraction | Mechanical Extraction | ||||
|---|---|---|---|---|---|---|
| Ethyl Acetate | Acetone | n-Hexane | Petroleum Ether | Hot-Pressing | Cold-Pressing | |
| Polyphenols (mg GAE/kg) | 38.01 ± 0.97 c | 140.27 ± 26.44 d | 24.04 ± 0.39 a | 27.88 ± 2.52 b | 24.52 ± 0.48 a | 23.36 ± 1.94 a |
| Carotenoids (mg/kg) | 5.43 ± 0.33 b | 42.95 ± 3.28 c | 3.51 ± 0.28 a | 3.35 ± 0.33 a | 5.03 ± 0.15 b | 4.72 ± 0.37 b |
| γ-tocopherol (mg/kg) | 669.98 ± 11.15 a | 712.98 ± 9.56 c | 688.61 ± 3.74 b | 720.10 ± 8.27 c | 708.83 ± 18.89 c | 722.05 ± 14.11 c |
| Parameters | Solvent Extraction | Mechanical Extraction | ||||
|---|---|---|---|---|---|---|
| Ethyl Acetate | Acetone | n-Hexane | Petroleum Ether | Hot-Pressing | Cold-Pressing | |
| DPPH (μmol TE/100 g) | 132.00 ± 1.26 d | 817.30 ± 13.73 e | 78.50 ± 3.73 b | 93.00 ± 11.36 c | 56.11 ± 10.04 a | 58.12 ± 7.56 a |
| ABTS (μmol TE/100 g) | 628.04 ± 6.11 d | 1238.80 ± 3.63 e | 561.05 ± 14.66 c | 547.90 ± 6.12 c | 507.10 ± 8.53 b | 450.38 ± 1.22 a |
| OSI (h) | 6.28 ± 0.39 a | 21.38 ± 0.53 d | 7.75 ± 0.07 c | 7.45 ± 0.21 b | 7.35 ± 0.28 b | 7.35 ± 0.07 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Guo, M.; Xue, Y.; Duan, Z. Effect of Extraction Methods on the Physicochemical Properties, Chemical Composition, and Antioxidant Activities of Samara Oil. Foods 2023, 12, 3163. https://doi.org/10.3390/foods12173163
Li X, Guo M, Xue Y, Duan Z. Effect of Extraction Methods on the Physicochemical Properties, Chemical Composition, and Antioxidant Activities of Samara Oil. Foods. 2023; 12(17):3163. https://doi.org/10.3390/foods12173163
Chicago/Turabian StyleLi, Xiujuan, Mimi Guo, Yalin Xue, and Zhangqun Duan. 2023. "Effect of Extraction Methods on the Physicochemical Properties, Chemical Composition, and Antioxidant Activities of Samara Oil" Foods 12, no. 17: 3163. https://doi.org/10.3390/foods12173163




