Exploitation of Selected Sourdough Saccharomyces cerevisiae Strains for the Production of a Craft Raspberry Fruit Beer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Culture Conditions
2.2. Microbiological Analysis
2.3. Screening of S. cerevisiae Strains for Technological Traits
2.3.1. Growth of Different Carbon Sources
2.3.2. Ethanol Tolerance
2.3.3. Hydrogen Sulphide Production
2.4. Wort Fermentation Ability of S. cerevisiae Strains
2.5. Small-Scale Brewing by Selected S. cerevisiae Strains
2.6. Chemical and Analytical Determination of Wort and Beers
2.7. Aromatic Profile Determination of Beers
Qualitative and Quantitative Analyses
2.8. Statistical and Data Analysis
3. Results and Discussion
3.1. Yeast Screening for Technological Characteristics
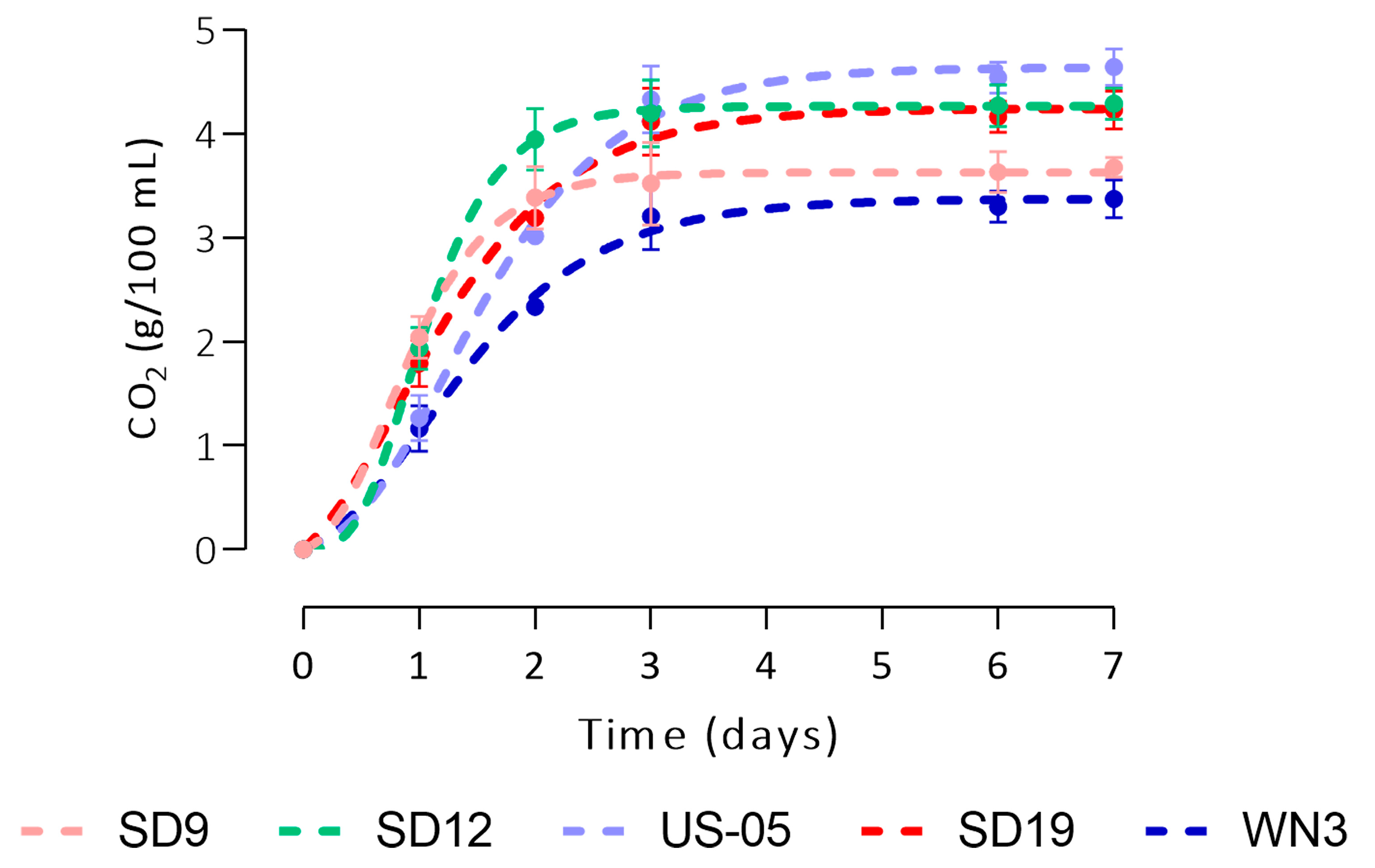
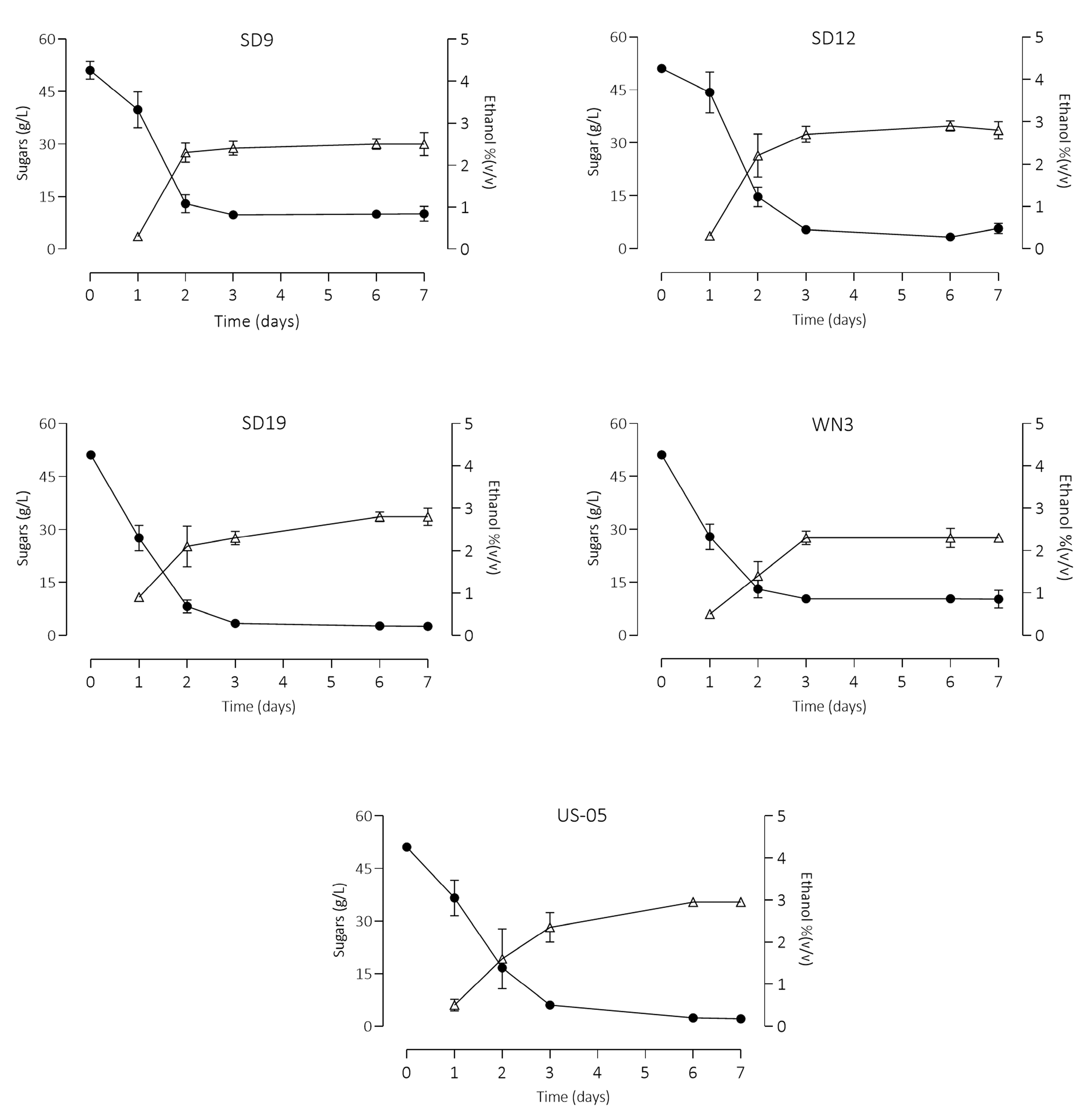
3.2. Wort Fermentation
3.3. Fruit and Control Beer Production
3.4. Physico-Chemical Characteristics of Control (CB) and Fruit (FB) Beers
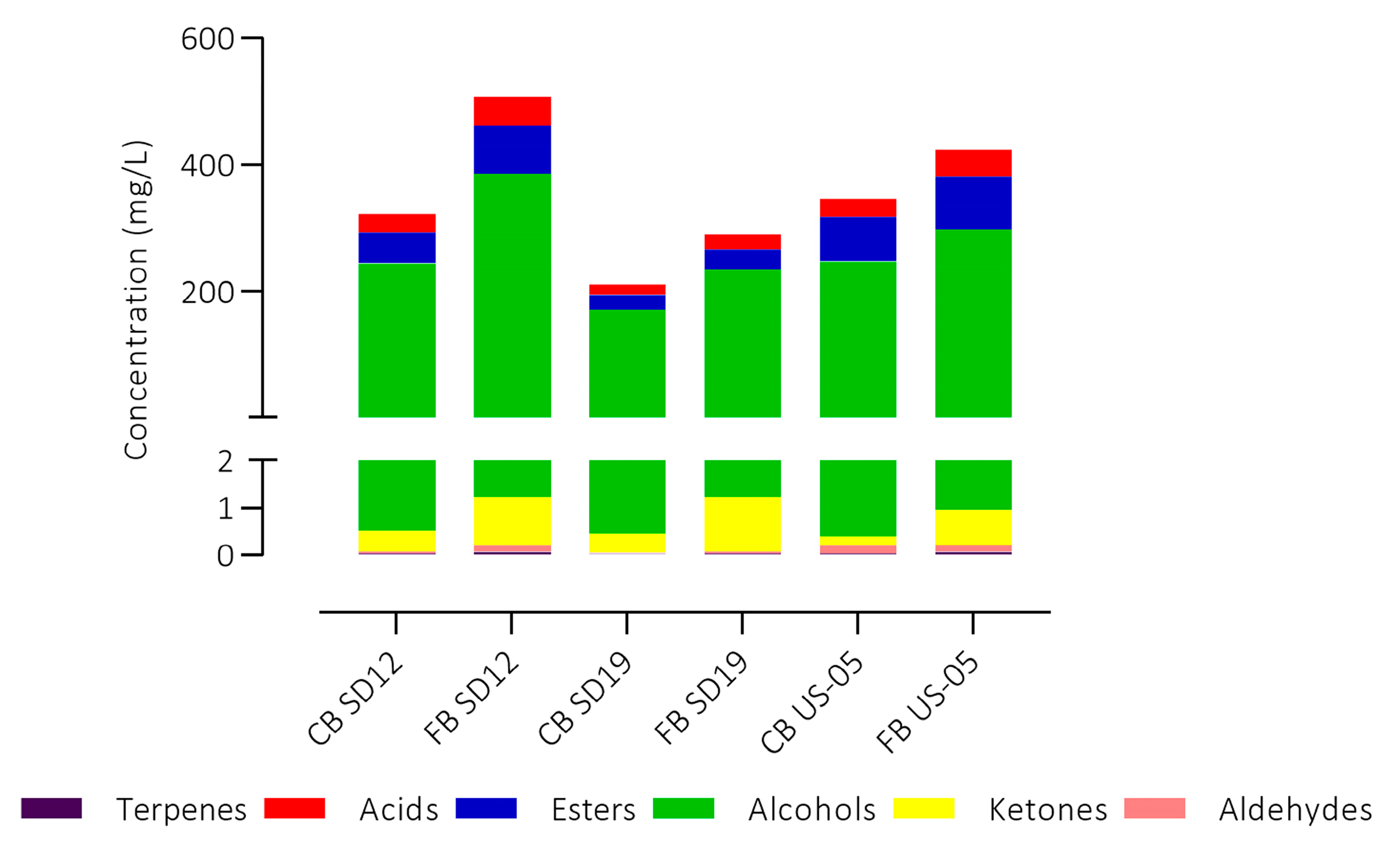
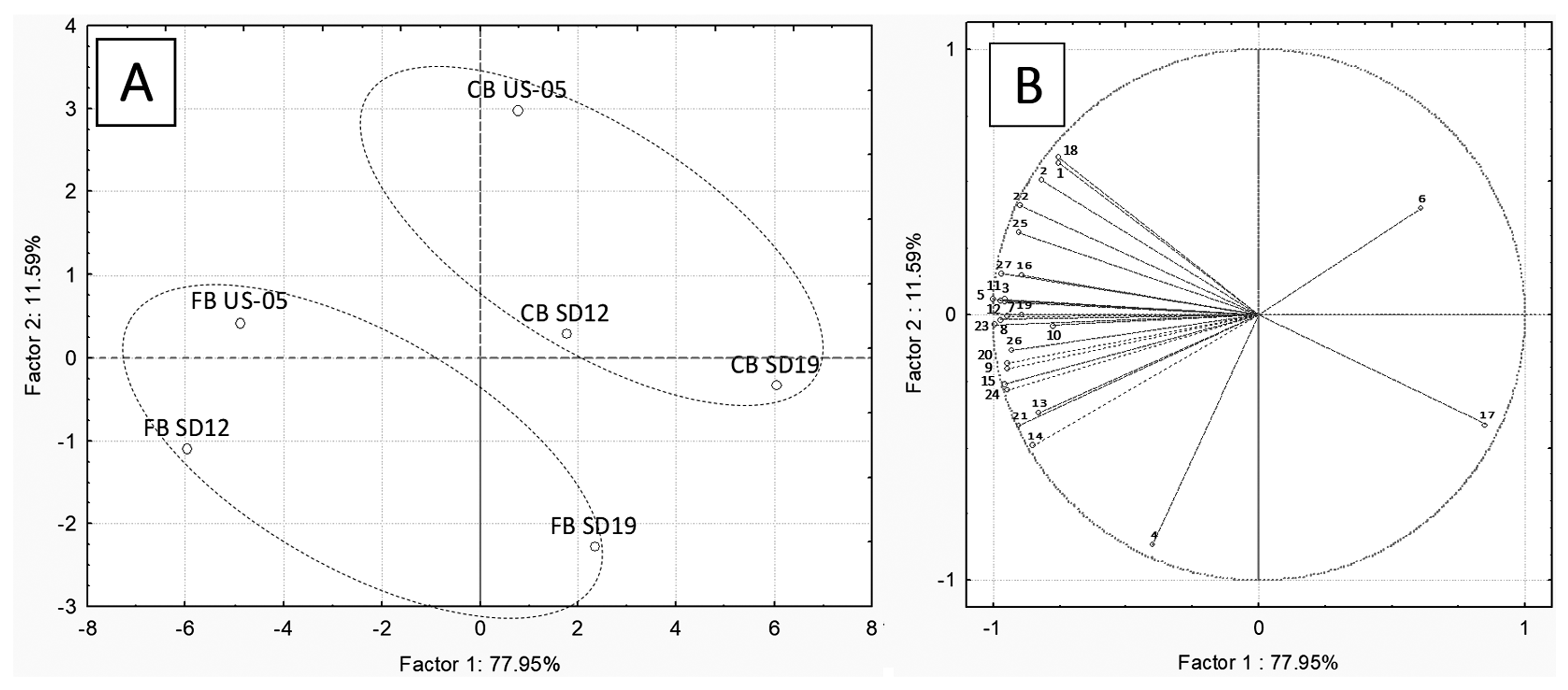
3.5. Volatile Organic Compounds (VOCs) Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seo, S.H.; Kim, E.J.; Park, S.E.; Park, D.H.; Park, K.M.; Na, C.S.; Son, H.S. GC/MS-based metabolomics study to investigate differential metabolites between ale and lager beers. Food Biosci. 2020, 36, 100671. [Google Scholar] [CrossRef]
- Zhao, X.; Yin, Y.; Fang, W.; Yang, Z. What happens when fruit married with beer? Int. J. Gastron. Food Sci. 2023, 32, 100716. [Google Scholar] [CrossRef]
- Fruit Beer Market Size, Growth, Share, Trends: 2022–2027. Market Data Forecast. Available online: https://www.marketdataforecast.com/market-reports/fruit-beer-market (accessed on 4 February 2023).
- Legge 28 Luglio n. 154. Art. 35, Denominazione di Birra Artigianale, Gazzetta Ufficiale Della Repubblica Italiana, GU Serie Generale n. 186. 2016. Available online: http://www.gazzettaufficiale.it/eli/id/2016/08/10/16G00169/sg%20 (accessed on 9 January 2023).
- Cho, J.H.; Kim, I.D.; Dhungana, S.K.; Do, H.M.; Shin, D.H. Persimmon fruit enhanced quality characteristics and antioxidant potential of beer. Food Sci. Biotechnol. 2018, 27, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Fanari, M.; Forteschi, M.; Sanna, M.; Piu, P.P.; Porcu, M.C.; D’hallewin, G.; Secchi, N.; Zinellu, M.; Pretti, L. Pilot plant production of craft fruit beer using Ohmic-treated fruit puree. J. Food Process. Preserv. 2020, 44, e14339. [Google Scholar] [CrossRef]
- Gasiński, A.; Kawa-Rygielska, J.; Szumny, A.; Czubaszek, A.; Gąsior, J.; Pietrzak, W. Volatile compounds content, physicochemical parameters, and antioxidant activity of beers with addition of mango fruit (Mangifera Indica). Molecules 2020, 25, 3033. [Google Scholar] [CrossRef] [PubMed]
- Valentoni, A.; Melis, R.; Sanna, M.; Porcu, M.C.; Rodolfi, M.; Braca, A.; Bianco, A.; Zara, G.; Budroni, M.; Anedda, R.; et al. Fruit Beer with the Bisucciu Sardinian Apricot Cultivar (Prunus armeniaca L.): A Technological and Analytical Approach. Fermentation 2023, 9, 305. [Google Scholar] [CrossRef]
- Ducruet, J.; Rébénaque, P.; Diserens, S.; Kosińska-Cagnazzo, A.; Héritier, I.; Andlauer, W. Amber ale beer enriched with goji berries–The effect on bioactive compound content and sensorial properties. Food Chem. 2017, 226, 109–118. [Google Scholar] [CrossRef]
- Yin, H.; Deng, Y.; Zhao, J.; Zhang, L.; Yu, J.; Deng, Y. Improving oxidative stability and sensory properties of ale beer by enrichment with dried red raspberries (Rubus idaeus L.). J. Am. Soc. Brew. Chem. 2021, 79, 370–377. [Google Scholar] [CrossRef]
- Rao, A.V.; Snyder, D.M. Raspberries and human health: A review. J. Agric. Food Chem. 2010, 58, 3871–3883. [Google Scholar] [CrossRef]
- Aprea, E.; Biasioli, F.; Gasperi, F. Volatile compounds of raspberry fruit: From analytical methods to biological role and sensory impact. Molecules 2015, 20, 2445–2474. [Google Scholar] [CrossRef]
- Vanderhaegen, B.; Neven, H.; Coghe, S.; Verstrepen, K.J.; Derdelinckx, G.; Verachtert, H. Bioflavoring and beer refermentation. Appl. Microbiol. Biotechnol. 2003, 62, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Cubillos, F.A.; Gibson, B.; Grijalva-Vallejos, N.; Krogerus, K.; Nikulin, J. Bioprospecting for brewers: Exploiting natural diversity for naturally diverse beers. Yeast 2019, 36, 383–398. [Google Scholar] [CrossRef]
- Araújo, T.M.; Souza, M.T.; Diniz, R.H.S.; Yamakawa, C.K.; Soares, L.B.; Lenczak, J.L.; de Castro Oliveira, J.V.; Goldman, G.H.; Barbosa, E.A.; Silva Campos, A.C.; et al. Cachaça yeast strains: Alternative starters to produce beer and bioethanol. Antonie Leeuwenhoek 2018, 111, 1749–1766. [Google Scholar] [CrossRef] [PubMed]
- Lentz, M.; Putzke, T.; Hessler, R.; Luman, E. Genetic and physiological characterization of yeast isolated from ripe fruit and analysis of fermentation and brewing potential. J. Inst. Brew. 2014, 120, 559–564. [Google Scholar] [CrossRef]
- Mascia, I.; Fadda, C.; Dostálek, P.; Karabín, M.; Zara, G.; Budroni, M.; Del Caro, A. Is it possible to create an innovative craft durum wheat beer with sourdough yeasts? A case study. J. Inst. Brew. 2015, 121, 283–286. [Google Scholar] [CrossRef]
- Iattici, F.; Catallo, M.; Solieri, L. Designing new yeasts for craft brewing: When natural biodiversity meets biotechnology. Beverages 2020, 6, 3. [Google Scholar] [CrossRef]
- Marongiu, A.; Zara, G.; Legras, J.L.; Del Caro, A.; Mascia, I.; Fadda, C.; Budroni, M. Novel starters for old processes: Use of Saccharomyces cerevisiae strains isolated from artisanal sourdough for craft beer production at a brewery scale. J. Indl. Microbiol. Biotechnol. 2015, 42, 85–92. [Google Scholar] [CrossRef]
- Canonico, L.; Comitini, F.; Ciani, M. Dominance and influence of selected Saccharomyces cerevisiae strains on the analytical profile of craft beer refermentation. J. Inst. Brew. 2014, 120, 262–267. [Google Scholar] [CrossRef]
- Matraxia, M.; Alfonzo, A.; Prestianni, R.; Francesca, N.; Gaglio, R.; Todaro, A.; Alfeo, V.; Perretti, G.; Columba, P.; Settanni, L.; et al. Non-conventional yeasts from fermented honey by-products: Focus on Hanseniaspora uvarum strains for craft beer production. Food Microbiol. 2021, 99, 103806. [Google Scholar] [CrossRef]
- Jiranek, V.; Langridge, P.; Henschke, P. Regulation of hydrogen sulfide liberation in wine-producing Saccharomyces cerevisiae strains by assimilable nitrogen. Appl. Environ. Microbiol. 1995, 61, 461–467. [Google Scholar] [CrossRef]
- Guerrini, S.; Galli, V.; Barbato, D.; Facchini, G.; Mangani, S.; Pierguidi, L.; Granchi, L. Effects of Saccharomyces cerevisiae and Starmerella bacillaris on the physicochemical and sensory characteristics of sparkling pear cider (Perry). Eur. Food Res. Technol. 2023, 249, 341–352. [Google Scholar] [CrossRef]
- Kawa-Rygielska, J.; Adamenko, K.; Kucharska, A.Z.; Prorok, P.; Piórecki, N. Physicochemical and antioxidative properties of Cornelian cherry beer. Food Chem. 2019, 281, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, L.; Angeloni, G.; Masella, P.; Calamai, L.; Parenti, A. A technological solution to modulate the aroma profile during beer fermentation. Food Bioproc. Technol. 2018, 11, 1259–1266. [Google Scholar] [CrossRef]
- Salema-Oom, M.; Valadão Pinto, V.; Gonçalves, P.; Spencer-Martins, I. Maltotriose utilization by industrial Saccharomyces strains: Characterization of a new member of the α-glucoside transporter family. Appl. Environ. Microbiol. 2005, 71, 5044–5049. [Google Scholar] [CrossRef]
- Meier-Dörnberg, T.; Hutzler, M.; Michel, M.; Methner, F.J.; Jacob, F. The importance of a comparative characterization of Saccharomyces cerevisiae and Saccharomyces pastorianus strains for brewing. Fermentation 2017, 3, 41. [Google Scholar] [CrossRef]
- Gonçalves, M.; Pontes, A.; Almeida, P.; Barbosa, R.; Serra, M.; Libkind, D.; Hutzler, M.; Gonçalves, P.; Sampaio, J.P. Distinct domestication trajectories in top-fermenting beer yeasts and wine yeasts. Curr. Biol. 2016, 26, 2750–2761. [Google Scholar] [CrossRef]
- Catallo, M.; Nikulin, J.; Johansson, L.; Krogerus, K.; Laitinen, M.; Magalhães, F.; Piironen, M.; Mikkelson, A.; Randazzo, C.L.; Solieri, L.; et al. Sourdough derived strains of Saccharomyces cerevisiae and their potential for farmhouse ale brewing. J. Inst. Brew. 2020, 126, 168–175. [Google Scholar] [CrossRef]
- Rossi, S.; Turchetti, B.; Sileoni, V.; Marconi, O.; Perretti, G. Evaluation of Saccharomyces cerevisiae strains isolated from non-brewing environments in beer production. J. Inst. Brew. 2018, 124, 381–388. [Google Scholar] [CrossRef]
- Siesto, G.; Pietrafesa, R.; Tufariello, M.; Gerardi, C.; Grieco, F.; Capece, A. Application of microbial cross-over for the production of Italian grape ale (IGA), a fruit beer obtained by grape must addition. Food Biosci. 2023, 52, 102487. [Google Scholar] [CrossRef]
- Zhao, X.; Procopio, S.; Becker, T. Flavor impacts of glycerol in the processing of yeast fermented beverages: A review. J. Food Sci. Technol. 2015, 52, 7588–7598. [Google Scholar] [CrossRef]
- Udom, N.; Chansongkrow, P.; Charoensawan, V.; Auesukaree, C. Coordination of the cell wall integrity and high-osmolarity glycerol pathways in response to ethanol stress in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2019, 85, 00551-19. [Google Scholar] [CrossRef] [PubMed]
- Nardini, M.; Garaguso, I. Characterization of bioactive compounds and antioxidant activity of fruit beers. Food Chem. 2020, 305, 125437. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.; Vegara, S.; Martí, N.; Valero, M.; Saura, D. Physicochemical characterization of special persimmon fruit beers using bohemian pilsner malt as a base. J. Ins. Brew. 2017, 123, 319–327. [Google Scholar] [CrossRef]
- He, Y.; Dong, J.; Yin, H.; Zhao, Y.; Chen, R.; Wan, X.; Chen, P.; Hou, X.; Liu, J.; Chen, L. Wort composition and its impact on the flavour-active higher alcohol and ester formation of beer–a review. J. Inst. Brew. 2014, 120, 157–163. [Google Scholar] [CrossRef]
- Stewart, G.G. Saccharomyces species in the Production of Beer. Beverages 2016, 2, 34. [Google Scholar] [CrossRef]
- Baigts-Allende, D.K.; Perez-Alva, A.; Ramirez-Rodrigues, M.A.; Palacios, A.; Ramirez-Rodrigues, M.M. A comparative study of polyphenolic and amino acid profiles of commercial fruit beers. J. Food Compost. Anal. 2021, 100, 103921. [Google Scholar] [CrossRef]
- Pires, E.J.; Teixeira, J.A.; Brányik, T.; Vicente, A.A. Yeast: The soul of beer’s aroma—A review of flavour-active esters and higher alcohols produced by the brewing yeast. Appl. Microbiol. Biotechnol. 2014, 98, 1937–1949. [Google Scholar] [CrossRef]
- Marconi, O.; Rossi, S.; Galgano, F.; Sileoni, V.; Perretti, G. Influence of yeast strain, priming solution and temperature on beer bottle conditioning. J. Sci. Food Agric. 2016, 96, 4106–4115. [Google Scholar] [CrossRef]
- Saerens, S.M.G.; Delvaux, F.; Verstrepen, K.J.; Van Dijck, P.; Thevelein, J.M.; Delvaux, F.R. Parameters affecting ethyl ester production by Saccharomyces cerevisiae during fermentation. Appl. Environ. Microbiol. 2008, 74, 454–461. [Google Scholar] [CrossRef]
- Olaniran, A.O.; Hiralal, L.; Mokoena, M.P.; Pillay, B. 2017 Flavour-active volatile compounds in beer: Production, regulation and control. J. Inst. Brew. 2020, 123, 13–23. [Google Scholar] [CrossRef]
- Ferreira, I.M.; Guido, L.F. Impact of wort amino acids on beer flavour: A review. Fermentation 2018, 4, 23. [Google Scholar] [CrossRef]
- Krogerus, K.; Gibson, B.R. 125th anniversary review: Diacetyl and its control during brewery fermentation. J. Inst. Brew. 2013, 119, 86–97. [Google Scholar] [CrossRef]
- Yeo, H.Q.; Liu, S.Q. An overview of selected specialty beers: Developments, challenges and prospects. Int. J. Food Sci. Technol. 2014, 49, 1607–1618. [Google Scholar] [CrossRef]
- Meilgaard, M.C. Flavor chemistry of beer: Part II: Flavor and threshold of 239 aroma volatiles. Tech. Q.—Master Brew. Assoc. Am. 1975, 12, 151–168. [Google Scholar]


| Source of Isolation | Strain |
|---|---|
| Sourdough | SD1; SD2; SD3; SD4; SD5; SD6; SD7; SD8; SD9; SD10; SD11; SD12; SD13; SD14; SD15; SD16; SD17; SD18; SD19 |
| Winery | WN1; WN2; WN3 |
| Commercial bakery yeast | ZEUS 45DB3 (Zeus Iba s.r.l, Florence, Italy) |
| Commercial brewing yeasts | Fermentis SafAle US-05 (Fermentis, LeSaffre, Italia S.p.A., Parma, Italy); FERMOALE AY3 (AEB group, San Polo (BS) Italy); Weiss arome+ (AEB group, San Polo (BS) Italy); Fermentis SafAle F2 (Fermentis, LeSaffre, Italia S.p.A., Parma, Italy) |
| Commercial wine yeasts | Lalvin S6U (Lallemand Inc., Montreal, QC, Canada), Lalvin EC1118 (Lallemand Inc., Montreal, QC, Canada) |
| S. cerevisiae Strain | Maltotriose (g/L) | Maltose (g/L) | Glucose (g/L) | Fructose (g/L) | Total Sugars (g/L) | Glycerol (g/L) | Acetic Acid (g/L) | Ethanol (%v/v) | Yeast Concentration (CFU/mL) |
|---|---|---|---|---|---|---|---|---|---|
| US-05 | 1.35 ± 0.07 a | 0.20 ± 0.00 | <0.10 | 0.55 ± 0.07 | 2.10 ± 0.00 a | 0.95 ± 0.07 a | <0.01 a | 2.95 ± 0.07 c | (2.81 ± 0.70) × 107 a |
| SD9 | 9.20 ± 0.14 d | 0.50 ± 0.00 | <0.10 | 0.40 ± 0.14 | 10.05 ± 0.07 b | 1.15 ± 0.07 b | 0.20 ± 0.10 c | 2.45 ± 0.20 ab | (9.25 ± 0.79) × 106 a |
| WN3 | 9.00 ± 0.00 d | 0.95 ± 0.07 | <0.10 | 0.50 ± 0.1 | 10.25 ± 0.07 b | 1.05 ± 0.07 ab | 0.14 ± 0.03 bc | 2.30 ± 0.21 a | (5.14 ± 0.48) × 107 ab |
| SD19 | 2.05 ± 0.07 b | 0.30 ± 0.01 | <0.10 | 0.30 ± 0.04 | 2.65 ± 0.07 a | 1.10 ± 0.00 ab | 0.08 ± 0.02 b | 2.80 ± 0.25 bc | (4.45 ± 0.14) × 107 ab |
| SD12 | 5.10 ± 0.00 c | 0.40 ± 0.00 | <0.10 | 0.50 ± 0.05 | 5.80 ± 0.00 ab | 1.05 ± 0.07 ab | 0.08 ± 0.02 b | 2.80 ± 0.14 bc | (6.98 ± 2.01) × 107 c |
| Sample | Maltotriose (g/L) | Maltose (g/L) | Glucose (g/L) | Fructose (g/L) | Total Sugars (g/L) | Glycerol (g/L) | Ethanol (%v/v) | pH | Total Phenolic Content (mg GAE/L) | FAN (mg/L) |
|---|---|---|---|---|---|---|---|---|---|---|
| FB US-05 | 4.15 ± 0.06 bc | 1.20 ± 0.07 abc | <0.10 | 0.81 ±0.05 b | 6.27 ± 0.04 c | 2.53 ± 0.05 b | 5.75 ± 0.21 bc | 3.55 ± 0.05 a | 748.07 ± 60.36 a | 14.65 ± 1.61 a |
| FB SD19 | 3.45 ± 0.07 a | 1.15 ± 0.07 ab | <0.10 | 0.42 ± 0.03 a | 5.10 ± 0.10 a | 3.06 ± 0.07 c | 6.05 ± 0.63 c | 3.69 ± 0.10 a | 824.05 ± 36.73 a | 17.78 ± 1.42 a |
| FB SD12 | 3.80 ± 0.01 ab | 0.98 ± 0.32 a | <0.10 | 0.42 ± 0.03 a | 5.27 ± 0.38 ab | 2.60 ± 0.07 b | 5.40 ± 0.15 abc | 3.78 ± 0.05 a | 767.02 ± 62.01 a | 21.02 ± 1.47 a |
| CB US-05 | 4.00 ± 0.14 bc | 1.65 ± 0.20 bc | <0.10 | 0.58 ± 0.08 a | 6.28 ± 0.20 c | 1.76 ± 0.15 a | 4.80 ± 0.30 abc | 4.12 ± 0.10 b | 743.82 ± 62.60 a | 24.00 ± 2.64 ab |
| CB SD19 | 4.25 ± 0.21 c | 1.25 ± 0.07 abc | <0.10 | 0.41 ± 0.01 a | 5.97 ± 0.30 bc | 1.92 ± 0.18 a | 4.60 ± 0.42 ab | 4.45 ± 0.05 bc | 700.30 ± 69.0 a | 31.54 ± 2.52 b |
| CB SD12 | 4.13 ± 0.04 bc | 1.85 ± 0.07 c | <0.10 | 0.43 ± 0.03 a | 6.52 ± 0.05 c | 1.87 ± 0.09 a | 4.05 ± 0.07 a | 4.58 ± 0.10 c | 716.61 ± 43.18 a | 64.60 ± 4.52 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galli, V.; Venturi, M.; Guerrini, S.; Mangani, S.; Barbato, D.; Vallesi, G.; Granchi, L. Exploitation of Selected Sourdough Saccharomyces cerevisiae Strains for the Production of a Craft Raspberry Fruit Beer. Foods 2023, 12, 3354. https://doi.org/10.3390/foods12183354
Galli V, Venturi M, Guerrini S, Mangani S, Barbato D, Vallesi G, Granchi L. Exploitation of Selected Sourdough Saccharomyces cerevisiae Strains for the Production of a Craft Raspberry Fruit Beer. Foods. 2023; 12(18):3354. https://doi.org/10.3390/foods12183354
Chicago/Turabian StyleGalli, Viola, Manuel Venturi, Simona Guerrini, Silvia Mangani, Damiano Barbato, Gianni Vallesi, and Lisa Granchi. 2023. "Exploitation of Selected Sourdough Saccharomyces cerevisiae Strains for the Production of a Craft Raspberry Fruit Beer" Foods 12, no. 18: 3354. https://doi.org/10.3390/foods12183354







