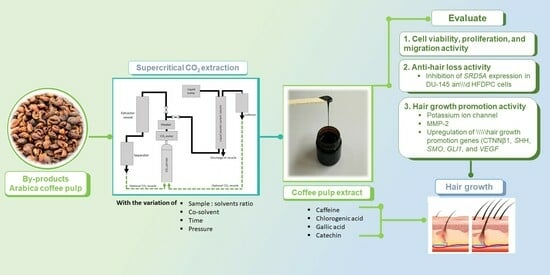
Hair Growth Promotion and Anti-Hair Loss Effects of By-Products Arabica Coffee Pulp Extracts Using Supercritical Fluid Extraction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Collection of Extract
2.3. Phytochemical Evaluations
2.3.1. Total Phenolic Content
2.3.2. Total Flavonoid Content
2.3.3. Quantitative Analysis of Phenolic Profiles and Caffeine Content by High-Performance Liquid Chromatography Analysis (HPLC)
2.3.4. Determination of Antioxidant Activities
DPPH Radical Scavenging Assay
ABTS Radical Scavenging Assay
2.4. In Vitro Cell Viability and Proliferation Assay
2.5. Cell Migration Assay
2.6. Cell-Based Potassium Ion Channel Assay
2.7. MMP-2 Activity Assay by Gelatinolytic Zymography
2.8. Semi-Quantitative Reverse Transcription and Polymerase Chain Reaction Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Extraction Process
3.2. Bioactive Constituents and Antioxidant Activities of Coffee Pulp Extracts
3.3. Effect of Coffee Pulp Extracts on Cell Viability and Proliferation
3.4. Effect of Coffee Pulp Extracts on Migration of HFDPCs
3.5. Effect of Coffee Pulp Extracts on Potassium Ion Channel in HFDPCs
3.6. Effect of Coffee Pulp Extracts on MMP-2 Activity Assay by Gelatinolytic Activity (Zymography)
3.7. Effects of Coffee Pulp Extracts on Genes Expression
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Organization, I.C. Coffee Market Report. Available online: https://www.ico.org/documents/cy2022-23/cmr-1222-e.pdf (accessed on 23 August 2023).
- Tran, H.T.; Ramaraj, T.; Furtado, A.; Lee, L.S.; Henry, R.J. Use of a draft genome of coffee (Coffea arabica) to identify SNP s associated with caffeine content. Plant Biotechnol. J. 2018, 16, 1756–1766. [Google Scholar] [CrossRef] [PubMed]
- Aristizábal-Marulanda, V.; Chacón-Perez, Y.; Alzate, C.A.C. The biorefinery concept for the industrial valorization of coffee processing by-products. In Handbook of Coffee Processing By-Products; Elsevier: Amsterdam, The Netherlands, 2017; pp. 63–92. [Google Scholar]
- Murthy, P.S.; Naidu, M.M. Recovery of phenolic antioxidants and functional compounds from coffee industry by-products. Food Bioprocess Technol. 2012, 5, 897–903. [Google Scholar] [CrossRef]
- Esquivel, P.; Viñas, M.; Steingass, C.B.; Gruschwitz, M.; Guevara, E.; Carle, R.; Schweiggert, R.M.; Jiménez, V.M. Coffee (Coffea arabica L.) by-products as a source of carotenoids and phenolic compounds—Evaluation of varieties with different peel color. Front. Sustain. Food Syst. 2020, 4, 590–597. [Google Scholar] [CrossRef]
- Fischer, T.; Herczeg-Lisztes, E.; Funk, W.; Zillikens, D.; Bíró, T.; Paus, R. Differential effects of caffeine on hair shaft elongation, matrix and outer root sheath keratinocyte proliferation, and transforming growth factor-β2/insulin-like growth factor-1-mediated regulation of the hair cycle in male and female human hair follicles in vitro. Br. J. Dermatol. 2014, 171, 1031–1043. [Google Scholar] [PubMed]
- Wang, W.; Rao, L.; Wu, X.; Wang, Y.; Zhao, L.; Liao, X. Supercritical carbon dioxide applications in food processing. Food Eng. Rev. 2021, 13, 570–591. [Google Scholar] [CrossRef]
- Shankar, D.K.; Chakravarthi, M.; Shilpakar, R. Male androgenetic alopecia: Population-based study in 1005 subjects. Int. J. Trichology 2009, 1, 131–133. [Google Scholar] [CrossRef]
- Ring, C.M.; Finney, R.; Avram, M. Lasers, lights, and compounds for hair loss in aesthetics. Clin. Dermatol. 2022, 40, 64–75. [Google Scholar] [CrossRef]
- Khantham, C.; Ruksiriwanich, W.; Sringarm, K.; Prom-u-thai, C.; Jamjod, S.; Arjin, C.; Muangsanguan, A.; Rachtanapun, P.; Jantanasakulwong, K.; Phimolsiripol, Y. Effects of bioactive composition in Oryza sativa L. cv. KDML105 bran extract on gene expression related to hair cycle in human hair follicle dermal papilla cells. Agronomy 2023, 13, 295. [Google Scholar] [CrossRef]
- Kwack, M.H.; Sung, Y.K.; Chung, E.J.; Im, S.U.; Ahn, J.S.; Kim, M.K.; Kim, J.C. Dihydrotestosterone-inducible dickkopf 1 from balding dermal papilla cells causes apoptosis in follicular keratinocytes. J. Investig. Dermatol. 2008, 128, 262–269. [Google Scholar] [CrossRef]
- Hou, C.; Miao, Y.; Wang, J.; Wang, X.; Chen, C.-Y.; Hu, Z.-Q. Collagenase IV plays an important role in regulating hair cycle by inducing VEGF, IGF-1, and TGF-β expression. Drug Des. Dev. Ther. 2015, 9, 5373–5383. [Google Scholar] [CrossRef]
- Shorter, K.; Farjo, N.P.; Picksley, S.M.; Randall1, V.A. Human hair follicles contain two forms of ATP-sensitive potassium channels, only one of which is sensitive to minoxidil. FASEB J. 2008, 22, 1725–1736. [Google Scholar] [CrossRef] [PubMed]
- Jaller, J.A.; MacQuhae, F.; Nichols, A. Chapter 26-Clinical Trials and Hair Loss; Alopecia, Miteva, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 267–284. [Google Scholar]
- Suchonwanit, P.; Thammarucha, S.; Leerunyakul, K. Minoxidil and its use in hair disorders: A review. Drug Des. Dev. Ther. 2019, 13, 2777–2786. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.Y. Targeting Wnt/β-Catenin Pathway for Developing Therapies for Hair Loss. Int. J. Mol. Sci. 2020, 21, 4915. [Google Scholar] [CrossRef]
- Hou, C.; Miao, Y.; Wang, X.; Chen, C.; Lin, B.; Hu, Z. Expression of matrix metalloproteinases and tissue inhibitor of matrix metalloproteinases in the hair cycle. Exp. Ther. Med. 2016, 12, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Valadez-Carmona, L.; Ortiz-Moreno, A.; Ceballos-Reyes, G.; Mendiola, J.A.; Ibáñez, E. Valorization of cacao pod husk through supercritical fluid extraction of phenolic compounds. J. Supercrit. Fluids 2018, 131, 99–105. [Google Scholar] [CrossRef]
- Sunanta, P.; Chung, H.H.; Kunasakdakul, K.; Ruksiriwanich, W.; Jantrawut, P.; Hongsibsong, S.; Sommano, S.R. Genomic relationship and physiochemical properties among raw materials used for Thai black garlic processing. Food Sci. Nutr. 2020, 8, 4534–4545. [Google Scholar] [CrossRef]
- Nazir, Y.; Linsaenkart, P.; Khantham, C.; Chaitep, T.; Jantrawut, P.; Chittasupho, C.; Rachtanapun, P.; Phimolsiripol, Y.; Sommano, S.R.; Tocharus, J.; et al. High efficiency in vitro wound healing of Dictyophora indusiata extracts via anti-Inflammatory and collagen stimulating (MMP-2 inhibition) mechanisms. J. Fungi 2021, 7, 1100. [Google Scholar] [CrossRef]
- Sangta, J.; Wongkaew, M.; Tangpao, T.; Withee, P.; Haituk, S.; Arjin, C.; Sringarm, K.; Hongsibsong, S.; Sutan, K.; Pusadee, T. Recovery of polyphenolic fraction from arabica coffee pulp and its antifungal applications. Plants 2021, 10, 1422. [Google Scholar] [CrossRef]
- Ruksiriwanich, W.; Khantham, C.; Sringarm, K.; Sommano, S.; Jantrawut, P. Depigmented Centella asiatica extraction by pretreated with supercritical carbon dioxide fluid for wound healing application. Processes 2020, 8, 277. [Google Scholar] [CrossRef]
- Adedapo, A.A.; Jimoh, F.O.; Afolayan, A.J.; Masika, P.J. Antioxidant activities and phenolic contents of the methanol extracts of the stems of Acokanthera oppositifolia and Adenia gummifera. BMC Complement. Altern. Med. 2008, 8, 54. [Google Scholar] [CrossRef]
- Khantham, C.; Yooin, W.; Sringarm, K.; Sommano, S.R.; Jiranusornkul, S.; Carmona, F.D.; Nimlamool, W.; Jantrawut, P.; Rachtanapun, P.; Ruksiriwanich, W. Effects on steroid 5-alpha reductase gene expression of Thai rice bran extracts and molecular dynamics study on SRD5A2. Biology 2021, 10, 319. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, S.N.; Jeong, G.; Hong, M.J.; Lee, Y.; Shin, S.H.; Park, H.; Jung, Y.C.; Kim, E.J.; Park, B.C. Efficacy of caffeine in promoting hair growth by enhancing intracellular activity of hair follicles. Korea J. Cosmet. Sci. 2019, 1, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Linsaenkart, P.; Ruksiriwanich, W.; Jantrawut, P.; Chittasupho, C.; Rachtanapun, P.; Jantanasakulwong, K.; Sommano, S.R.; Prom-U-Thai, C.; Jamjod, S.; Arjin, C. Natural melanogenesis inhibitor, antioxidant, and collagen biosynthesis stimulator of phytochemicals in rice Bran and husk extracts from purple glutinous rice (Oryza sativa L. cv. Pieisu 1 CMU) for cosmetic application. Plants 2023, 12, 970. [Google Scholar] [CrossRef]
- Ruksiriwanich, W.; Khantham, C.; Muangsanguan, A.; Phimolsiripol, Y.; Barba, F.J.; Sringarm, K.; Rachtanapun, P.; Jantanasakulwong, K.; Jantrawut, P.; Chittasupho, C. Guava (Psidium guajava L.) leaf extract as bioactive substances for anti-androgen and antioxidant activities. Plants 2022, 11, 3514. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.J.; Nathia-Neves, G.; Vardanega, R.; Meireles, M.A.A.; da Silva, E.A.; Vieira, M.G.A. Supercritical CO2 extraction of α-/β-amyrin from uvaia (Eugenia pyriformis Cambess.): Effects of pressure and co-solvent addition. J. Supercrit. Fluids 2019, 153, 104595. [Google Scholar] [CrossRef]
- Radzali, S.A.; Markom, M.; Saleh, N.M. Co-solvent selection for supercritical fluid extraction (SFE) of phenolic compounds from Labisia pumila. Molecules 2020, 25, 5859. [Google Scholar] [CrossRef]
- Dassoff, E.S.; Li, Y.O. Mechanisms and effects of ultrasound-assisted supercritical CO2 extraction. Trends Food Sci. Technol. 2019, 86, 492–501. [Google Scholar] [CrossRef]
- Kim, D.O.; Lee, C.Y. Extraction and isolation of polyphenolics. Curr. Protoc. Food Anal. Chem. 2002, 6, I1.2.1–I1.2.12. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Phenolics in cereals, fruits and vegetables: Occurrence, extraction and analysis. J. Pharm. Biomed. Anal. 2006, 41, 1523–1542. [Google Scholar] [CrossRef]
- Saewan, N. Effect of coffee berry extract on anti-aging for skin and hair—In vitro approach. Cosmetics 2022, 9, 66. [Google Scholar] [CrossRef]
- Matsubara, T.; Taniguchi, S.; Morimoto, S.; Yano, A.; Hara, A.; Wataoka, I.; Urakawa, H.; Yasunaga, H. Relationship between Dyeing Condition and Dyeability in Hair Colouring by Using Catechinone Prepared Enzymatically or Chemically from (+)-Catechin. J. Cosmet. Dermatol. Sci. Appl. 2015, 5, 94. [Google Scholar] [CrossRef]
- Barajas-Álvarez, P.; Castillo-Herrera, G.A.; Guatemala-Morales, G.M.; Corona-González, R.I.; Arriola-Guevara, E.; Espinosa-Andrews, H. Supercritical CO2-ethanol extraction of oil from green coffee beans: Optimization conditions and bioactive compound identification. J. Food Sci. Technol. 2021, 58, 4514–4523. [Google Scholar] [CrossRef] [PubMed]
- Andrade, K.S.; Gonçalvez, R.T.; Maraschin, M.; Ribeiro-do-Valle, R.M.; Martínez, J.; Ferreira, S.R. Supercritical fluid extraction from spent coffee grounds and coffee husks: Antioxidant activity and effect of operational variables on extract composition. Talanta 2012, 88, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.J.; Chang, S. A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J. Food Sci. 2007, 72, S159–S166. [Google Scholar] [CrossRef]
- Santana, Á.L.; Macedo, G.A. Effects of hydroalcoholic and enzyme-assisted extraction processes on the recovery of catechins and methylxanthines from crude and waste seeds of guarana (Paullinia cupana). Food Chem. 2019, 281, 222–230. [Google Scholar] [CrossRef]
- ISO 10993-5; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneve, Switzerland, 2009.
- Williams, R.; Pawlus, A.D.; Thornton, M.J. Getting under the skin of hair aging: The impact of the hair follicle environment. Exp. Dermatol. 2020, 29, 588–597. [Google Scholar] [CrossRef]
- Elliott, K.; Messenger, A.G.; Stephenson, T.J. Differences in hair follicle dermal papilla volume are due to extracellular matrix volume and cell number: Implications for the control of hair follicle size and androgen responses. J. Investig. Dermatol. 1999, 113, 873–877. [Google Scholar] [CrossRef]
- Park, S.; Kang, W.; Choi, D.; Son, B.; Park, T. Nonanal stimulates growth factors via cyclic adenosine monophosphate (cAMP) signaling in human hair follicle dermal papilla cells. Int. J. Mol. Sci. 2020, 21, 8054. [Google Scholar] [CrossRef]
- Wang, J.; Shen, H.; Chen, T.; Ma, L. Hair growth-promoting effects of Camellia seed cake extract in human dermal papilla cells and C57BL/6 mice. J. Cosmet. Dermatol. 2022, 21, 5018–5025. [Google Scholar] [CrossRef]
- Ramos, P.M.; Sinclair, R.D.; Kasprzak, M.; Miot, H.A. Minoxidil 1 mg oral versus minoxidil 5% topical solution for the treatment of female-pattern hair loss: A randomized clinical trial. J. Am. Acad. Dermatol. 2020, 82, 252–253. [Google Scholar] [CrossRef] [PubMed]
- Urrego, D.; Tomczak, A.P.; Zahed, F.; Stühmer, W.; Pardo, L.A. Potassium channels in cell cycle and cell proliferation. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130094. [Google Scholar] [CrossRef] [PubMed]
- Wuttisin, N.; Nararatwanchai, T.; Sarikaphuti, A. Matrix metalloproteinase-2 inhibition activity of Plukenetia volubilis L. leaves extract for anti-aging application. Food Res. 2021, 5, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Ruksiriwanich, W.; Linsaenkart, P.; Khantham, C.; Muangsanguan, A.; Sringarm, K.; Jantrawut, P.; Prom-U-Thai, C.; Jamjod, S.; Yamuangmorn, S.; Arjin, C. Regulatory effects of thai rice by-product extracts from Oryza sativa L. cv. Bue Bang 3 CMU and Bue Bang 4 CMU on melanin production, nitric oxide secretion, and steroid 5α-reductase inhibition. Plants 2023, 12, 653. [Google Scholar] [CrossRef] [PubMed]
- Dhariwala, M.Y.; Ravikumar, P. An overview of herbal alternatives in androgenetic alopecia. J. Cosmet. Dermatol. 2019, 18, 966–975. [Google Scholar] [CrossRef]
- Wisetkomolmat, J.; Arjin, C.; Satsook, A.; Seel-Audom, M.; Ruksiriwanich, W.; Prom-u-Thai, C.; Sringarm, K. Comparative analysis of nutritional components and phytochemical attributes of selected Thai rice bran. Front. Nutr. 2022, 9, 833730. [Google Scholar] [CrossRef]
- Manosroi, A.; Ruksiriwanich, W.; Abe, M.; Sakai, H.; Aburai, K.; Manosroi, W.; Manosroi, J. Physico-chemical properties of cationic niosomes loaded with fraction of rice (Oryza sativa) bran extract. J. Nanosci. Nanotechnol. 2012, 12, 7339–7345. [Google Scholar] [CrossRef]
- Manosroi, A.; Ruksiriwanich, W.; Manosroi, W.; Abe, M.; Manosroi, J. In vivo hair growth promotion activity of gel containing niosomes loaded with the Oryza sativa bran fraction (OSF3). Adv. Sci. Lett. 2012, 16, 222–228. [Google Scholar] [CrossRef]
- Khantham, C.; Linsaenkart, P.; Chaitep, T.; Jantrawut, P.; Chittasupho, C.; Rachtanapun, P.; Jantanasakulwong, K.; Phimolsiripol, Y.; Sommano, S.R.; Prom-u-thai, C.; et al. Antioxidation, Anti-Inflammation, and Regulation of SRD5A Gene Expression of Oryza sativa cv. Bue Bang 3 CMU Husk and Bran Extracts as Androgenetic Alopecia Molecular Treatment Substances. Plants 2022, 11, 330. [Google Scholar] [CrossRef]
- Ruksiriwanich, W.; Khantham, C.; Linsaenkart, P.; Chaitep, T.; Jantrawut, P.; Chittasupho, C.; Rachtanapun, P.; Jantanasakulwong, K.; Phimolsiripol, Y.; Sommano, S.R.; et al. In Vitro and in Vivo regulation of SRD5A mRNA expression of supercritical carbon dioxide extract from Asparagus racemosus willd. root as anti-sebum and pore-minimizing active ingredients. Molecules 2022, 27, 1535. [Google Scholar] [CrossRef]
- Schmidt-Ullrich, R.; Paus, R. Molecular principles of hair follicle induction and morphogenesis. Bioessays 2005, 27, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Ruksiriwanich, W.; Khantham, C.; Muangsanguan, A.; Chittasupho, C.; Rachtanapun, P.; Jantanasakulwong, K.; Phimolsiripol, Y.; Sommano, S.R.; Sringarm, K.; Ferrer, E. Phytochemical constitution, anti-inflammation, anti-androgen, and hair growth-promoting potential of shallot (Allium ascalonicum L.) extract. Plants 2022, 11, 1499. [Google Scholar] [CrossRef] [PubMed]
- Huelsken, J.; Vogel, R.; Erdmann, B.; Cotsarelis, G.; Birchmeier, W. β-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 2001, 105, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, E.; Merrill, B.J.; Jamora, C.; DasGupta, R. At the roots of a never-ending cycle. Dev. Cell 2001, 1, 13–25. [Google Scholar] [CrossRef]
- Widelitz, R.B. Wnt signaling in skin organogenesis. Organogenesis 2008, 4, 123–133. [Google Scholar] [CrossRef]
- Suzuki, K.; Yamaguchi, Y.; Villacorte, M.; Mihara, K.; Akiyama, M.; Shimizu, H.; Taketo, M.M.; Nakagata, N.; Tsukiyama, T.; Yamaguchi, T.P. Embryonic hair follicle fate change by augmented β-catenin through Shh and Bmp signaling. Development 2009, 136, 367–372. [Google Scholar] [CrossRef]
- Zhang, H.; Nan, W.; Wang, S.; Song, X.; Si, H.; Li, T.; Li, G. Epigallocatechin-3-gallate promotes the growth of mink hair follicles through sonic hedgehog and protein kinase B signaling pathways. Front. Pharmacol. 2018, 9, 674. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, H.; Jing, J.; Yu, L.; Wu, X.; Lu, Z. Regulation of hair follicle development by exosomes derived from dermal papilla cells. Biochem. Biophys. Res. Commun. 2018, 500, 325–332. [Google Scholar] [CrossRef]
- Paladini, R.D.; Saleh, J.; Qian, C.; Xu, G.-X.; Rubin, L.L. Modulation of hair growth with small molecule agonists of the hedgehog signaling pathway. J. Investig. Dermatol. 2005, 125, 638–646. [Google Scholar] [CrossRef]
- Cebe-Suarez, S.; Zehnder-Fjällman, A.; Ballmer-Hofer, K. The role of VEGF receptors in angiogenesis; complex partnerships. Cell. Mol. Life Sci. 2006, 63, 601–615. [Google Scholar] [CrossRef]
- Kubanov, A.; Gallyamova, Y.A.; Korableva, O. The study of growth factors in patients with androgenic alopecia. Biomed. Pharmacol. J. 2017, 10, 1219–1228. [Google Scholar] [CrossRef]
- de Oliveira Formiga, R.; Júnior, E.B.A.; Vasconcelos, R.C.; Araújo, A.A.; de Carvalho, T.G.; de Araújo Junior, R.F.; Guerra, G.B.C.; Vieira, G.C.; de Oliveira, K.M.; Diniz, M.d.F.F.M. Effect of p-cymene and rosmarinic acid on gastric ulcer healing–Involvement of multiple endogenous curative mechanisms. Phytomedicine 2021, 86, 153497. [Google Scholar] [CrossRef] [PubMed]
- Herman, A.; Herman, A.P. Mechanism of action of herbs and their active constituents used in hair loss treatment. Fitoterapia 2016, 114, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.B. Male hormone stimulation is prerequisite and an incitant in common baldness. Am. J. Anat. 1942, 71, 451–480. [Google Scholar] [CrossRef]
- Bejaoui, M.; Villareal, M.O.; Isoda, H. β-catenin-mediated hair growth induction effect of 3, 4, 5-tri-O-caffeoylquinic acid. Aging 2019, 11, 4216. [Google Scholar] [CrossRef] [PubMed]
- Hussein, R.M.; Anwar, M.M.; Farghaly, H.S.; Kandeil, M.A. Gallic acid and ferulic acid protect the liver from thioacetamide-induced fibrosis in rats via differential expression of miR-21, miR-30 and miR-200 and impact on TGF-β1/Smad3 signaling. Chem. Biol. Interact. 2020, 324, 109098. [Google Scholar] [CrossRef]
- Trakoolthong, P.; Ditthawuttikul, N.; Sivamaruthi, B.S.; Sirilun, S.; Rungseevijitprapa, W.; Peerajan, S.; Chaiyasut, C. Antioxidant and 5α-Reductase inhibitory activity of Momordica charantia extract, and development and characterization of microemulsion. Appl. Sci. 2022, 12, 4410. [Google Scholar] [CrossRef]





| No. | Ratio (Sample: Co-Solvent) | Co-Solvent | Time (min) | Pressure (bar) |
|---|---|---|---|---|
| SFE-1 | 1:0 | - | 10 | 300 |
| SFE-2 | 1:2 | 95% (v/v) Ethanol | 10 | 300 |
| SFE-3 | 1:2 | 50% (v/v) Ethanol | 30 | 300 |
| SFE-4 | 1:2 | 50% (v/v) Ethanol | 30 | 500 |
| SFE-5 | 1:2 | 25% (v/v) Ethanol | 30 | 500 |
| SFE-6 | 1:2 | Distilled water | 30 | 500 |
| Pathway | Gene | Accession Number | Forward Sequence | Reverse Sequence |
|---|---|---|---|---|
| Sonic Hedgehog | SHH | NM_000193.4 | AAAAGCTGACCCCTTTAGCC | GCTCCGGTGTTTTCTTCATC |
| SMO | NM_005631.5 | GAAGTGCCCTTGGTTCGGACA | CCGCCAGTCAGCCACGAAT | |
| GLI1 | NM_005269.3 | GCAGGGAGTGCAGCCAATACAG | GAGCGGCGGCTGACAGTATA | |
| Wnt/β-catenin | CTNNB1 | NM_001330729.2 | CCCACTAATGTCCAGCGTTT | AACCAAGCATTTTCACCAGG |
| Angiogenesis | VEGF | NM_001025366.3 | CTACCTCCACCATGCCAAGT | GCGAGTCTGTGTTTTTGCAG |
| 5α-reductase | SRD5A1 | NM_001047.4 | AGCCATTGTGCAGTGTATGC | AGCCTCCCCTTGGTATTTTG |
| SRD5A2 | NM_000348.4 | TGAATACCCTGATGGGTGG | CAAGCCACCTTGTGGAATC | |
| SRD5A3 | NM_024592.5 | TCCTTCTTTGCCCAAACATC | TCCTTCTTTGCCCAAACATC | |
| Internal control | GAPDH | NM_001289745.3 | GGAAGGTGAAGGTCGGAGTC | CTCAGCCTTGACGGTGCCATG |
| Extract No. | Extraction Yield (%) | Total Phenolic Content | Total Flavonoid Content | Caffeine Content | Chlorogenic Acid Content | Gallic Acid Content | Catechin Gallate Content | Antioxidant Activities (%) | |
|---|---|---|---|---|---|---|---|---|---|
| (mg GAE/g Dry Weight) | (mg EGCG/g Dry Weight) | (mg CAF/g Dry Weight) | mg CGA/Dry Weight | mg GAE/g Dry Weight | mg CG/g Dry Weight | ABTS | DPPH | ||
| SFE-1 | 0.70 ± 0.14 a | 0.10 ± 0.01 a | 0.05 ± 0.01 a | 5.18 ± 0.84 c | 0.08 ± 0.01 b | 0.06 ± 0.01 b | 0.08 ± 0.01 c | 8.75 ± 0.26 a | 9.50 ± 0.96 a |
| SFE-2 | 6.15 ± 0.63 b | 3.14 ± 0.31 b | 2.12 ± 0.12 c | 15.13 ± 1.12 e | 0.02 ± 0.01 a | 0.01 ± 0.01 a | 0.02 ± 0.01 a | 46.41 ± 2.96 c | 34.25 ± 0.22 c |
| SFE-3 | 5.70 ± 0.95 b | 3.30 ± 0.20 b | 1.99 ± 0.13 b | 4.07 ± 0.56 a | 0.15 ± 0.01 c | 0.12 ± 0.01 c | 0.06 ± 0.01 b | 51.59 ± 2.95 d | 34.66 ± 2.17 d |
| SFE-4 | 8.60 ± 0.13 c | 5.78 ± 0.03 c | 7.43 ± 0.13 e | 19.49 ± 1.04 f | 0.42 ± 0.02 e | 0.24 ± 0.01 d | 0.12 ± 0.01 d | 56.63 ± 1.13 e | 36.49 ± 1.24 e |
| SFE-5 | 11.50 ± 0.66 d | 5.67 ± 0.35 c | 2.99 ± 0.11 d | 4.91 ± 0.62 b | 0.55 ± 0.02 f | 0.31 ± 0.02 e | 0.26 ± 0.01 e | 46.20 ± 2.86 c | 33.47 ± 1.87 b,c |
| SFE-6 | 16.40 ± 0.28 e | 3.27 ± 0.29 b | 2.15 ± 0.10 c | 8.13 ± 1.01 d | 0.18 ± 0.01 d | 0.13 ± 0.01 c | 0.07 ± 0.01 b, c | 44.22 ± 1.52 b | 32.35 ± 1.62 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muangsanguan, A.; Linsaenkart, P.; Chaitep, T.; Sangta, J.; Sommano, S.R.; Sringarm, K.; Arjin, C.; Rachtanapun, P.; Jantanasakulwong, K.; Phimolsiripol, Y.; et al. Hair Growth Promotion and Anti-Hair Loss Effects of By-Products Arabica Coffee Pulp Extracts Using Supercritical Fluid Extraction. Foods 2023, 12, 4116. https://doi.org/10.3390/foods12224116
Muangsanguan A, Linsaenkart P, Chaitep T, Sangta J, Sommano SR, Sringarm K, Arjin C, Rachtanapun P, Jantanasakulwong K, Phimolsiripol Y, et al. Hair Growth Promotion and Anti-Hair Loss Effects of By-Products Arabica Coffee Pulp Extracts Using Supercritical Fluid Extraction. Foods. 2023; 12(22):4116. https://doi.org/10.3390/foods12224116
Chicago/Turabian StyleMuangsanguan, Anurak, Pichchapa Linsaenkart, Tanakarn Chaitep, Jiraporn Sangta, Sarana Rose Sommano, Korawan Sringarm, Chaiwat Arjin, Pornchai Rachtanapun, Kittisak Jantanasakulwong, Yuthana Phimolsiripol, and et al. 2023. "Hair Growth Promotion and Anti-Hair Loss Effects of By-Products Arabica Coffee Pulp Extracts Using Supercritical Fluid Extraction" Foods 12, no. 22: 4116. https://doi.org/10.3390/foods12224116
APA StyleMuangsanguan, A., Linsaenkart, P., Chaitep, T., Sangta, J., Sommano, S. R., Sringarm, K., Arjin, C., Rachtanapun, P., Jantanasakulwong, K., Phimolsiripol, Y., Castagnini, J. M., & Ruksiriwanich, W. (2023). Hair Growth Promotion and Anti-Hair Loss Effects of By-Products Arabica Coffee Pulp Extracts Using Supercritical Fluid Extraction. Foods, 12(22), 4116. https://doi.org/10.3390/foods12224116