The Authentication of Gayo Arabica Green Coffee Beans with Different Cherry Processing Methods Using Portable LED-Based Fluorescence Spectroscopy and Chemometrics Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Green Coffee Bean Samples
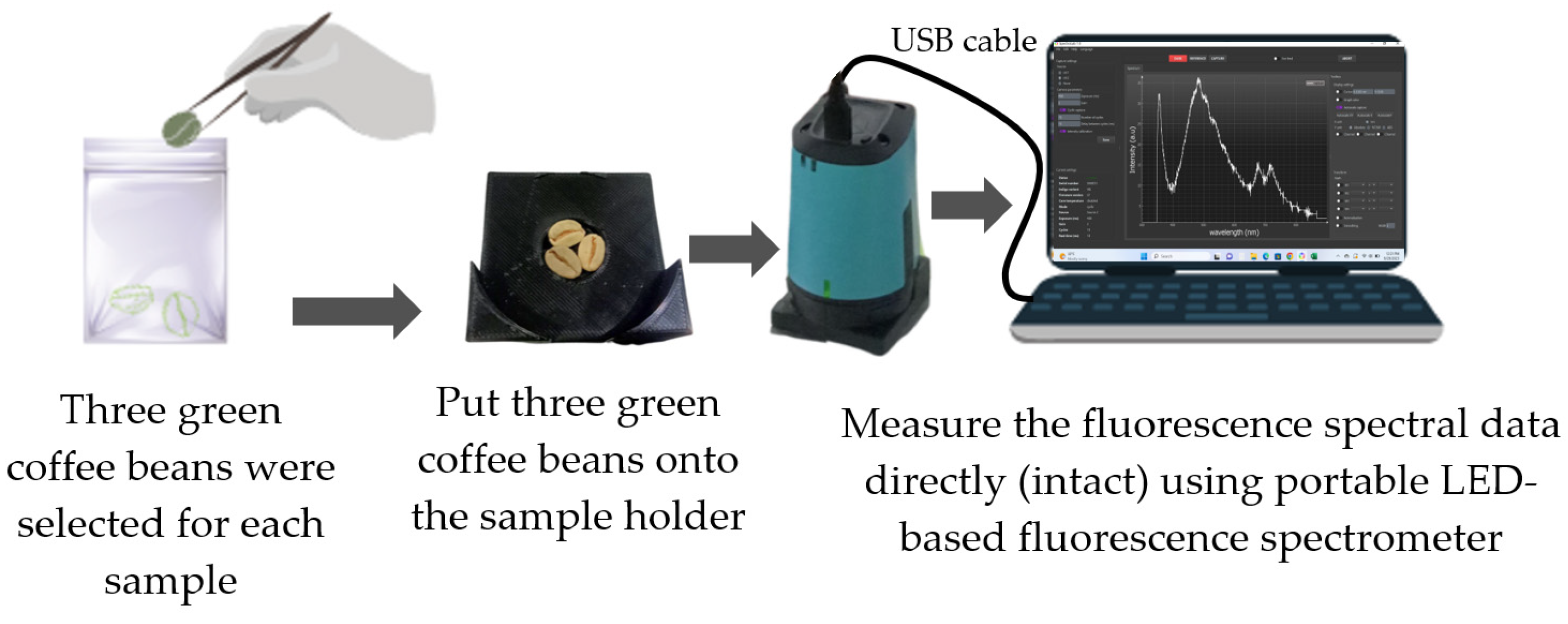
2.2. Spectral Acquisition
2.3. Chemometrics Analysis
3. Results and Discussion
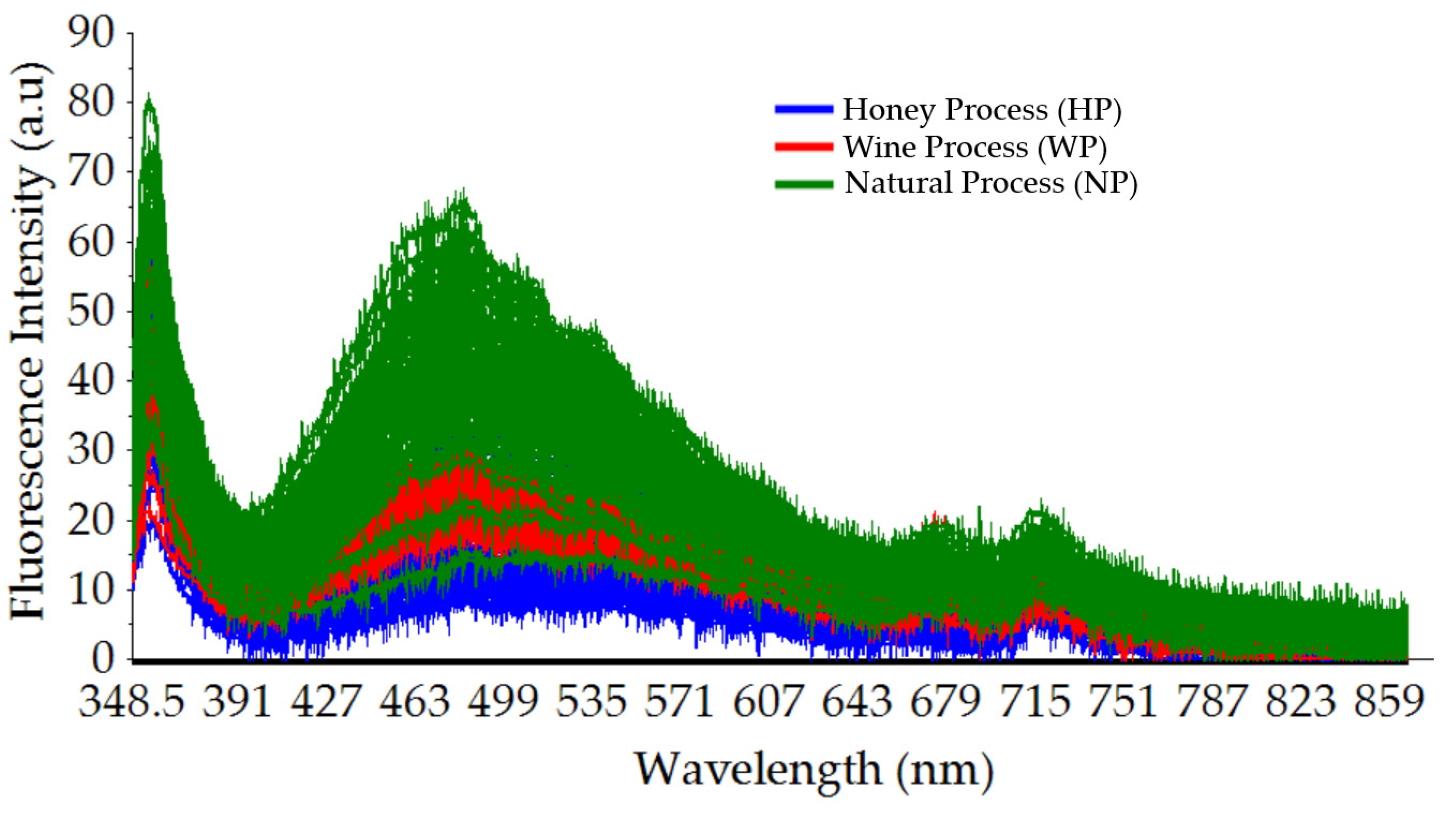
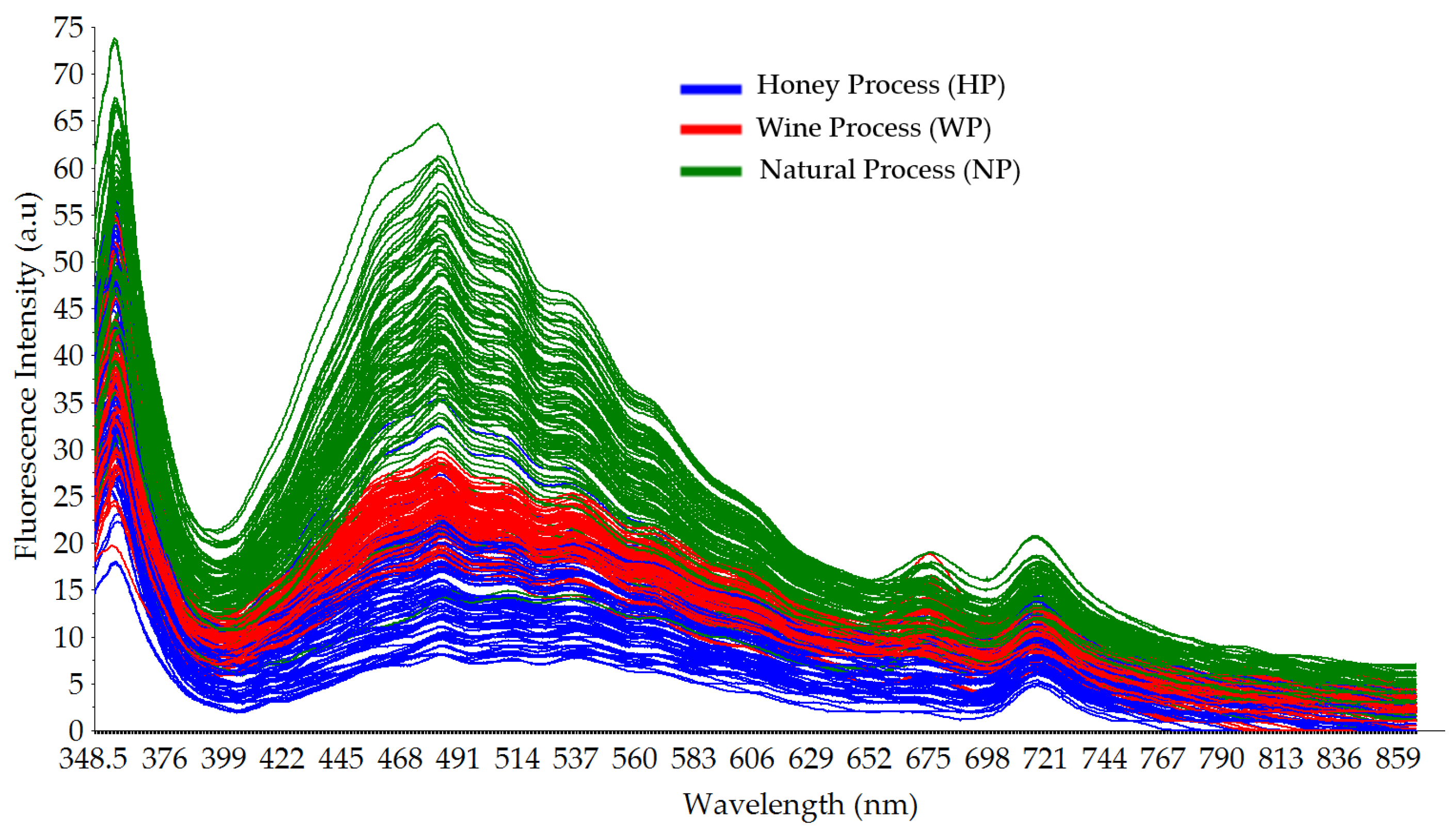
3.1. Typical Fluorescence Spectral Data
3.2. PCA Analysis
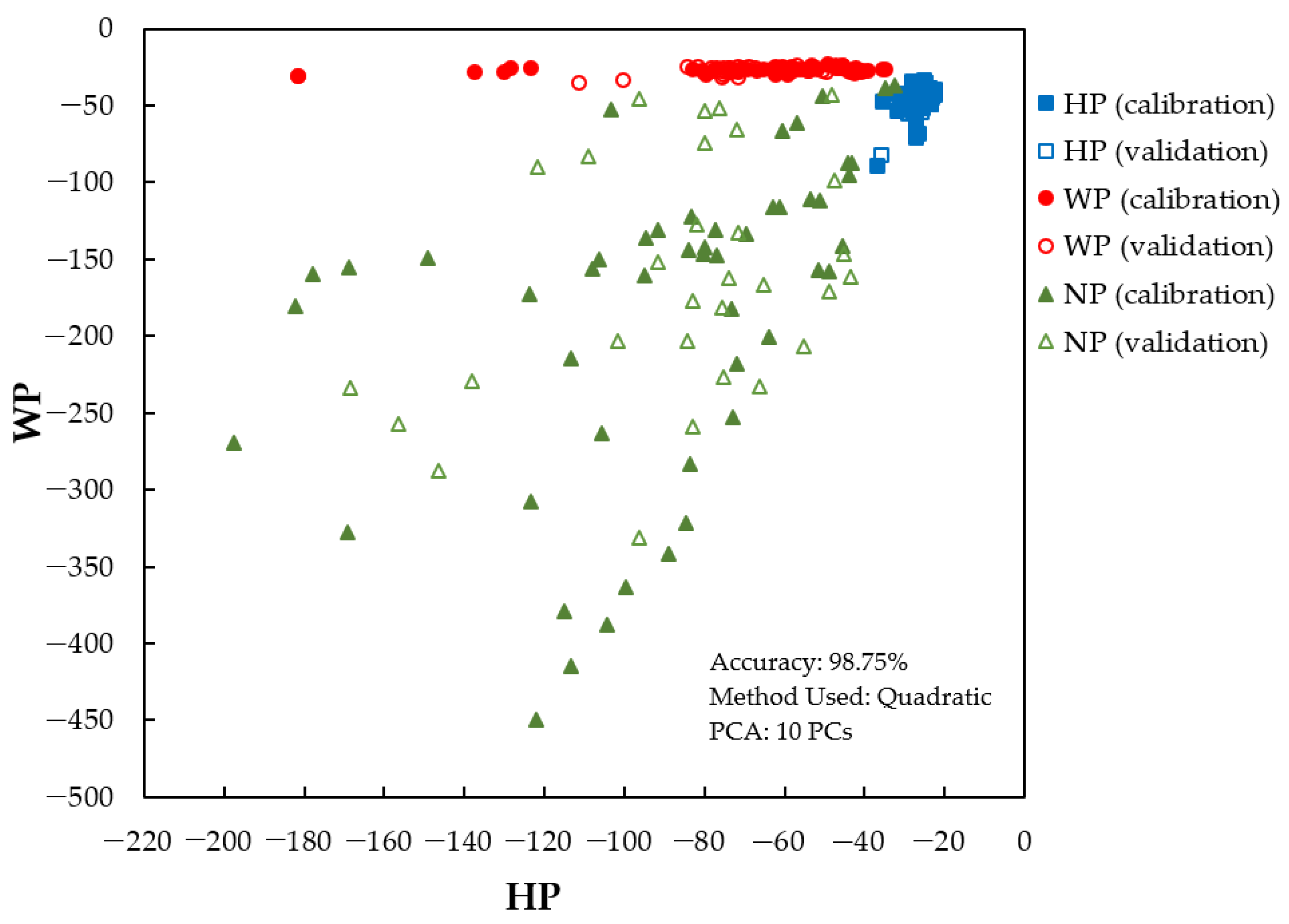
3.3. Classification Model Development Result Using PLS-DA, LDA, and PCA-LDA
3.4. The Result of Predictions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahmah, D.M.; Putra, A.S.; Ishizaki, R.; Noguchi, R.; Ahamed, T. A Life Cycle Assessment of Organic and Chemical Fertilizers for Coffee Production to Evaluate Sustainability toward the Energy–Environment–Economic Nexus in Indonesia. Sustainability 2022, 14, 3912. [Google Scholar] [CrossRef]
- Fadhil, R.; Maarif, M.S.; Bantacut, T.; Hermawan, A. A prospective Strategy for Institutional Development of Gayo Coffee Agroindustry in Aceh Province, Indonesia. Bulg. J. Agric. Sci. 2018, 24, 959–966. [Google Scholar]
- Suhandy, D.; Yulia, M. The Classification of Arabica Gayo Wine Coffee using UV-Visible Spectroscopy and PCA-DA Method. In Proceedings of the 3rd Annual Applied Science and Engineering Conference (AASEC 2018), Bandung, Indonesia, 18 April 2018. [Google Scholar] [CrossRef]
- Taufiqurrahman; Fajri; Hakim, L. The Value-Added Analysis of Gayo Arabica Coffee Based on Processing. Int. J. Multicult. Multirelig. Underst. 2020, 7, 56–59. [Google Scholar]
- Suhandy, D.; Yulia, M. Classification of Lampung Robusta Specialty Coffee According to Differences in Cherry Processing Methods Using UV Spectroscopy and Chemometrics. Agriculture 2021, 11, 109. [Google Scholar] [CrossRef]
- García, M.; Candelo-Becerra, J.E.; Hoyos, F.E. Quality and Defect Inspection of Green Coffee Beans Using a Computer Vision System. Appl. Sci. 2019, 9, 4195. [Google Scholar] [CrossRef]
- Wang, X.; Lim, L.-T.; Fu, Y. Review of Analytical Methods to Detect Adulteration in Coffee. J. AOAC Int. 2020, 103, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Núñez, N.; Collado, X.; Martínez, C.; Saurina, J.; Núñez, O. Authentication of the Origin, Variety and Roasting Degree of Coffee Samples by Non-Targeted HPLC-UV Fingerprinting and Chemometrics. Application to the Detection and Quantitation of Adulterated Coffee Samples. Foods 2020, 9, 378. [Google Scholar] [CrossRef]
- Weldegebreal, B.; Redi-Abshiro, M.; Chandravanshi, B.S. Development of New Analytical Methods for the Determination of Caffeine Content in Aqueous Solution of Green Coffee Beans. Chem. Cent. J. 2017, 11, 126. [Google Scholar] [CrossRef]
- El-Abassy, R.M.; Donfack, P.; Materny, A. Discrimination Between Arabica and Robusta Green Coffee Using Visible Micro Raman Spectroscopy and Chemometric Analysis. Food Chem. 2011, 126, 1443–1448. [Google Scholar] [CrossRef]
- Luna, A.S.; da Silva, A.P.; da Silva, C.S.; Lima, I.C.A.; de Gois, J.S. Chemometric Methods for Classification of Clonal Varieties of Green Coffee Using Raman Spectroscopy and Direct Sample Analysis. J. Food Compos. Anal. 2019, 76, 44–50. [Google Scholar] [CrossRef]
- Okubo, N.; Kurata, Y. Nondestructive Classification Analysis of Green Coffee Beans by Using Near-Infrared Spectroscopy. Foods 2019, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- Mutz, Y.S.; do Rosario, D.; Galvan, D.; Schwan, R.F.; Bernardes, P.C.; Conte-Junior, C.A. Feasibility of NIR Spectroscopy Coupled with Chemometrics for Classification of Brazilian Specialty Coffee. Food Control 2023, 149, 109696. [Google Scholar] [CrossRef]
- Guerrero, A.M.D.; Cabrera, L.V.P.; Reyes, T.F.; Izaguirre, R.O. Laser-Induced Breakdown Spectroscopy (LIBS) Applied in the Differentiation of Arabica and Robusta Coffee. Opt. Photon. J. 2017, 7, 79965. [Google Scholar] [CrossRef]
- Wei, F.; Furihata, K.; Koda, M.; Hu, F.; Kato, R.; Miyakawa, T.; Tanokura, M. 13C NMR-Based Metabolomics for the Classification of Green Coffee Beans According to Variety and Origin. J. Agric. Food Chem. 2012, 60, 10118–10125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, C.; Liu, F.; He, Y. Mid-Infrared Spectroscopy for Coffee Variety Identification: Comparison of Pattern Recognition Methods. J. Spectrosc. 2016, 2016, 7927286. [Google Scholar] [CrossRef]
- Giraudo, A.; Grassi, S.; Savorani, F.; Gavoci, G.; Casiraghi, E.; Geobaldo, F. Determination of the Geographical Origin of Green Coffee Beans Using NIR Spectroscopy and Multivariate Data Analysis. Food Control 2019, 99, 137–145. [Google Scholar] [CrossRef]
- Minh, Q.N.; Lai, Q.D.; Minh, H.N.; Kieu, M.T.T.; Gia, N.L.; Le, U.; Hang, M.P.; Nguyen, H.D.; Chau, T.D.A.; Doan, N.T.T. Authenticity Green Coffee Bean Species and Geographical Origin Using Near-Infrared Spectroscopy Combined with Chemometrics. Int. J. Food Sci. Technol. 2022, 57, 4507–4517. [Google Scholar] [CrossRef]
- Dharmawan, A.; Masithoh, R.E.; Amanah, H.Z. Development of PCA-MLP Model Based on Visible and Shortwave Near Infrared Spectroscopy for Authenticating Arabica Coffee Origins. Foods 2023, 12, 2112. [Google Scholar] [CrossRef]
- Yang, S.; Li, C.; Mei, Y.; Liu, W.; Liu, R.; Chen, W.; Han, D.; Xu, K. Determination of the Geographical Origin of Coffee Beans Using Terahertz Spectroscopy Combined with Machine Learning Methods. Front. Nutr. 2021, 8, 2021. [Google Scholar] [CrossRef]
- Quan, N.M.; Phung, H.M.; Uyen, L.; Dat, L.Q.; Ngoc, L.G.; Hoang, N.M.; Tu, T.K.M.; Dung, N.H.; Ai, C.T.D.; Trinh, D.N.T. Species and Geographical Origin Authenticity of Green Coffee Beans Using UV–VIS Spectroscopy and PLS-DA Prediction Model. Food Chem. Adv. 2023, 2, 100281. [Google Scholar] [CrossRef]
- dos Santos, L.B.; Tarabal, J.; Sena, M.M.; Almeida, M.R. UV-Vis Spectroscopy and One-Class Modeling for the Authentication of the Geographical Origin of Green Coffee Beans from Cerrado Mineiro, Brazil. J. Food Compos. Anal. 2023, 123, 105555. [Google Scholar] [CrossRef]
- Adnan, A.; Naumann, M.; Mörlein, D.; Pawelzik, E. Reliable Discrimination of Green Coffee Beans Species: A Comparison of UV-Vis-Based Determination of Caffeine and Chlorogenic Acid with Non-Targeted Near-Infrared Spectroscopy. Foods 2020, 9, 788. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.-Y.; Tan, J. Front-Face Synchronous Fluorescence Spectroscopy: A Rapid and Non-Destructive Authentication Method for Arabica Coffee Adulterated with Maize and Soybean Flours. J. Consum. Prot. Food Saf. 2022, 17, 209–219. [Google Scholar] [CrossRef]
- Naito, H.; Suhandy, D.; Morio, Y.; Murakami, K. Discrimination Between Normal Coffee Beans and Peaberries Using Excitation-Emission Matrix Measured by a Hand-Held Optical System. In Proceedings of the 3rd International Conference on Agricultural Postharvest Handling and Processing (ICAPHP 2021), Bogor, Indonesia, 12–14 October 2021. [Google Scholar] [CrossRef]
- Graf, A.; Claßen, J.; Solle, D.; Hitzmann, B.; Rebner, K.; Hoehse, M. A Novel LED-Based 2D-Fluorescence Spectroscopy System for In-Line Monitoring of Chinese Hamster Ovary Cell Cultivations—Part I. Eng. Life Sci. 2019, 19, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.-H.; Gutierrez-Wing, M.T.; Choi, J.-W. Review—Recent Progress in Portable Fluorescence Sensors. J. Electrochem. Soc. 2021, 168, 017502. [Google Scholar] [CrossRef]
- Hart, S.J.; Jiji, R.D. Light Emitting Diode Excitation Emission Matrix Fluorescence Spectroscopy. Analyst 2002, 127, 1693–1699. [Google Scholar] [CrossRef] [PubMed]
- Suhandy, D.; Al Riza, D.F.; Yulia, M.; Kusumiyati, K. Non-Targeted Detection and Quantification of Food Adulteration of High-Quality Stingless Bee Honey (SBH) via a Portable LED-Based Fluorescence Spectroscopy. Foods 2023, 12, 3067. [Google Scholar] [CrossRef]
- Luo, S.; Yan, C.; Chen, D. Preliminary Study on Coffee Type Identification and Coffee Mixture Analysis by Light Emitting Diode Induced Fluorescence Spectroscopy. Food Control 2022, 138, 109044. [Google Scholar] [CrossRef]
- Febrianto, N.A.; Zhu, F. Coffee Bean Processing: Emerging Methods and Their Effects on Chemical, Biological and Sensory Properties. Food Chem. 2023, 412, 135489. [Google Scholar] [CrossRef]
- Sulaiman, I.; Hasni, D.; Alkausar, R. Effect of Moisture Contents and Roasting Degree on Quality of Wine Coffee from Arabica Gayo. Int. J. Adv. Sci. Eng. Inf. Technol. 2022, 12, 1586–1592. [Google Scholar] [CrossRef]
- Pereira, G.V.M.; Neto, D.P.C.; Júnior, A.I.M.; Vásquez, Z.S.; Medeiros, A.B.P.; Vandenberghe, L.P.S.; Soccol, C.R. Exploring the Impacts of Postharvest Processing on the Aroma Formation of Coffee Beans—A Review. Food Chem. 2019, 272, 441–452. [Google Scholar] [CrossRef]
- Robert, J.V.; de Gois, J.S.; Rocha, R.B.; Luna, A.S. Direct Solid Sample Analysis Using Synchronous Fluorescence Spectroscopy Coupled with Chemometric Tools for the Geographical Discrimination of Coffee Samples. Food Chem. 2022, 371, 131063. [Google Scholar] [CrossRef] [PubMed]
- Minas, I.S.; Blanco-Cipollone, F.; Sterle, D. Accurate Non-Destructive Prediction of Peach Fruit Internal Quality and Physiological Maturity with A Single Scan Using Near Infrared Spectroscopy. Food Chem. 2021, 335, 127626. [Google Scholar] [CrossRef] [PubMed]
- Moon, E.J.; Kim, Y.; Xu, Y.; Na, Y.; Giaccia, A.J.; Lee, J.H. Evaluation of Salmon, Tuna, and Beef Freshness Using a Portable Spectrometer. Sensors 2020, 20, 4299. [Google Scholar] [CrossRef] [PubMed]
- Muñiz-Valencia, R.; Jurado, J.M.; Ceballos-Magaña, S.G.; Alcázar, A.; Reyes, J. Geographical Differentiation of Green Coffees According to Their Metal Content by Means of Supervised Pattern Recognition Techniques. Food Anal. Methods 2013, 6, 1271–1277. [Google Scholar] [CrossRef]
- Mendes, G.A.; de Oliveira, M.A.L.; Rodarte, M.P.; dos Anjos, V.C.; Bell, M.J.V. Origin Geographical Classification of Green Coffee Beans (Coffea arabica L.) Produced in Different Regions of the Minas Gerais State by FT-MIR and Chemometric. Curr. Res. Food Sci. 2022, 5, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Sim, J.; Dixit, Y.; Mcgoverin, C.; Oey, I.; Frew, R.; Reis, M.M.; Kebede, B. Machine Learning-Driven Hyperspectral Imaging for Non-Destructive Origin Verification of Green Coffee Beans Across Continents, Countries, and Regions. Food Control 2024, 156, 110159. [Google Scholar] [CrossRef]
- Jia, S.; Yang, L.; An, D.; Liu, Z.; Yan, Y.; Li, S.; Zhang, X.; Zhu, D.; Gu, J. Feasibility of Analyzing Frost-Damaged and Non-Viable Maize Kernels Based on Near Infrared Spectroscopy and Chemometrics. J. Cereal Sci. 2016, 69, 145–150. [Google Scholar] [CrossRef]
- Girolamo, A.D.; Cortese, M.; Cervellieri, S.; Lippolis, V.; Pascale, M.; Logrieco, A.F.; Suman, M. Tracing the Geographical Origin of Durum Wheat by FT-NIR Spectroscopy. Foods 2019, 8, 450. [Google Scholar] [CrossRef]
- Hassoun, A.; Måge, I.; Schmidt, W.F.; Temiz, H.T.; Li, L.; Kim, H.Y.; Nilsen, H.; Biancolillo, A.; Aït-Kaddour, A.; Sikorski, M.; et al. Fraud in Animal Origin Food Products: Advances in Emerging Spectroscopic Detection Methods over the Past Five Years. Foods 2020, 9, 1069. [Google Scholar] [CrossRef]
- Kennard, R.W.; Stone, L.A. Computer Aided Design of Experiments. Technometrics 1969, 11, 137–148. [Google Scholar] [CrossRef]
- Harvey, T.J.; Gazi, E.; Henderson, A.; Snook, R.D.; Clarke, N.W.; Brown, M.; Gardner, P. Factors Influencing the Discrimination and Classification of Prostate Cancer Cell Lines by FTIR Microspectroscopy. Analyst 2009, 134, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Basri, K.N.; Hussain, M.N.; Bakar, J.; Sharif, Z.; Khir, M.F.A.; Zoolfakar, A.S. Classification and Quantification of Palm Oil Adulteration Via Portable NIR Spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 173, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Belay, A.; Kim, H.K.; Hwang, Y.-H. Binding of Caffeine with Caffeic Acid and Chlorogenic Acid Using Fluorescence Quenching, UV/vis and FTIR Spectroscopic Techniques. Luminescence 2016, 31, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Botelho, B.G.; Oliveira, L.S.; Franca, A.S. Fluorescence Spectroscopy as Tool for the Geographical Discrimination of Coffees Produced in Different Regions of Minas Gerais State in Brazil. Food Control 2017, 77, 25–31. [Google Scholar] [CrossRef]
- Suhandy, D.; Yulia, M. Discrimination of Several Indonesian Specialty Coffees Using Fluorescence Spectroscopy Combined with SIMCA Method. In Proceedings of the 3rd International Conference on Chemical Engineering Sciences and Applications (the 3rd ICChESA 2017), Banda Aceh, Indonesia, 20–21 September 2017. [Google Scholar] [CrossRef]
- Pimpley, V.; Patil, S.; Srinivasan, K.; Desai, N.; Murthy, P.S. The Chemistry of Chlorogenic Acid from Green Coffee and Its Role in Attenuation of Obesity and Diabetes. Prep. Biochem. Biotechnol. 2020, 50, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Perrone, D.; Farah, A.; Donangelo, C.M.; de Paulis, T.; Martin, P.R. Comprehensive Analysis of Major and Minor Chlorogenic Acids and Lactones in Economically Relevant Brazilian Coffee Cultivars. Food Chem. 2008, 106, 859–867. [Google Scholar] [CrossRef]
- Shraddha, T.; Neha, M.; Neetu, M. Pharmacological Perspective of Green Coffee Beans and their Metabolites: A Review. Curr. Nutr. Food Sci. 2023, 19, 346–356. [Google Scholar] [CrossRef]
- Farah, A.; Monteiro, M.C.; Calado, V.; Franca, A.S.; Trugo, L.C. Correlation Between Cup Quality and Chemical Attributes of Brazilian Coffee. Food Chem. 2006, 98, 373–380. [Google Scholar] [CrossRef]
- Mills, C.E.; Oruna-Concha, M.J.; Mottram, D.S.; Gibson, G.R.; Spencer, J.P.E. The Effect of Processing on Chlorogenic Acid Content of Commercially Available Coffee. Food Chem. 2013, 141, 3335–3340. [Google Scholar] [CrossRef]
- Mintesnot, A.; Dechassa, N. Effect of Altitude, Shade, and Processing Methods on the Quality and Biochemical Composition of Green Coffee Beans in Ethiopia. East Afr. J. Sci. 2018, 12, 87–100. [Google Scholar]
- Franca-Silva, F.; Cicero, S.M.; Gomes-Junior, F.G.; Medeiros, A.D.; Franca-Neto, J.B.; Dias, D.C.F.S. Quantification of Chlorophyll Fluorescence in Soybean Seeds by Multispectral Images and Their Relationship with Physiological Potential. J. Seed Sci. 2022, 44, e202244023. [Google Scholar] [CrossRef]
- Li, W.; Huang, W.; Fan, D.; Gao, X.; Zhang, X.; Meng, Y.; Liu, T.C. Rapid Quantification of Goat Milk Adulteration with Cow Milk Using Raman Spectroscopy and Chemometrics. Anal. Methods 2023, 15, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Suhandy, D.; Yulia, M. Peaberry Coffee Discrimination Using UV-Visible Spectroscopy Combined with SIMCA and PLS-DA. Int. J. Food Prop. 2017, 20 (Suppl. S1), S331–S339. [Google Scholar] [CrossRef]
- Lapcharoensuk, R.; Fhaykamta, C.; Anurak, W.; Chadwut, W.; Sitorus, A. Nondestructive Detection of Pesticide Residue (Chlorpyrifos) on Bok Choi (Brassica rapa subsp. Chinensis) Using a Portable NIR Spectrometer Coupled with a Machine Learning Approach. Foods 2023, 12, 955. [Google Scholar] [CrossRef] [PubMed]
- Yulia, M.; Ningtyas, K.R.; Kuncoro, S.; Aurum, F.S.; Suhandy, D. A Low-Cost and Eco-Friendly Approach for Rapid Discrimination Between Authentic and Feeding Luwak Arabica Coffees. In Proceedings of the 5th International Conference on Sustainable Agriculture (ICoSA 2022), Yogyakarta, Indonesia, 20–21 July 2022. [Google Scholar] [CrossRef]
- Fodor, M.; Jókai, Z.; Matkovits, A.; Benes, E. Assessment of Maturity of Plum Samples Using Fourier Transform Near-Infrared Technique Combined with Chemometric Methods. Foods 2023, 12, 3059. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.M.; Ferreira, M.V.S.; Kamruzzaman, M.; Barbin, D.F. Prediction of Impurities in Cocoa Shell Powder Using NIR Spectroscopy. J. Pharm. Biomed. Anal. Open 2023, 2, 100015. [Google Scholar] [CrossRef]
- Kurniawan, F.; Budiastra, I.W.; Sutrisno, S.; Widyotomo, S. Classification of Arabica Java Coffee Beans Based on Their Origin using NIR Spectroscopy. In Proceedings of the 2nd International Conference on Agriculture Postharvest Handling and Processing (ICAPHP 2018), Bali, Indonesia, 29–31 August 2018. [Google Scholar] [CrossRef]
- Bona, E.; Marquetti, I.; Link, J.V.; Makimori, G.Y.F.; Arca, V.C.; Lemes, A.L.G.; Ferreira, J.M.G.; Scholz, M.G.S.; Valderrama, P.; Poppi, R.J. Support Vector Machines in Tandem with Infrared Spectroscopy for Geographical Classification of Green Arabica Coffee. LWT Food Sci. Technol. 2017, 76 Pt B, 330–336. [Google Scholar] [CrossRef]
- Diniz, P.H.G.D.; Barbosa, M.F.; Milanez, K.D.T.M.; Pistonesi, M.F.; de Araújo, M.C.U. Using UV–Vis Spectroscopy for Simultaneous Geographical and Varietal Classification of Tea Infusions Simulating a Home-Made Tea Cup. Food Chem. 2016, 192, 374–379. [Google Scholar] [CrossRef]
- Szabó, E.; Gergely, S.; Salgó, A. Linear Discriminant Analysis, Partial Least Squares Discriminant Analysis, and Soft Independent Modeling of Class Analogy of Experimental and Simulated Near-Infrared Spectra of a Cultivation Medium for Mammalian Cells. J. Chemom. 2018, 32, e3005. [Google Scholar] [CrossRef]
- Lasalvia, M.; Capozzi, V.; Perna, G. A Comparison of PCA-LDA and PLS-DA Techniques for Classification of Vibrational Spectra. Appl. Sci. 2022, 12, 5345. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |



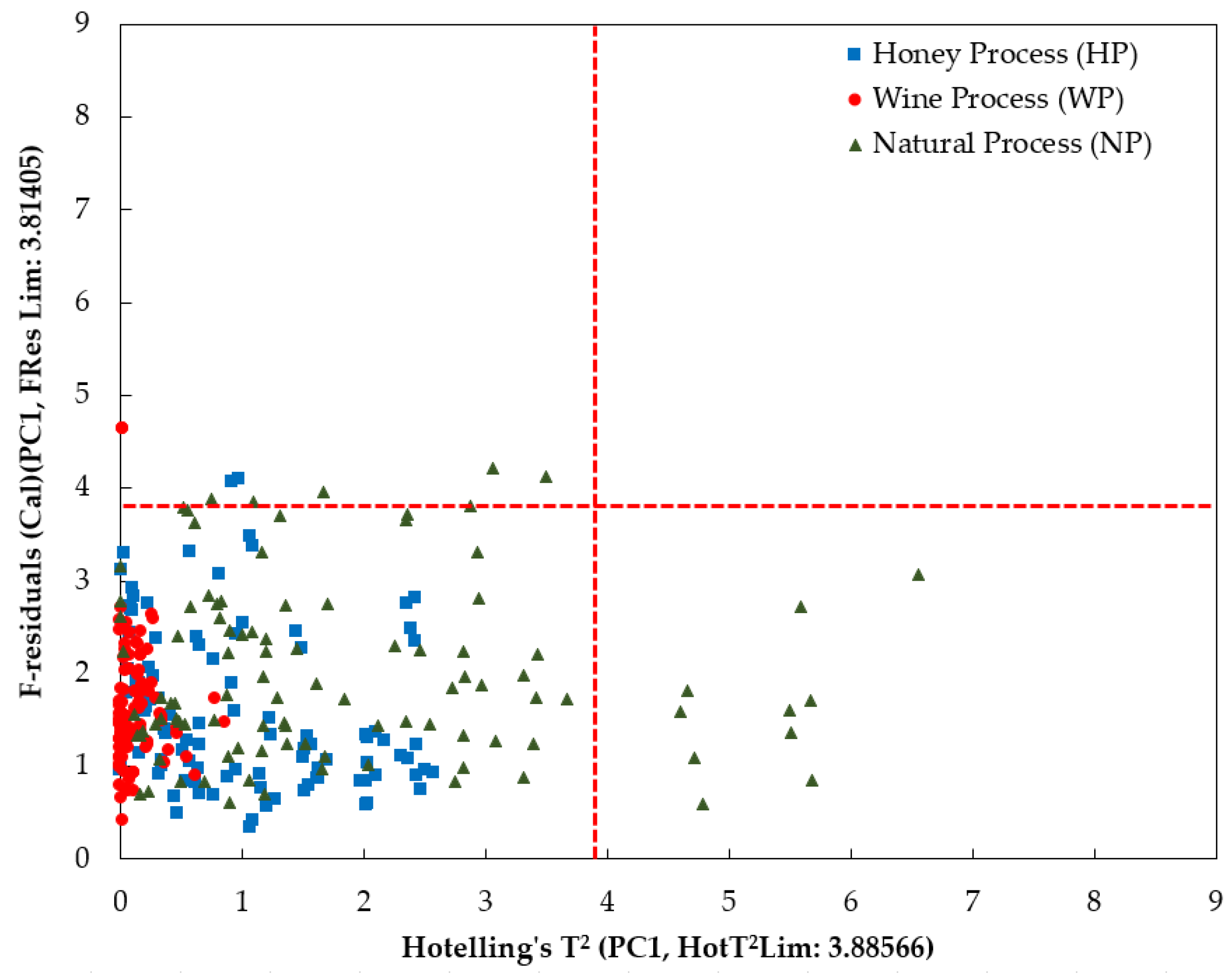
| Principal Components (PCs) | Cumulative Percentages Variance (CPV) (%) | |
|---|---|---|
| Calibration | Validation | |
| PC1 | 91.16 | 91.00 |
| PC2 | 96.14 | 96.03 |
| PC3 | 98.58 | 98.52 |
| PC4 | 99.30 | 99.27 |
| PC5 | 99.56 | 99.53 |
| PC6 | 99.73 | 99.71 |
| PC7 | 99.88 | 99.87 |
| PC8 | 99.92 | 99.92 |
| PC9 | 99.95 | 99.94 |
| PC10 | 99.96 | 99.96 |
| Classification Model | Samples | Actual | Accuracy Rate (%) | |||
|---|---|---|---|---|---|---|
| HP | WP | NP | ||||
| PLS-DA | Predicted | HP | 16 | 0 | 0 | 83.33 |
| WP | 4 | 20 | 6 | |||
| NP | 0 | 0 | 14 | |||
| LDA | Predicted | HP | 20 | 2 | 0 | 95.00 |
| WP | 0 | 17 | 0 | |||
| NP | 0 | 1 | 20 | |||
| PCA-LDA | Predicted | HP | 20 | 0 | 0 | 96.67 |
| WP | 0 | 18 | 0 | |||
| NP | 0 | 2 | 20 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yulia, M.; Analianasari, A.; Widodo, S.; Kusumiyati, K.; Naito, H.; Suhandy, D. The Authentication of Gayo Arabica Green Coffee Beans with Different Cherry Processing Methods Using Portable LED-Based Fluorescence Spectroscopy and Chemometrics Analysis. Foods 2023, 12, 4302. https://doi.org/10.3390/foods12234302
Yulia M, Analianasari A, Widodo S, Kusumiyati K, Naito H, Suhandy D. The Authentication of Gayo Arabica Green Coffee Beans with Different Cherry Processing Methods Using Portable LED-Based Fluorescence Spectroscopy and Chemometrics Analysis. Foods. 2023; 12(23):4302. https://doi.org/10.3390/foods12234302
Chicago/Turabian StyleYulia, Meinilwita, Analianasari Analianasari, Slamet Widodo, Kusumiyati Kusumiyati, Hirotaka Naito, and Diding Suhandy. 2023. "The Authentication of Gayo Arabica Green Coffee Beans with Different Cherry Processing Methods Using Portable LED-Based Fluorescence Spectroscopy and Chemometrics Analysis" Foods 12, no. 23: 4302. https://doi.org/10.3390/foods12234302







