Effect of Apple Cultivar and Selected Technological Treatments on the Quality of Apple Distillate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials, Microorganisms, and Supplements
- -
- Pasteurization—samples of apple pulp were heated to 70–75 °C and kept at this temperature for 30 min;
- -
- Depectinization—a pectolytic enzyme preparation, ROHAPECT 10 L (AB Enzymes GmbH, Darmstadt, Germany), was added in the amount of 0.1 mL/kg of apple pulp and the pulp was heated at 50 °C for 30 min;
- -
- Deacidification—the pH of the apple pulp samples was adjusted with a sodium hydroxide solution (30% w/w) from natural value (approx. 3.5) to 5.0.
2.2. Fermentation
2.3. Distillation
2.4. Analytical Methods
2.4.1. Analysis of Apple Pulp before and after Fermentation
- -
- Total extract, using a digital refractometer (Atago, Tokyo, Japan) calibrated in the weight percentage (% w/w) of sucrose [19];
- -
- Titratable acidity, expressed in grams of malic acid/100 g apple pulp. The assay involved diluting the portion of apple pulp (10 g) by adding 90 mL of distilled water and heating just to the boiling point. After cooling down, the solution was filtered and titrated with 0.1 M of NaOH solution against phenolphthalein [19]. The acidity of the apple pulp was calculated according to the following formula:
- -
- Sugars (glucose and fructose), by the HPLC technique [20]. To prepare apple pulp for sugar analysis, a sample weight of 10 g of apple pulp was centrifuged at 910× g for 10 min. The obtained supernatant was then clarified by using Carrez reagents and centrifuged again at the above-given conditions. The clarified solution was used for the determination of glucose and fructose. To determine the sucrose content in the apple pulp, the supernatant obtained after clarification was subjected to the inversion of sucrose by 36% w/w hydrochloric acid at 68–70 °C for 5 min. The solution after sucrose inversion was cooled to approx. 20 °C, neutralized with 25% (w/w) NaOH solution, and the content of the total sugars (i.e., the sum of glucose and fructose after the inversion of sucrose) was determined. Sucrose content was calculated by multiplying the difference between the sum of glucose and fructose after and before inversion, by the coefficient 0.95.
2.4.2. Analysis of Apple Distillates
2.5. Statistical Analysis
3. Results and Discussion
3.1. Characteristics of the Tested Apple Cultivars
3.2. Influence of Apple Cultivar on the Results of Raw Pulp Fermentation
3.3. Influence of Apple Pulp Processing Method on Fermentation Results
3.4. The Chemical Composition of the Obtained Distillates
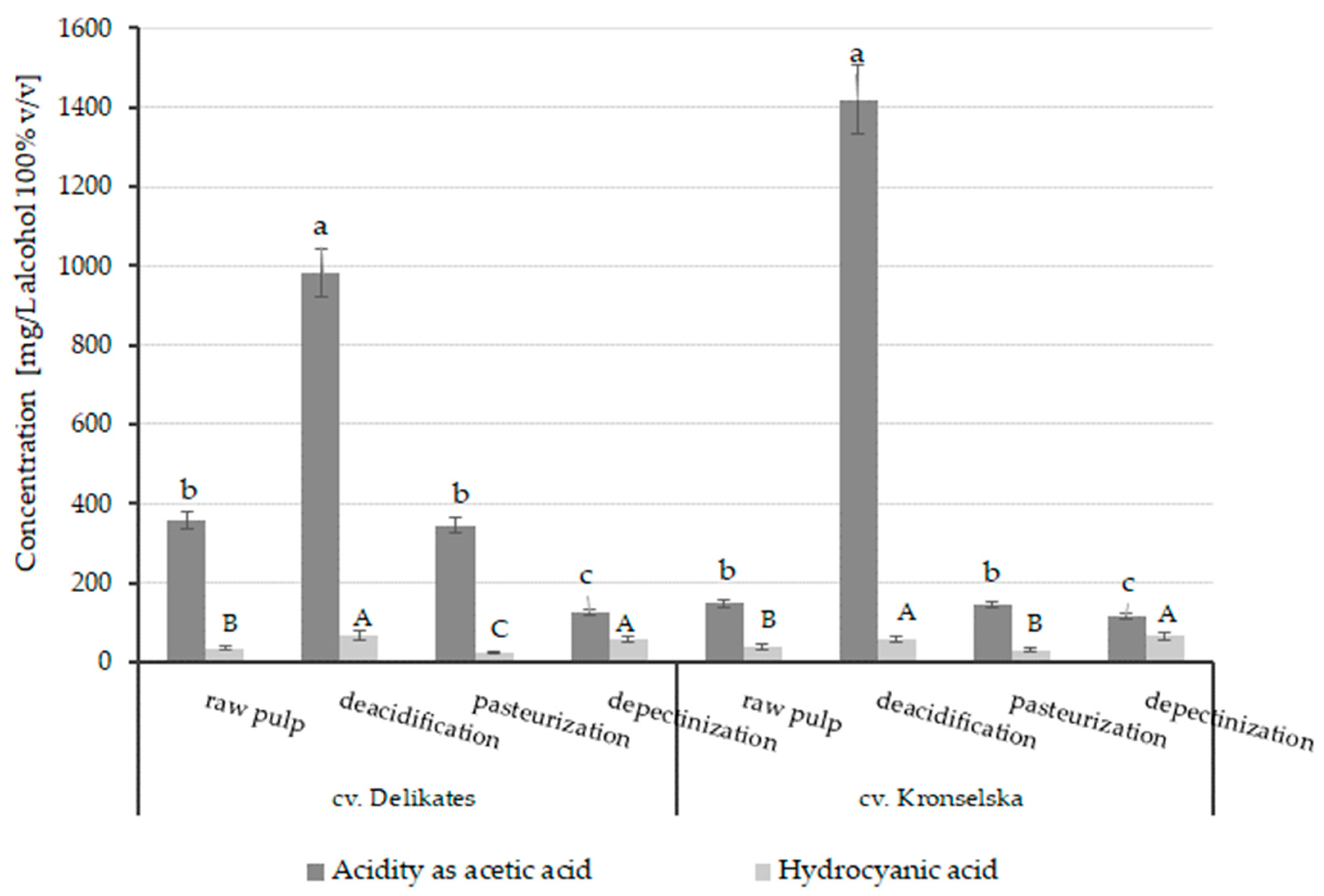
3.4.1. Acidity and Hydrocyanic Acid
3.4.2. Volatile Compounds Determined by GC-MS
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Albrecht, W. Die Obstbrennerei in Deutschland und ihre Produkte. Alk. Ind. 1998, 111, 4–21. [Google Scholar]
- Satora, P.; Tuszyński, T. Chemical characteristics of Śliwowica Łącka and other plum brandies. J. Sci. Food Agric. 2008, 88, 167–174. [Google Scholar] [CrossRef]
- Balcerek, M.; Pielech-Przybylska, K.; Patelski, P.; Sapińska, E.; Księżopolska, M. The usefulness of intermediate products of plum processing for alcoholic fermentation and chemical composition of the obtained distillates. Food Sci. 2013, 78, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Żurawicz, E.; Kubik, J.; Lewandowski, M.; Rutkowski, K.P.; Zmarlicki, K. The apple industry in Poland. Acta Hortic. 2019, 1261, 13–20. [Google Scholar] [CrossRef]
- Pietrzak, M.; Chlebicka, A.; Kraciński, P.; Malak-Rawlikowska, A. Information asymmetry as a barrier in upgrading the position of local producers in the global value chain—Evidence from the apple sector in Poland. Sustainability 2020, 12, 7857. [Google Scholar] [CrossRef]
- Makosz, E. The future of Polish apples. Bulletin. Agric. Mark. Agency 2015, 3, 10–16. (In Polish) [Google Scholar]
- Kostrz, M.; Satora, P. Apple—A raw material for the production of alcoholic beverages. In The Role of Technological Processes in Shaping of Food Quality; Duda-Chodak, A., Najgebauer-Lejko, D., Drożdż, I., Tarko, T., Eds.; Polish Society of Food Technologists—Małopolska Branch: Krakow, Poland, 2016; pp. 118–126. (In Polish) [Google Scholar]
- Guiné, R.P.F.; Barroca, M.J.; Coldea, T.E.; Bartkiene, E.; Ofélia, A. Apple fermented products: An overview of technology, properties and health effects. Processes 2021, 9, 223. [Google Scholar] [CrossRef]
- Zhang, H.; Woodams, E.E.; Hang, Y.D. Influence of pectinase treatment on fruit spirits from apple mash, juice and pomace. Process. Biochem. 2011, 46, 1909–1913. [Google Scholar] [CrossRef]
- Renard, C.M.G.C.; Maingonnat, J.F. Thermal Processing of Fruits and Fruit Juices. In Thermal Food Processing: New Technologies and Qualities, 2nd ed.; Sun, D.-W., Ed.; CRC Press Boca Raton: New York, NY, USA, 2012; pp. 417–420. [Google Scholar]
- Hang, Y.D.; Woodams, E.E. Influence of apple cultivar and juice pasteurization on hard cider and eau-de-vie methanol content. Bioresour. Technol. 2010, 101, 1396–1398. [Google Scholar] [CrossRef]
- Liu, X.; Jia, B.; Sun, X.; Ai, J.; Wang, L.; Wang, C.; Zhao, F.; Zhan, J.; Huang, W. Effect of initial pH on growth characteristics and fermentation properties of Saccharomyces cerevisiae. J. Food Sci. 2015, 80, 800–808. [Google Scholar] [CrossRef]
- Joshi, V.K.; Sharma, R.; Abrol, G.S. Stone Fruit: Wine and Brandy. In Handbook of Plant-Based Fermented Food and Beverage Technology; Hui, Y.H., Evranuz, E.Ö.Ü., Arroyo-López, F.N., Fan, L., Hansen, S., Jaramillo-Flores, M.E., Rakin, M., Schwan, R.F., Zhou, W., Eds.; CRC Press: Boca Raton, FL, USA, 2012; Available online: https://www.routledgehandbooks.com/doi/10.1201/b12055-16 (accessed on 25 November 2023).
- Balcerek, M.; Szopa, J.S. Optimierung der Gewinnungstechnologie von Aronia Spirituosen—Teil 1: Auswahl der Gärungsbedingungen für Aronia—Maischen. Dtsch. Lebensm. Rundsch. 2002, 98, 326–331. [Google Scholar]
- Yang, X.; Song, X.; Yang, L.; Zhao, J.; Zhu, X. Effect of Deacidification treatment on the flavor quality of zaosu pear–kiwifruit wine. Foods 2022, 11, 2007. [Google Scholar] [CrossRef] [PubMed]
- IOC. Activit. Data Sheet. Available online: https://www.perdomini-ioc.com/wp-content/uploads/2017/08/ST_ACTIVIT_(EN).pdf (accessed on 15 October 2023).
- Pielech-Przybylska, K.; Balcerek, M.; Nowak, A.; Patelski, P.; Dziekońska-Kubczak, U. Influence of yeast on the yield of fermentation and volatile profile of ‘Węgierka Zwykła’ plum distillates. J. Inst. Brew. 2016, 122, 612–623. [Google Scholar] [CrossRef]
- Krell, E. Handbook of Laboratory Distillation, 2nd ed.; Elsevier: Amsterdam, Holland, 1982; p. 365. [Google Scholar]
- Clark, D.H. Fruits and Fruit Products. In Official Methods of Analysis of AOAC International; Latimer, G.W., Jr., Ed.; AOAC Publications: New York, NY, USA, 2023. [Google Scholar] [CrossRef]
- Dziekońska-Kubczak, U.; Berłowska, J.; Dziugan, P.; Patelski, P.; Pielech-Przybylska, K.; Balcerek, M. Nitric acid pretreatment of jerusalem artichoke stalks for enzymatic saccharification and bioethanol production. Energies 2018, 11, 2153. [Google Scholar] [CrossRef]
- Polish Standard PN-A-79528-7; Spirit (Ethyl Alcohol). Test Methods. Determination of Acidity. Polish Committee for Standardization: Warsaw, Poland, 2001. (In Polish)
- Standard Methods for the Examination of Water and Wastewater, 24th ed.; Lipps, W.C.; Braun-Howland, E.B.; Baxter, T.E. (Eds.) APHA Press: Washington, DC, USA, 2023; Method no 8027, 2000. [Google Scholar]
- Epstein, J. Estimation of microquanties of cyanide. Anal. Chem. 1947, 19, 272–274. [Google Scholar] [CrossRef]
- Błaszczyk, J. The influence of harvest date on the storability of ‘Topaz’ apples. Zesz. Nauk. Inst. Sadow. Kwiac. Skiern. 2006, 14, 87–93. (In Polish) [Google Scholar]
- Tarko, T.; Kostrz, M.; Duda-Chodak, A.; Semik-Szczurak, D.; Sroka, P.; Senczyszyn, T. The effect of apple cultivars and yeast strains on selected quality parameters and antioxidant activity of fermented apple beverages. CyTA-J. Food 2018, 16, 892–900. [Google Scholar] [CrossRef]
- Spaho, N.; Dürr, P.; Grba, S.; Velagić-Habul, E.; Blesić, M. Effects of distillation cut on the distribution of higher alcohols and esters in brandy produced from three plum varieties. J. Inst. Brew. 2013, 119, 48–56. [Google Scholar] [CrossRef]
- Jordão, A.M.; Vilela, A.; Cosme, F. From sugar of grape to alcohol of wine: Sensorial impact of alcohol in wine. Beverages 2015, 1, 292–310. [Google Scholar] [CrossRef]
- Lee, C.Y. Common nutrients and nutraceutical quality of apples. NYFQ 2012, 20, 3–8. [Google Scholar]
- Maicas, S. The role of yeasts in fermentation processes. Microorganisms 2020, 8, 1142. [Google Scholar] [CrossRef] [PubMed]
- Januszek, M.; Satora, P.; Tarko, T. Oenological characteristics of fermented apple musts and volatile profile of brandies obtained from different apple cultivars. Biomolecules 2020, 10, 853. [Google Scholar] [CrossRef] [PubMed]
- Remize, F.; Sablayrolles, J.M.; Dequin, S. Re-assessment of the influence of yeast strain and environmental factors on glycerol production in wine. J. Appl. Microbiol. 2000, 88, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, S.K.; Ozbas, Z.Y. Effects of pH and temperature on growth and glycerol production kinetics of two indigenous wine strains of Saccharomyces cerevisiae from Turkey. Braz. J. Microbiol. 2008, 39, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Baroň, M.; Fiala, J. Chasing after minerality, relationship to yeast nutritional stress and succinic acid production. Czech J. Food Sci. 2012, 30, 188–193. [Google Scholar] [CrossRef]
- Carr, F.J.; Chill, D.; Maida, N. The lactic acid bacteria: A literature survey. Crit. Rev. Microbiol. 2002, 28, 281–370. [Google Scholar] [CrossRef] [PubMed]
- Cousin, F.J.; Le Guellec, R.; Schlusselhuber, M.; Dalmasso, M.; Laplace, J.M.; Cretenet, M. Microorganisms in fermented apple beverages: Current knowledge and future directions. Microorganisms 2017, 5, 39. [Google Scholar] [CrossRef] [PubMed]
- Graça, A.; Santo, D.; Esteves, E.; Nunes, C.; Abadias, M.; Quintas, C. Evaluation of microbial quality and yeast diversity in fresh-cut apple. Food Microbiol. 2015, 51, 179–185. [Google Scholar] [CrossRef]
- Hauka, F.I.A.; El-Sawah, M.M.A.; Kassem, M.M.; El-Kady, S.M. Some factors affecting citric acid production from sugar cane molasses by Aspergillus niger. In Proceeding of the 2nd Arab Mansoura Conference on Food and Dairy Science & Technology; Mansoura University: Mansoura, Egypt, 2005; pp. 1–13. [Google Scholar]
- Dziubak, M. Old apple cultivars and their origin. Rocz. Dendrol. 2006, 54, 51–66. (In Polish) [Google Scholar]
- Coulter, A. Ask the AWRI: The tricks and traps of deacidification. Aust. N.Z. Grapegrow. Winemak. 2017, 642, 56–57. [Google Scholar]
- Vera-Peña, M.Y.; Rodriguez Rodriguez, W.L. Effect of pH on the growth of three lactic acid bacteria strains isolated from sour cream. Univ. Sci. 2020, 25, 341–358. [Google Scholar] [CrossRef]
- Berthels, N.J.; Cordero Otero, R.R.; Bauer, F.F.; Thevelein, J.M.; Pretorius, I.S. Discrepancy in glucose and fructose utilisation during fermentation by Saccharomyces cerevisiae wine yeast strains. FEMS Yeast Res. 2004, 4, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.H.; Kim, N.H.; Shim, M.B.; Jeon, Y.W.; Ahn, J.H.; Lee, S.H.; Hwang, I.G.; Rhee, M.S. Microbiological diversity and prevalence of spoilage and pathogenic bacteria in commercial fermented alcoholic beverages (beer, fruit wine, refined rice wine, and yakju). J. Food Protect. 2015, 78, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Galabova, M.; Stoyanov, N.; Mitev, P. Primary studies of the composition of distillate beverages produced from Sorbus Domestica fruits. BIO Web Conf. 2022, 45, 01012. [Google Scholar] [CrossRef]
- Lilly, M.; Lambrechts, M.G.; Pretorius, I.S. Effect of increased yeast alcohol acetyltransferase activity on flavor profiles of wine and distillates. Appl. Environ. Microbiol. 2000, 66, 744–753. [Google Scholar] [CrossRef]
- Balcerek, M.; Szopa, J. Ethanol biosynthesis and hydrocyanic acid liberation during fruit mashes fermentation. Czech J. Food Sci. 2012, 30, 144–152. [Google Scholar] [CrossRef]
- Regulation (EU) 2019/787 of the European Parliament and of the Council of 17 April 2019 on the Definition, Description, Presentation and Labelling of Spirit Drinks, the Use of the Names of Spirit Drinks in the Presentation and Labelling of Other Foodstuffs, the Protection of Geographical Indications for Spirit Drinks, the Use of Ethyl Alcohol and Distillates of Agricultural Origin in Alcoholic Beverages, and Repealing Regulation (EC) No 110/2008. OJL 130, 17.5.2019, pp. 1–54. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32019R0787 (accessed on 10 October 2023).
- Balcerek, M. Ethyl carbamate and its precursors in fruit distillates. In Scientific Journals of the Lodz University of Technology No. 1180, Scientific Dissertations no. 478, Pub; Lodz University of Technology: Lodz, Poland, 2013. (In Polish) [Google Scholar]
- Madrera, R.R.; Mangas, A.J.J. Typification of cider brandy on the basis of cider used in its manufacture. J. Agric. Food Chem. 2005, 53, 3071–3075. [Google Scholar] [CrossRef]
- Blumenthal, P.; Steger, M.C.; Einfalt, D.; Rieke-Zapp, J.; Quintanilla Bellucci, A.; Sommerfeld, K.; Lachenmeier, D.W. Methanol mitigation during manufacturing of fruit spirits with special consideration of novel coffee cherry spirits. Molecules 2021, 26, 2585. [Google Scholar] [CrossRef]
- Do Amaral, S.H.; De Assis, S.A.; De Faria Oliveira, O.M.M. Partial purification and characterization of pectin methylesterase from orange (citrus sinensis) cv. Perario. J. Food Biochem. 2005, 29, 367–380. [Google Scholar] [CrossRef]
- Satora, P.; Tuszyński, T. Influence of indigenous yeasts on the fermentation and volatile profile of plum brandies. Food Microbiol. 2010, 27, 418–424. [Google Scholar] [CrossRef]
- Glatthar, J.; Senn, T.; Pieper, H.J. Investigations on reducing the methanol content in distilled spirits made of Bartlett pears. Dtsch. Lebensm. Rundsch. 2001, 97, 209–216. [Google Scholar]
- Chaiyasut, C.; Jantavong, S.; Kruatama, C.; Peerajan, S.; Sirilun, S.; Shank, L. Factors affecting methanol content of fermented plant beverage containing Morinda citrifolia. Afr. J. Biotechnol. 2013, 12, 4356–4363. [Google Scholar]
- Cabaroglu, T.; Yilmaztekin, M. Methanol and major volatile compounds of Turkish Raki and effect of distillate source. J. Inst. Brew. 2011, 117, 98–105. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Bartowsky, E.J.; Henschke, P.A.; Pretorius, I.S. Yeast and bacterial modulation of wine aroma flavor. Aust. J. Grape Wine Res. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- Wongfhun, P.; Gordon, M.H.; Apichartsrangkoon, A. Flavour characterisation of fresh and processed pennywort (Centella asiatica L.) juices. Food Chem. 2010, 119, 69–74. [Google Scholar] [CrossRef]
- Soufleros, E.H.; Mygdalia, A.S.; Natskouli, P. Characterization and safety evaluation of the traditional Greek fruit distillate “Mouro” by flavor compounds and mineral analysis. Food Chem. 2004, 86, 625–636. [Google Scholar] [CrossRef]
- Ferreira, V.; Hernandez-Orte, P.; Escudero, A.; Lopez, R.; Cacho, J. Semipreperative reversed-phase liquid chromatographic fractionation of aroma extracts from wine and other alcoholic beverages. J. Chromotogr. A 1999, 864, 77–88. [Google Scholar] [CrossRef]
- Cortés, S.; Rodríguez, R.; Salgado, J.M.; Domínguez, J.M. Comparative study between Italian and Spanish grape marc spirits in terms of major volatile compounds. Food Control 2011, 22, 673–680. [Google Scholar] [CrossRef]
- Medina, S.; Perestrelo, R.; Pereira, R.; Câmara, J.S. Evaluation of volatilomic fingerprint from apple fruits to ciders: A useful tool to find putative biomarkers for each apple variety. Foods 2020, 9, 1830. [Google Scholar] [CrossRef]
- Akhtar, M.K.; Dandapani, H.; Thiel, K.; Jones, P.R. Microbial production of 1-octanol: A naturally excreted biofuel with diesel-like properties. Metab. Eng. Commun. 2015, 2, 1–5. [Google Scholar] [CrossRef]
- Liu, S.Q.; Pilone, G.J. An overview of formation and roles of acetaldehyde in winemaking with emphasis on microbiological implications. Int. J. Food Sci. Technol. 2000, 35, 49–61. [Google Scholar] [CrossRef]
- Melgarejo, P.; Sanchez, A.C.; Hernandez, F.; Szumny, A.; Martinez, J.J.; Legua, P.; Martinez, R.; Carbonell-Barrachina, A.A. Chemical, functional and quality properties of Japanese plum (Prunus salicina Lindl.) as affected by mulching. Sci. Horticult. 2012, 134, 114–120. [Google Scholar] [CrossRef]
- Apostolopoulou, A.A.; Flouros, A.I.; Demertzis, P.G.; Akrida-Demertzi, K. Differences in concentration of principal volatile constituents in traditional Greek distillates. Food Control 2005, 16, 57–164. [Google Scholar] [CrossRef]
- Regodón Mateos, J.A.; Pérez-Nevado, F.; Ramírez Fernández, M. Influence of Saccharomyces cerevisiae yeast strain on the major volatile compounds of wine. Enzym. Microb. Technol. 2006, 40, 151–157. [Google Scholar] [CrossRef]
- Ladauphin, J.; Basset, B.; Cohen, S.; Payot, T.; Barillier, D. Identification of trace volatile compounds in freshly distilled Calvados and Cognac: Carbonyl and sulfur compounds. J. Food Compos. Anal. 2006, 19, 28–40. [Google Scholar] [CrossRef]
- Nykänen, L.; Nykänen, I. Distilled beverages. In Volatile Compounds in Foods and Beverages, 1st ed.; Maarse, H., Ed.; Marcell Dekker, Inc.: New York, NY, USA, 1991; pp. 547–558. [Google Scholar]
- Zea, L.; Serratosa, M.P.; Mérida, J.; Moyano, L. Acetaldehyde as key compound for the authenticity of sherry wines: A study covering 5 decades. Compr. Rev. Food Sci. Food Saf. 2015, 14, 681–693. [Google Scholar] [CrossRef]
- Zohre, D.E.; Erten, H. The influence of Kloeckera apiculata and Candida pulcherrima yeasts on wine fermentation. Process Biochem. 2002, 38, 319–324. [Google Scholar] [CrossRef]
- Jackson, R.S. Postfermentation Treatments and Related Topics. In Wine Science: Principles and Applications, 3rd ed.; Jackson, R.S., Ed.; Academic Press: Cambridge, MA, USA, 2008; pp. 418–519. [Google Scholar]
- Satora, P.; Tuszyński, T. Biodiversity of yeasts during plum Wegierka Zwykla spontaneous fermentation. Food Technol. Biotechnol. 2005, 43, 277–282. [Google Scholar]
- Arrieta-Garay, Y.; López-Vázquez, C.; Blanco, P.; Pérez-Correa, J.R.; Orriols, I.; López, F. Kiwi spirits with stronger floral and fruity characters were obtained with a packed column distillation system. J. Inst. Brew. 2014, 120, 111–118. [Google Scholar] [CrossRef]
- Rodríguez Madrera, R.; García Hevia, A.; Valles, B.S. Comparative study of two aging systems for cider brandy making. Changes in chemical composition. LWT—Food Sci. Technol. 2013, 54, 513–520. [Google Scholar] [CrossRef]
- Bougas, N.V. Evaluation the Effect of Pot Still Design on the Resultant Distillate. Master’s Thesis, Stellenbosch University, Faculty of AgriScience, Matieland, South Africa, 2009. [Google Scholar]

| Apple Cultivar | Extract [% w/w] | Titratable Acidity [g Malic Acid/100 g] | Glucose [g/100 g] | Fructose [g/100 g] | Sucrose [g/100 g] |
|---|---|---|---|---|---|
| Antonówka | 15.0 ± 0.5 b | 0.70 ± 0.04 a | 2.59 ± 0.30 b | 7.62 ± 0.44 ab | 4.22 ± 0.13 c |
| Delikates | 15.2 ± 0.4 b | 0.36 ± 0.02 c | 2.51 ± 0.27 b | 6.59 ± 0.41 b | 4.17 ± 0.13 c |
| Kosztela | 23.2 ± 0.7 a | 0.16 ± 0.01 d | 3.97 ± 0.36 a | 8.27 ± 0.68 a | 9.87 ± 0.29 a |
| Kronselska | 15.5 ± 0.3 b | 0.54 ± 0.03 b | 2.34 ± 0.28 b | 7.13 ± 0.46 ab | 4.97 ± 0.15 b |
| Apple Cultivar | Extract [% w/w] | Titratable Acidity [g Malic Acid/100 g] | Glucose [g/100 g] | Fructose [g/100 g] | Sucrose [g/100 g] |
|---|---|---|---|---|---|
| Antonówka | 3.4 ± 0.2 b | 2.51 ± 0.12 a | 0.14 ± 0.02 c | 0.34 ± 0.03 b | 0.15 ± 0.01 b |
| Delikates | 3.9 ± 0.1 a | 1.73 ± 0.08 b | 0.10 ± 0.01 d | 0.11 ± 0.01 c | 0.02 ± 0.01 c |
| Kosztela | 4.1 ± 0.2 a | 1.88 ± 0.05 b | 0.31 ± 0.02 b | 0.34 ± 0.02 b | 0.03 ± 0.01 c |
| Kronselska | 4.0 ± 0.1 a | 2.79 ± 0.22 a | 0.46 ± 0.02 a | 0.71 ± 0.03 a | 0.23 ± 0.01 a |
| Compound | Antonówka | Delikates | Kosztela | Kronselska |
|---|---|---|---|---|
| Ethanol [g/kg] | 58.03 ± 2.14 b | 55.97 ± 3.23 b | 87.39 ± 5.42 a | 57.29 ± 3.12 b |
| Ethanol yield [% of the theoretical] | 77.72 ± 2.87 a | 81.36 ± 4.69 a | 75.77 ± 4.69 a | 76.48 ± 4.16 a |
| By-products [g/kg] | ||||
| Glycerol | 2.20 ± 0.07 c | 3.02 ± 0.09 b | 3.71 ± 0.11 a | 3.07 ± 0.09 b |
| Malic acid | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a |
| Citric acid | n.d. | n.d. | 0.39 ± 0.02 | n.d. |
| Tartaric acid | 0.09 ± 0.01 d | 0.18 ± 0.01 b | 0.26 ± 0.01 a | 0.14 ± 0.01 c |
| Succinic acid | 0.08 ± 0.01 b | 0.05 ± 0.01 c | 0.20 ± 0.01 a | 0.03 ±0.00 d |
| Lactic acid | 0.05 ± 0.00 d | 0.12 ± 0.01 c | 1.08 ± 0.03 a | 0.55 ± 0.02 b |
| Formic acid | 0.20 ± 0.03 b | 0.05 ± 0.01 c | 0.07 ± 0.01 c | 0.32 ± 0.03 a |
| Acetic acid | 0.12 ± 0.01 b | 0.20 ± 0.01 b | 0.34 ± 0.02 a | 0.10 ± 0.01 c |
| Cultivar | Pretreatment | Extract [% w/w] | Titratable Acidity [g Malic Acid/100 g] | Glucose [g/100 g] | Fructose [g/100 g] | Sucrose [g/100 g] |
|---|---|---|---|---|---|---|
| Delikates | Raw pulp | 4.1 ± 0.4 b | 0.57 ± 0.02 a | 0.20 ± 0.01 b | 0.26 ± 0.02 b | 0.03 ± 0.00 b |
| Deacidification | 5.5 ± 0.5 a | 0.51 ± 0.02 bc | 0.41 ± 0.06 a | 1.12 ± 0.06 a | 0.02 ± 0.00 b | |
| Pasteurization | 4.0 ± 0.2 b | 0.53 ± 0.03 ab | 0.09 ±0.01 c | 0.21 ± 0.01 b | 0.12 ± 0.00 a | |
| Depectinization | 4.3 ± 0.4 b | 0.47 ± 0.01 c | 0.15 ± 0.02 bc | 0.22 ± 0.03 b | 0.02 ± 0.00 b | |
| Kronselska | Raw pulp | 4.2 ± 0.2 b | 0.65 ± 0.03 b | 0.13 ± 0.01 b | 0.36 ± 0.02 a | 0.07 ± 0.01 a |
| Deacidification | 6.1 ± 0.5 a | 1.92 ± 0.07 a | 0.13 ± 0.01 b | 0.17 ± 0.02 c | 0.04 ± 0.01 b | |
| Pasteurization | 4.0 ± 0.2 b | 0.64 ± 0.03 b | 0.12 ± 0.01 b | 0.25 ± 0.02 b | 0.03 ± 0.01 b | |
| Depectinization | 4.2 ± 0.2 b | 0.67 ± 0.03 b | 0.18 ± 0.01 a | 0.26 ± 0.02 b | 0.02 ± 0.00 c |
| Compound [g/kg] | cv. Delikates | cv. Kronselska | ||||||
|---|---|---|---|---|---|---|---|---|
| Raw Pulp | Deacidification | Pasteurization | Depectinization | Raw pulp | Deacidification | Pasteurization | Depectinization | |
| Ethanol | 53.33 ± 2.18 b | 35.33 ± 1.76 c | 55.59 ± 1.23 b | 61.28 ± 1.93 a | 56.30 ± 3.79 b | 30.57 ± 1.62 c | 55.73 ± 0.77 b | 63.70 ± 0.83 a |
| Ethanol yield [% of theoretical] | 77.52 ± 3.17 b | 51.36 ± 2.56 c | 80.81 ± 1.79 b | 88.57 ± 2.00 a | 75.16 ± 5.06 b | 40.81 ± 2.16 c | 74.39 ± 1.03 b | 85.03 ± 1.11 a |
| By-products [g/kg] | ||||||||
| Glycerol | 3.84 ± 0.12 b | 3.32 ± 0.09 c | 3.17 ± 0.09 c | 4.23 ± 0.13 a | 2.84 ± 0.09 b | 2.65 ± 0.08 c | 2.29 ± 0.07 d | 5.03 ± 0.15 a |
| Glucose | 1.26 ± 0.03 b | 1.49 ± 0.04 a | 0.64 ± 0.02 c | 0.15 ± 0.01 d | 0.53 ± 0.02 a | 0.48 ± 0.01 b | 0.46 ± 0.01 b | 0.18 ± 0.01 c |
| Fructose | 3.37 ± 0.10 c | 6.61 a ± 0.41 | 2.37 d ± 0.07 | 5.15 b ± 0.15 | 2.76 d ± 0.08 | 7.83 a ± 0.03 | 3.20 c ± 0.09 | 4.62 b ± 0.14 |
| Arabinose | n.d. | n.d. | n.d. | 0.36 ± 0.01 | n.d. | n.d. | n.d. | n.d. |
| Malic acid | n.d. | 1.01 ± 0.03 | n.d. | n.d. | n.d. | 0.49± 0.01 | n.d. | n.d. |
| Citric acid | 0.25 ± 0.01 b | 0.35 ± 0.01 a | n.d. | n.d. | n.d. | 0.34 ± 0.01 a | n.d. | 0.16 ± 0.01 c |
| Tartaric acid | 0.13 ± 0.01 c | 0.76 ± 0.02 b | 0.10 ± 0.01 d | 1.77 ± 0.05 a | 0.19 ± 0.01 c | 0.70 ± 0.03 b | n.d. | 1.16 ± 0.03 a |
| Succinic acid | 0.49 ± 0.02 a | 0.18 ± 0.01 b | 0.52 ± 0.02 a | n.d. | 0.59 ± 0.02 c | 0.66 ± 0.02 b | 0.41 ± 0.01 d | 0.76 ± 0.02 a |
| Lactic acid | 1.07 ± 0.03 a | 1.14 ± 0.04 a | 0.17 ± 0.02 b | 0.13 ± 0.02 b | 1.79 ± 0.05 b | 3.13 ± 0.06 a | 0.06 ± 0.00 c | n.d. |
| Acetic acid | 3.68 ± 0.11 a | 1.95 ± 0.06 c | 2.39 ± 0.07 b | 0.51 ± 0.02 d | 3.20 ± 0.10 b | 15.14 ± 0.45 a | 1.20 ± 0.03 c | 1.07 ± 0.03 d |
| Volatile Compound [mg/L of alcohol 100% v/v] | cv. Antonówka | cv. Delikates | cv. Kosztela | cv. Kronselska |
|---|---|---|---|---|
| Methanol | 935.60 ± 28.07 a | 1061.90 ± 31.86 b | 1416.40 ± 42.49 c | 1084.60 ± 32.54 b |
| 2-Propanol | 3.33 ± 0.10 b | 6.35 ± 0.19 c | 8.44 ± 0.25 d | 0.99 ± 0.03 a |
| 1-Propanol | 251.10 ± 7.53 a | 232.40 ±6.97 a | 394.30 ± 11.83 c | 290.20 ± 8.71 b |
| 2-Methyl-1-propanol | 1152.20 ± 34.57 c | 1052.60 ± 31.58 b | 776.90 ± 23.31 a | 1110.60 ± 33.32 bc |
| 1-Butanol | 450.70 ± 13.52 c | 86.40 ± 2.59 a | 167.70 ± 5.03 b | 470.10 ± 14.10 c |
| 2-Methyl-1-butanol | 859.00 ± 25.77 c | 707.60 ± 21.23 a | 891.20 ± 26.74 c | 779.00 ± 23.37 b |
| 3-Methyl-1-butanol | 3786.70 ± 113.60 c | 2685.80 ± 80.57 a | 3098.00 ± 92.94 b | 2820.50 ± 84.61 a |
| 1-Pentanol | 8.53 ± 0.26 d | 4.60 ± 0.14 b | 6.86 ± 0.21 c | 3.99 ± 0.12 a |
| 2-Hexanol | 4.75 ± 0.14 | n.d. | n.d. | n.d. |
| 1-Hexanol | 205.83 ± 6.17 d | 45.14 ± 1.35 a | 89.97 ± 2.70 b | 171.42 ± 5.14 c |
| Furfuryl alcohol | 3.15 ± 0.09 b | 2.47 ± 0.07 a | 4.06 ± 0.12 c | 7.21 ± 0.22 d |
| Benzyl alcohol | 17.68 ± 0.53 b | 14.47 ± 0.43 a | 23.47 ± 0.70 c | 16.57 ± 0.50 b |
| 2-Phenyletanol | 105.33 ± 3.16 c | 69.08 ± 2.07 a | 176.58 ± 5.30 d | 84.43 ± 2.53 b |
| 1-Nonanol | 37.98 ± 1.14 b | 45.47 ± 1.36 c | 34.47 ± 1.03 a | 47.03 ± 1.41 c |
| 2-Butanol | 0.53 ± 0.02 d | 0.25 ± 0.01 a | 0.46 ± 0.01 c | 0.29 ± 0.01 b |
| 1-Octanol | 25.19 ± 0.76 b | 20.63 ± 0.62 a | 33.45 ± 1.12 c | 23.62 ± 0.71 b |
| Acetaldehyde | 169.16 ± 5.07 d | 91.32 ± 2.74 b | 146.30 ± 4.39 c | 72.63 ± 2.18 a |
| Isovaleraldehyde | 2.30 ± 0.07 d | 0.94 ± 0.03 b | 1.99 ± 0.06 c | 0.67 ± 0.02 a |
| 2-Methylbutyraldehyde | 1.01 ± 0.05 c | 0.87 ± 0.03 b | 1.08 ± 0.07 c | 0.44 ± 0.01 a |
| Valeraldehyde | 0.26 ± 0.01 b | 0.43 ± 0.01 d | 0.35 ± 0.01 c | 0.17 ± 0.01 a |
| Heksanal | 6.09 ± 0.48 b | 3.65 ± 0.21 a | n.d. | 3.20 ± 0.24 a |
| Phenylacetaldehyde | 1.10 ± 0.13 c | 0.84 ± 0.03 b | 1.26 ± 0.14 c | 0.58 ± 0.02 a |
| Nonanal | 26.34 ± 1.79 b | 14.15 ± 0.92 a | 12.72 ± 0.88 a | 14.49 ± 0.83 a |
| Decanal | 11.36 ± 0.34 b | 9.35 ± 0.28 a | 13.81 ± 0.41 c | 9.52 ± 0.29 a |
| Furfural | 111.10 ± 3.33 b | 18.26 ± 0.55 a | 17.92 ± 0.54 a | 20.82 ± 0.62 a |
| Benzaldehyde | 8.15 ± 0.24 a | 11.12 ± 0.33 b | 11.23 ± 0.34 b | 12.72 ± 0.38 c |
| Isobutyraldehyde | 44.17 ± 1.33 a | 44.30 ± 1.33 a | n.d. | 43.16 ± 1.29 a |
| Trans-2-heptanal | 18.24 ± 0.55 a | n.d. | n.d. | 19.10 ± 0.57 a |
| 3-Etoksypropionaldehyde | 16.60 ± 0.50 a | n.d. | n.d. | 16.71 ± 0.50 a |
| 2-Propanone (acetone) | 5.50 ± 0.17 b | 7.79 ± 0.23 d | 4.88 ± 0.15 a | 7.07 ± 0.21 c |
| 2,3-Butanedione (diacetyl) | 3.63 ± 0.11 a | 3.57 ± 0.11 a | 3.72 ± 0.11 a | 3.03 ± 0.09 b |
| Acetaldehyde diethyl acetal | 1355.10 ± 40.65 b | 526.70 ± 15.80 a | 1310.60 ± 39.32 b | 582.90 ± 17.49 a |
| 2.3-Pentanodione | n.d. | 10.58 ± 0.32 a | n.d. | 8.51 ± 0.26 a |
| 3-Octanone | 25.18 ± 0.76 a | 27.90 ± 2.84 a | 28.43 ± 2.85 a | 29.36 ± 1.88 a |
| Ethyl formate | n.d. | n.d. | 1.05 ± 0.05 | n.d. |
| Ethyl acetate | 397.80 ± 26.90 b | 295.60 ± 14.80 a | 1141.20 ± 23.10 c | 390.60 ± 17.50 b |
| Isobutyl acetate | 0.26 ± 0.11 a | 0.13 ± 0.05 a | 0.33 ± 0.08 a | 0.19 ± 0.05 a |
| Butyl acetate | 1.08 ±0.05 b | n.d. | 4.88 ± 0.24 c | 0.14 ± 0.01 a |
| 3-Methylbutyl acetate | 16.47 ± 0.32 c | 11.11 ± 0.25 b | 2.78 ± 0.14 a | 11.57 ± 0.28 b |
| 2-Methylbutyl acetate | 1.09 ± 0.03 a | 1.12 ± 0.03 a | 1.28 ± 0.06 b | 1.09 ± 0.05 a |
| Hexyl acetate | 0.16 ± 0.03 a | n.d. | 0.11 ± 0.04 a | 0.14 ± 0.04 a |
| 2-Phenylethyl acetate | 3.41 ± 0.18 a | 3.26 ± 0.07 a | 5.69 ± 0.23 b | 8.20 ± 0.04 c |
| Methyl acetate | 2.41 ± 0.18 a | 2.15 ± 0.35 a | 3.48 ± 0.23 b | 11.65 ± 0.75 c |
| Ethyl propanoate | 2.28 ± 0.11 b | 0.94 ± 0.18 a | 2.05 ± 0.13 b | 1.32 ± 0.27 a |
| Isoamyl propanoate | 0.07 ± 0.04 | n.d. | n.d. | n.d. |
| Ethyl isobutyrate | 0.10 ± 0.03 a | 0.13 ± 0.05 a | 0.15 ± 0.04 a | 0.11 ± 0.03 a |
| Ethyl butyrate | 0.95 ± 0.09 a | 0.81 ± 0.14 a | 1.28 ± 0.36 a | 1.08 ± 0.15 a |
| Ethyl 2-methylbutyrate | 0.15 ± 0.04 a | 0.24 ± 0.06 a | 0.72 ± 0.14 b | 0.22 ± 0.08 a |
| Ethyl 3-methylbutyrate | n.d. | 0.07 ± 0.04 a | 0.03 ± 0.02 a | n.d. |
| Ethyl hexanoate | 2.78 ± 0.14 a | 2.98 ± 0.15 a | 2.99 ± 0.15 a | 5.35 ± 0.27 b |
| Ethyl heptanoate | 0.09 ± 0.03 a | 0.10 ± 0.01 a | 0.10 ± 0.01 a | 0.15 ± 0.06 a |
| Ethyl octanoate | 16.96 ± 1.85 ab | 13.70 ± 1.69 a | 20.14 ± 1.15 b | 14.07 ± 1.70 a |
| Ethyl nonanoate | 0.54 ± 0.06 a | 0.41 ± 0.09 a | 0.53 ± 0.08 a | 0.45 ± 0.07 a |
| Ethyl decanoate | 25.40 ± 2.27 b | 31.65 ± 2.58 bc | 38.89 ± 4.94 c | 16.52 ± 1.83 a |
| Ethyl lactate | 2.37 ± 0.42 a | 1.62 ± 0.48 a | 61.92 ± 3.10 c | 14.32 ± 0.72 b |
| Ethyl benzoate | 21.38 ± 1.07 b | 13.75 ± 0.69 a | 14.20 ± 0.71 a | 13.27 ± 0.66 a |
| Ethyl tetradecanoate | 6.18 ± 0.81 ab | 14.08 ± 2.57 c | 10.54 ± 2.53 bc | 5.29 ± 0.86 a |
| Compound [mg/L of alcohol 100% v/v] | Delikates | Kronselska | ||||||
| Raw Pulp | Deacidification | Pasteurization | Depectinization | Raw Pulp | Deacidification | Pasteurization | Depectinization | |
| Methanol | 1231.80 ± 36.95 c | 12,185.10 ± 365.55 b | 495.90 ± 35.85 d | 13,569.20 ± 407.108 a | 1412.10 ± 42.36 b | 15,166.50 ± 455.00 a | 339.70 ± 10.19 b | 14,891.10 ± 446.73 a |
| 2-Propanol | 3.48 ±0.42 a | n.d. | 1.14 ± 0.23 b | 2.86 ±0.29 a | n.d. | 2.75 ± 0.08 a | n.d. | n.d. |
| 1-Propanol | 264.20 ± 17.93 a | 271.80 ± 18.15 a | 198.50 ± 15.96 b | 144.70 ± 14.34 c | 385.40 ± 21.56 bc | 439.80 ± 33.19 a | 394.50 ± 21.84 bc | 368.50 ± 25.06 c |
| 2-Methyl-1-propanol | 894.50 ± 26.84 b | 1127.70 ± 33.83 a | 746.40 ± 22.39 c | 718.10 ±21.54 c | 592.90 ± 17.79 d | 1038.70 ± 42.19 a | 678.20 ± 20.35 b | 747.30 ± 22.42 b |
| 1-Butanol | 116.00 ± 5.50 a | 145.90 ± 47.40 a | 87.80 ± 26.60 a | 97.60 ± 25.90 a | 376.50 ± 11.30 b | 369.30 ± 11.10 b | 233.10 ± 6.90 c | 596.80 ± 17.90 a |
| 2-Methyl-1-butanol | 575.60 ± 17.30 c | 685.16 ± 20.56 ab | 643.80 ± 19.30 b | 714.80 ± 21.40 a | 677.10 ± 20.30 b | 1107.60 ± 33.21 a | 549.60 ± 16.50 c | 583.60 ± 17.52 c |
| 3-Methylbutanol | 1820.90 ± 120.60 bc | 2089.20 ± 162.68 ab | 1689.90 ± 70.70 c | 2292.50 ± 188.30 a | 2000.20 ± 160.01 ab | 2325.40 ± 169.76 a | 1555.20 ± 146.66 c | 1647.40 ± 149.42 bc |
| 1-Pentanol | 3.34 ±0.37 b | 6.59 ±0.78 a | 2.58 ± 0.48 b | 3.91 ± 0.82 b | 5.67 ± 0.77 a | 6.40 ± 0.19 a | 3.81 ± 0.16 b | 5.45 ± 0.16 a |
| 2-Hexanol | 0.52 ± 0.22 b | 0.29 ± 0.18 b | 12.24 ± 1.37 a | 15.42 ± 2.46 a | 10.05 ± 1.30 b | 76.17 ± 12.29 a | 9.57 ± 1.29 b | 7.38 ± 1.22 b |
| 1-Hexanol | 47.22 ± 5.42 b | 63.50 ± 8.71 a | 37.67 ± 5.13 b | 53.59 ± 4.61 ab | 127.26 ± 13.82 a | 138.89 ± 14.17 a | 71.59 ± 12.15 b | 91.76 ± 10.75 b |
| Furfuryl alcohol | 2.68 ± 0.68 b | 5.89 ± 2.18 ab | 7.11 ± 1.21 a | 9.42 ± 1.28 a | 7.55 ± 1.23 ab | 7.44 ± 1.22 b | 10.49 ± 1.31 a | 3.16 ± 0.59 c |
| Benzyl alcohol | 14.94 ± 1.45 a | 16.01 ± 10.48 a | 11.84 ± 1.36 a | 15.46 ± 2.46 a | 20.24 ± 2.61 a | 18.11 ± 2.54 a | 14.27 ± 3.43 a | 17.60 ± 3.53 a |
| 2-Phenyletanol | 67.51 ± 5.03 b | 70.44 ± 5.11 b | 61.84 ± 16.86 b | 218.71 ± 16.56 a | 70.63 ±5.12 b | 76.08 ± 5.28 b | 84.46 ± 7.53 b | 114.86 ± 3.45 a |
| 1-Nonanol | 40.34 ± 2.21 a | 43.37 ± 3.30 a | 43.32 ± 3.30 a | 46.15 ± 4.38 a | 34.87 ± 4.05 a | 35.34 ± 4.06 a | 37.26 ± 3.12 a | 41.83 ± 3.25 a |
| 2-Butanol | 0.43 ± 0.08 ab | 0.60 ± 0.10 a | 0.35 ± 0.08 b | 0.34 ± 0.02 b | 0.17 ± 0.08 b | 0.58 ± 0.06 a | 0.17 ± 0.03 b | 0.23 ± 0.01 b |
| 1-Octanol | 21.29 ± 0.64 a | 22.81 ± 0.68 a | 16.87 ± 0.51 b | 22.03 ± 0.66 a | 28.80 ± 3.86 a | 25.81 ± 1.77 ab | 20.34 ± 1.61 b | 25.08 ± 2.75 ab |
| Acetaldehyde | 171.74 ±22.15 bc | 278.70 ± 18.36 a | 197.09 ± 18.91 b | 132.54 ± 23.98 c | 216.66 ± 16.5 c | 1491.71 ± 15.75 a | 343.55 ±19.31 b | 349.86 ±14.5 b |
| Isovaleraldehyde | 1.08 ± 0.09 a | 1.17 ± 0.09 a | 0.57 ± 0.08 b | 1.03 ± 0.04 a | 1.11 ± 0.09 b | 1.75 ± 0.15 a | 0.83 ± 0.18 b | 1.13 ± 0.13 b |
| 2-Methylbutyraldehyde | 1.13 ± 0.15 a | 0.62 ± 0.08 b | 0.73 ± 0.07 b | 1.29 ±0.12 a | 1.11 ± 0.13 a | 1.33 ± 0.14 a | 1.41 ± 0.24 a | 2.02 ± 0.16 b |
| Valeraldehyde | 0.33 ± 0.05 a | 0.27 ± 0.05 a | 0.34 ± 0.07 a | 0.22 ± 0.06 a | 0.38 ± 0.07 bc | 1.15 ± 0.12 a | 0.45 ± 0.09 b | 0.22 ± 0.05 c |
| Heksanal | 2.98 ± 0.39 b | 4.70 ± 0.54 a | 2.48 ± 0.27 b | 3.50 ± 0.31 b | 5.59 ± 0.57 a | 7.15 ± 0.81 a | 5.71 ± 0.67 a | 3.86 ± 0.42 b |
| Phenylacetaldehyde | 1.02 ± 0.13 b | 0.79 ± 0.12 bc | 0.45 ± 0.06 c | 3.16 ± 0.29 a | 1.27 ± 0.14 b | 3.17 ± 0.24 a | 0.66 ± 0.52 b | 0.51 ± 0.42 b |
| Nonanal | 19.35 ± 0.88 a | 17.78 ± 0.63 ab | 17.19 ± 0.82 b | 13.02 ± 0.79 c | 17.93 ± 2.54 c | 43.62 ± 1.31 a | 23.67 ± 2.71 b | 16.56 ± 1.50 c |
| Decanal | 6.56 ± 0.60 a | 7.29 ± 0.62 a | 6.86 ± 0.71 a | 7.30 ± 0.52 a | 4.67 ± 0.54 b | 12.38 ± 1.37 a | 5.63 ± 0.67 b | 3.87 ± 0.42 b |
| Furfural | 10.67 ± 1.32 c | 31.92 ± 2.96 b | 6.35 ± 0.89 c | 51.45 ± 3.54 | 33.78 ± 2.01 c | 113.48 ± 8.40 a | 19.44 ± 1.58 d | 63.80 ± 4.91 b |
| Benzaldehyde | 21.22 ± 2.64 b | 34.58 ± 4.04 a | 8.55 ± 1.26 c | 10.31 ± 1.11 c | 11.89 ± 1.36 b | 21.15 ± 1.63 a | 7.47 ± 0.98 c | 9.88 ± 1.52 bc |
| Isobutyraldehyde | 43.48 ± 2.30 a | n.d. | 44.21 ± 3.33 a | 43.28 ± 3.30 a | 41.48 ± 3.24 a | 39.86 ± 3.20 a | 43.41 ± 3. a 30 | 48.91 ± 4.47 a |
| Trans-2-heptanal | 20.98 ± 3.63 a | 18.79 ± 2.56 a | 18.57 ± 2.56 a | 20.18 ± 2.61 a | 19.91 ± 1.60 a | 20.05 ± 2.18 a | 19.01 ± 1.57 a | 19.63 ± 1.59 a |
| 3-Etoksypropionaldehyde | 16.61 ± 1.50 a | 16.62 ± 1.23 a | 16.38 ± 1.49 a | n.d. | n.d. | 19.82 ± 1.60 a | 18.47 ± 1.55 a | 17.09 ± 1.51 a |
| Compound [mg/L alcohol 100% v/v] | Delikates | Kronselska | ||||||
| Raw pulp | Deacidification | Pasteurization | Raw pulp | Deacidification | Pasteurization | Raw pulp | Deacidification | |
| 2-Propanone | n.d. | 8.53 ± 0.76 a | 4.30 ± 0.52 b | 5.33 ± 0.76 b | 4.32 ± 0.63 b | 9.74 ± 0.89 a | 4.41 ± 0.33 b | 3.48 ± 0.67 b |
| 2,3-Butanedione | 3.81 ± 0.51 a | 0.48 ± 0.16 c | 0.22 ± 0.09 c | 2.03 ± 0.36 b | 5.93 ± 0.38 b | 18.28 ± 1.55 a | 0.75 ± 0.08 c | 2.15 ± 0.46 c |
| Acetaldehyde diethyl acetal | 1131.90 ± 33.96 c | 1627.30 ± 48.82 b | 1856.1 ± 55.68 a | 823.4 ± 24.7 d | 1616.2 b ± 48.5 | 1208.9 c ± 36.3 | 2419.4 a ± 72.6 | 583.6 d ± 17.5 |
| 2,3-Pentanodione | 10.39 ab ±1.31 | 8.28 b ± 0.95 | 11.87 a ± 1.36 | 10.01 ab ± 1.30 | 17.37 ± 2.52 a | 12.96 ± 1.39 a | 16.35 ± 1.49 a | 22.50 ± 1.68 b |
| 3-Octanone | 37.13 ± 15.11 ab | 50.27 ± 4.51 a | 22.59 ± 3.68 b | 27.16 ± 3.81 b | 28.63 ± 3.86 ab | 37.06 ± 5.11 a | 24.55 ± 3.74 b | 26.76 ± 3.80 ab |
| Ethyl formate | 1.37 ± 0.07 c | 4.76 ± 0.24 a | 0.42 ± 0.02 d | 2.39 ± 0.12 b | 3.83 ± 0.19 a | 1.83 ± 0.39 b | 1.49 ± 0.27 b | 1.42 ± 0.27 b |
| Ethyl acetate | 339.80 ± 41.99 b | 712.00 ± 59.60 a | 367.30 ± 40.62 b | 314.70 ± 25.89 b | 383.00 ±34.15 b | 869.20 ± 34.56 a | 355.80 ± 39.79 b | 301.50 ± 65.08 b |
| Isobutyl acetate | 2.03 ± 0.29 a | 1.36 ± 0.17 b | 0.75 ± 0.08 c | 0.40 ± 0.09 c | 1.36 ± 0.27 b | 9.61 ± 0.48 a | 0.88 ± 0.09 b | 1.51 ± 0.28 b |
| Butyl acetate | 1.26 ± 0.26 b | 0.78 ± 0.19 bc | 1.91 ± 0.35 a | 0.53 ± 0.12 c | 6.71 ± 0.94 b | 8.43 ± 1.42 b | 8.01 ± 0.40 b | 13.56 ± 2.68 a |
| 3-Methylbutyl acetate | 14.32 ± 1.72 a | 14.15 ± 0.71 ab | 9.73 ± 2.49 bc | 9.34 ± 1.47 c | 14.31 ± 1.72 c | 37.01 ± 2.85 b | 38.04 ± 1.90 b | 76.45 ± 3.82 a |
| 2-Methylbutyl acetate | 1.22 ± 0.16 a | 1.16 ± 0.16 a | 1.42 ± 0.17 a | 0.64 ± 0.09 b | 1.98 ± 0.15 c | 9.44 ± 0.47 a | 1.89 ± 0.19 c | 3.08 ± 0.15 b |
| Hexyl acetate | 0.20 ± 0.01 b | 0.20 ± 0.01 b | 0.60 ± 0.03 a | 0.12 ± 0.01 c | 0.98 ± 0.049 c | 2.33 ± 0.12 b | 3.28 ± 0.16 a | 3.24 ± 0.16 a |
| 2-Phenylethyl acetate | 2.40 ± 0.35 b | 2.35 ± 0.32 b | 3.90 ± 0.27 a | 2.96 ± 0.35 b | 7.72 ± 0.69 c | 8.89 ± 1.94 bc | 13.85 ± 2.69 ab | 18.31 ± 2.12 a |
| Methyl acetate | 2.28 ± 0.11 c | 30.85 ± 1.54 a | 9.12 ± 0.46 b | 30.32 ± 1.52 a | 12.58 ±0.63 c | 428.23 ±21.41 a | n.d. | 230.95 ±11.54 b |
| Ethyl propanoate | 1.93 ± 0.35 b | 1.52 ± 0.18 b | 2.76 ± 0.14 a | 2.60 ± 0.13 a | 8.75 ± 0.94 b | 5.13 ± 0.26 c | 7.27 ± 0.86 bc | 11.65 ± 1.58 a |
| Isoamyl propanoate | 0.08 ± 0.01 a | 0.06 ± 0.01 a | 0.08 ± 0.02 a | 0.07 ± 0.01 a | 0.14 ± 0.02 a | 0.18 ± 0.04 a | 0.15 ± 0.01 a | 0.21 ± 0.05 a |
| Ethyl isobutyrate | 0.41 ± 0.11 a | 0.48 ± 0.12 a | 0.30 ± 0.07 ab | 0.14 ± 0.01 b | 0.19 ± 0.03 b | 4.89 ± 0.24 a | 0.29 ± 0.07 b | 0.39 ± 0.09 b |
| Ethyl butyrate | 1.00 ± 0.08 a | 1.14 ± 0.06 a | 0.78 ± 0.07 b | 0.66 ± 0.09 b | 0.86 ± 0.14 b | 2.85 ± 0.14 a | 0.64 ± 0.13 b | 0.54 ± 0.11 b |
| Ethyl 2-methylbutyrate | 0.26 ± 0.05 a | 0.33 ± 0.05 a | 0.06 ± 0.01 b | 0.09 ± 0.03 b | 0.13 ± 0.04 b | 0.70 ± 0.14 a | 0.03 ± 0.01 b | 0.05 ± 0.02 b |
| Ethyl 3-methylbutyrate | 0.28 ± 0.01 b | 0.54 ± 0.03 a | 0.13 ± 0.04 c | 0.07 ± 0.03 c | 0.05 ± 0.02 b | 2.50 ± 0.13 a | 0.07 ± 0.02 b | 0.05 ± 0.01 b |
| Ethyl hexanoate | 3.62 ± 0.88 ab | 4.93 ± 0.75 a | 1.92 ± 0.10 c | 2.21 ± 0.27 bc | 2.74 ± 0.36 b | 7.85 ± 0.89 a | 2.10 ± 0.11 b | 1.61 ± 0.39 b |
| Ethyl heptanoate | 0.18 ± 0.02 a | 0.22 ± 0.05 a | 0.13 ± 0.06 a | 0.10 ± 0.05 a | 0.11 ± 0.01 c | 0.26 ± 0.04 a | 0.19 ± 0.03 b | 0.12 ± 0.01 c |
| Ethyl octanoate | 9.42 ± 1.47 a | 11.44 ± 2.57 a | 10.32 ± 1.52 a | 10.91 ± 1.55 a | 6.90 ± 1.35 a | 8.20 ± 2.91 a | 8.82 ± 2.44 a | 6.45 ± 2.32 a |
| Ethyl nonanoate | 0.31 ± 0.02 b | 0.61 ± 0.03 a | 0.27 ± 0.04 b | 0.31 ± 0.03 b | 0.26 ± 0.05 b | 0.86 ± 0.04 a | 0.33 ± 0.04 b | 0.33 ± 0.02 b |
| Ethyl decanoate | 12.30 ± 1.62 b | 13.46 ± 1.67 ab | 15.98 ± 2.80 ab | 19.26 ± 2.96 a | 8.27 ±0.41 c | 27.69 ± 2.38 a | 15.71 ± 2.79 b | 12.51 ± 1.63 bc |
| Ethyl lactate | 12.23 ± 1.61 a | 10.13 ± 1.36 a | 0.24 ± 0.03 b | n.d. | 49.83 ± 3.49 a | 46.42 ± 2.82 a | n.d. | n.d. |
| Ethyl benzoate | 8.96 ± 0.84 a | 9.52 ± 0.45 a | 10.41 ± 1.52 a | 11.90 ± 2.60 a | 5.98 ± 0.30 c | 2.14 ± 0.11 c | 10.93 ± 1.55 a | 8.71 ± 1.44 ab |
| Ethyl tetradecanoate | 6.59 ± 0.83 a | 4.96 ± 0.72 a | 5.38 ± 0.27 a | 5.07 ± 0.75 a | 9.19 ± 1.46 b | 18.68 ± 1.93 a | 7.40 ± 1.36 b | 6.59 ± 0.33 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balcerek, M.; Pielech-Przybylska, K.; Dziekońska-Kubczak, U.; Bartosik, A. Effect of Apple Cultivar and Selected Technological Treatments on the Quality of Apple Distillate. Foods 2023, 12, 4494. https://doi.org/10.3390/foods12244494
Balcerek M, Pielech-Przybylska K, Dziekońska-Kubczak U, Bartosik A. Effect of Apple Cultivar and Selected Technological Treatments on the Quality of Apple Distillate. Foods. 2023; 12(24):4494. https://doi.org/10.3390/foods12244494
Chicago/Turabian StyleBalcerek, Maria, Katarzyna Pielech-Przybylska, Urszula Dziekońska-Kubczak, and Anita Bartosik. 2023. "Effect of Apple Cultivar and Selected Technological Treatments on the Quality of Apple Distillate" Foods 12, no. 24: 4494. https://doi.org/10.3390/foods12244494