Influence of Supercritical Carbon Dioxide on the Activity and Conformational Changes of α-Amylase, Lipase, and Peroxidase in the Solid State Using White Wheat Flour as an Example
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Samples
2.2. Supercritical Carbon Dioxide Treatment
2.3. Extraction Protocol and Determination of Total Protein Content
2.4. Determination of Enzymes Residual Activities
2.5. Circular Dichroism Spectroscopy
2.6. Determination of Zeta Potential
2.7. Determination of Particle Size Distribution
2.8. Statistical Analysis
3. Results and Discussion
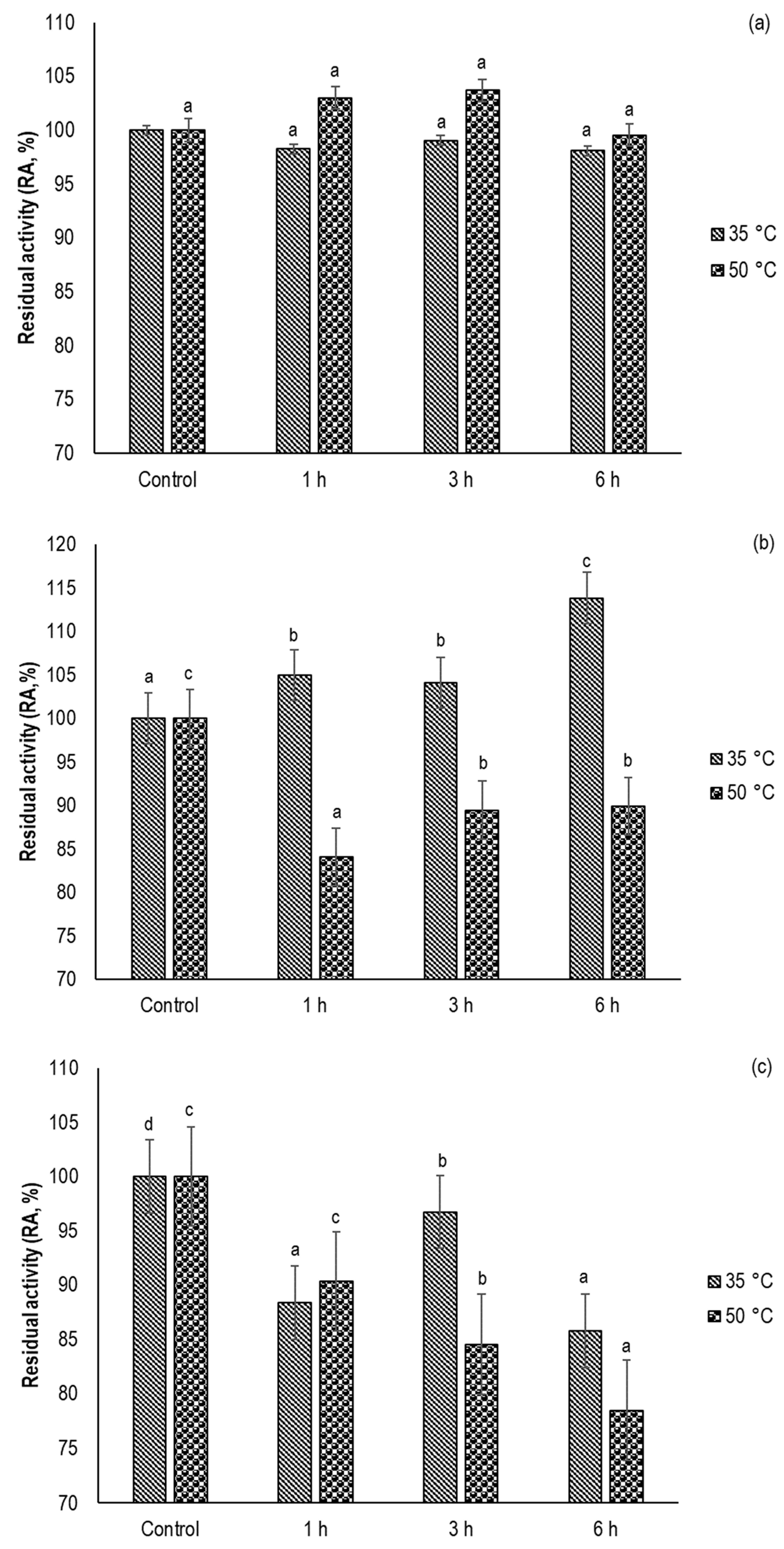
3.1. Inactivation Rate of α-amylase, Lipase, and POD in White Wheat Flour
3.2. Effect of sc-CO2 Treatment on the Native Enzyme Activity
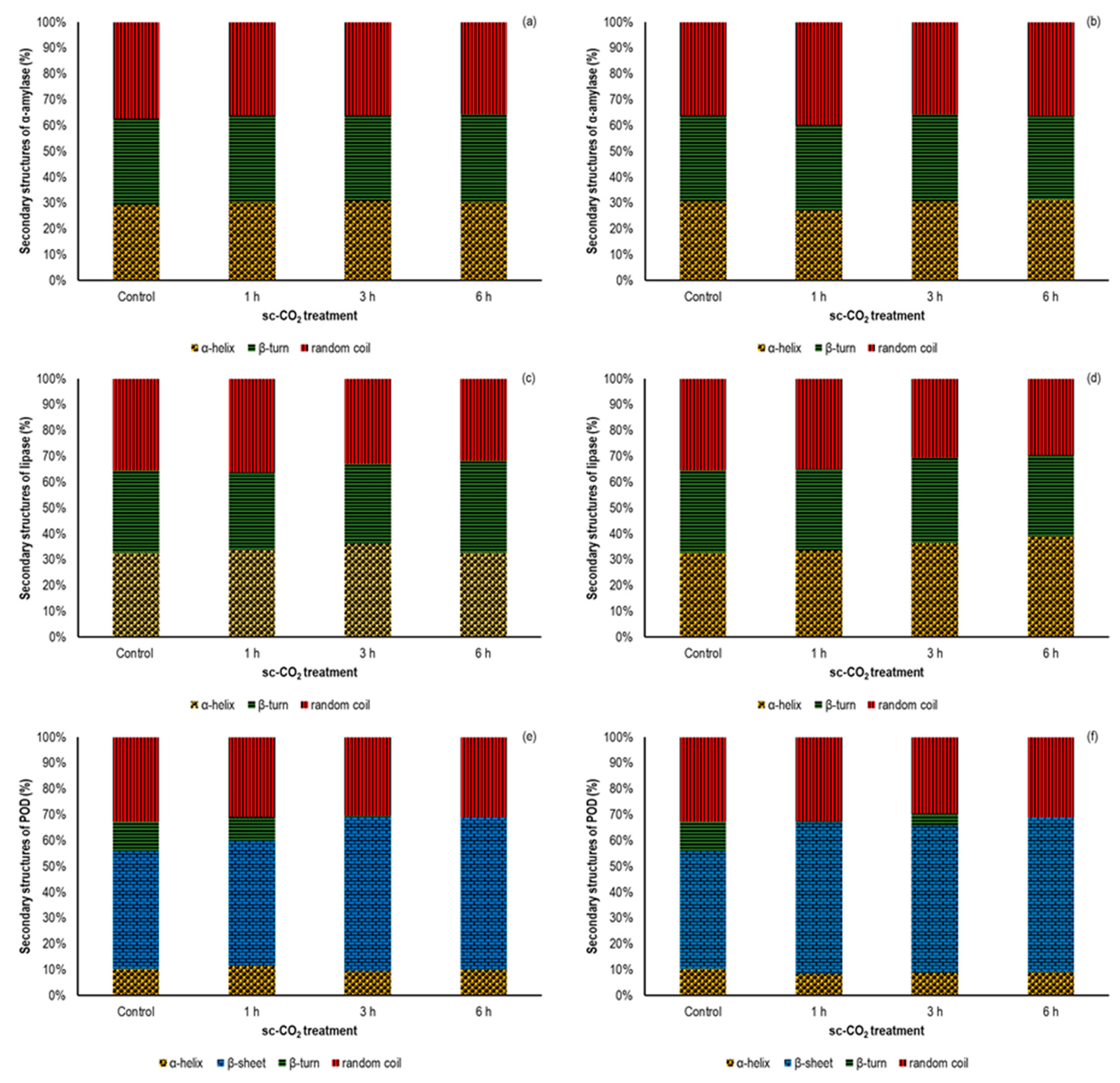
3.3. Effect of sc-CO2 on the Secondary Structure of the Investigated Enzymes
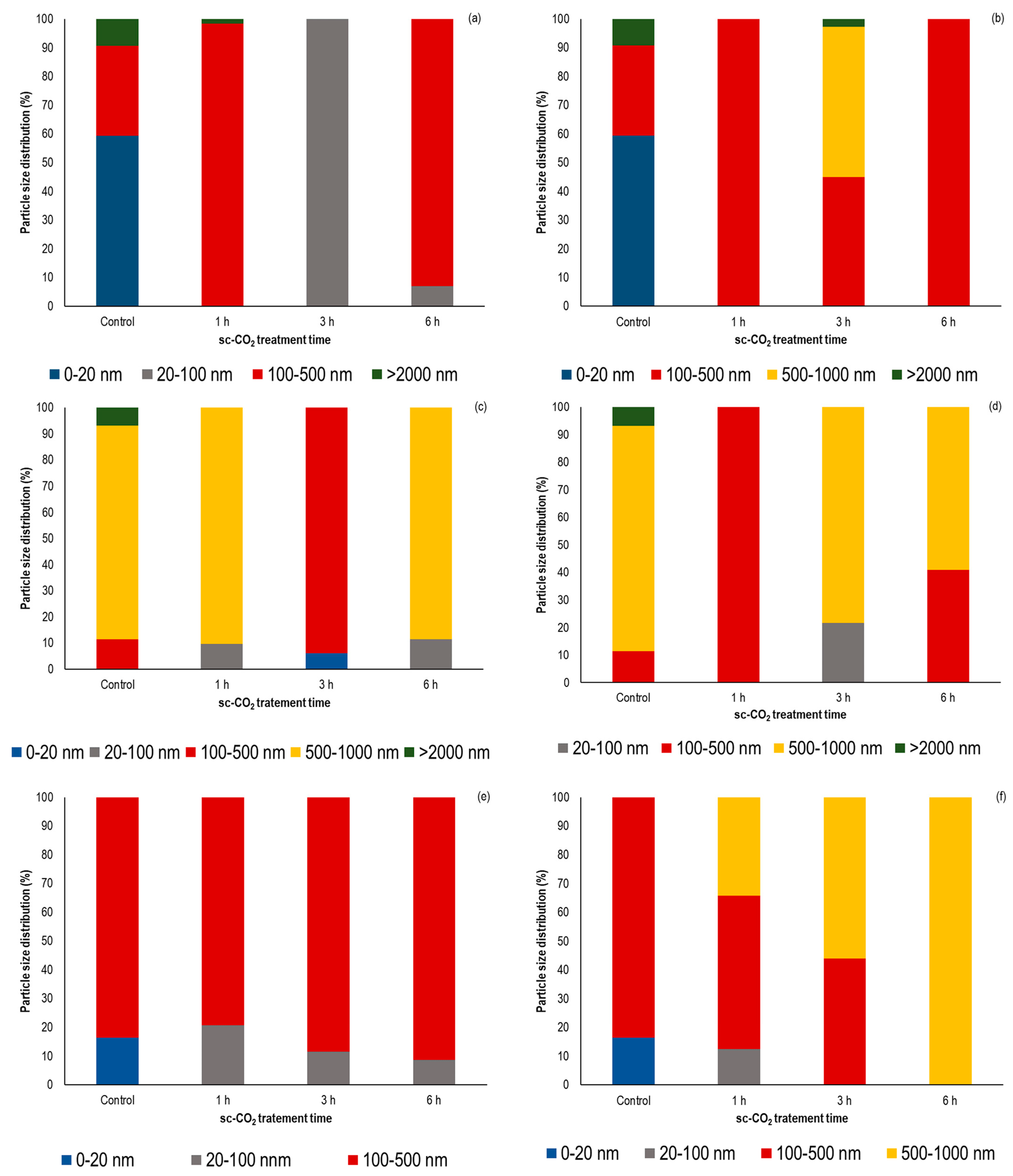
3.4. Influence of sc-CO2 Treatment on the Particle Size Distribution
3.5. Influence of sc-CO2 Treatment on the ζ-Potential
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shouket, S.; Khurshid, S.; Khan, J.; Batool, R.; Sarwar, A.; Aziz, T.; Alhomrani, M.; Alamri, A.S.; Sameeh, M.Y.; Zubair Filimban, F. Enhancement of shelf-life of food items via immobilized enzyme nanoparticles on varied supports. A sustainable approach towards food safety and sustainability. Food Res. Int. 2023, 169, 112940. [Google Scholar] [CrossRef] [PubMed]
- Leitgeb, M.; Knez, Ž.; Hojnik Podrepšek, G. Effect of Green Food Processing Technology on the Enzyme Activity in Spelt Flour. Foods 2022, 11, 3832. [Google Scholar] [CrossRef] [PubMed]
- Li, M.J.; Wang, H.R.; Tong, L.T.; Fan, B.; Yang, X.J.; Sun, R.Q.; Liu, L.Y.; Wang, F.Z.; Wang, L.L. A comparison study of three heating assisted enzyme inactivation pretreatments on the physicochemical properties and edible quality of highland barley grain and flour. J. Cereal Sci. 2022, 104, 103404. [Google Scholar] [CrossRef]
- Lancelot, E.; Fontaine, J.; Grua-Priol, J.; Le-Bail, A. Effect of long-term storage conditions on wheat flour and bread baking properties. Food Chem. 2021, 346, 128902. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Qian, X.; Sun, B.; Tian, X.; Wang, X.; Ma, S. Effect of roasting treatment on the micromorphology, gelatinization, structure, and digestibility of whole oat flour. LWT 2022, 168, 113828. [Google Scholar] [CrossRef]
- Zhao, B.; Shang, J.; Liu, L.; Tong, L.T.; Zhou, X.; Wang, S.; Zhang, Y.; Wang, L.; Zhou, S. Effect of roasting process on enzymes inactivation and starch properties of highland barley. Int. J. Biol. Macromol. 2020, 165, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Bulhões Bezerra Cavalcante, T.A.; dos Santos Funcia, E.; Wilhelms Gut, J.A. Inactivation of polyphenol oxidase by microwave and conventional heating: Investigation of thermal and non-thermal effects of focused microwaves. Food Chem. 2021, 340, 127911. [Google Scholar] [CrossRef]
- Kubo, M.T.K.; Curet, S.; Augusto, P.E.D.; Boillereaux, L. Multiphysics modeling of microwave processing for enzyme inactivation in fruit juices. J. Food Eng. 2019, 263, 366–379. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, L.; Li, Z. Superheated steam treatment on wheat bran: Enzymes inactivation and nutritional attributes retention. LWT 2018, 91, 446–452. [Google Scholar] [CrossRef]
- Wu, Y.; Li, W.; Martin, G.J.O.; Ashokkumar, M. Mechanism of low-frequency and high-frequency ultrasound-induced inactivation of soy trypsin inhibitors. Food Chem. 2021, 360, 130057. [Google Scholar] [CrossRef]
- Yu, Z.L.; Zeng, W.C.; Zhang, W.H.; Liao, X.P.; Shi, B. Effect of ultrasound on the activity and conformation of α-amylase, papain and pepsin. Ultrason. Sonochem. 2014, 21, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhang, B.; Pang, H.; Wang, Y.; Fu, H.; Chen, X.; Wang, Y. The effect of radio frequency heating on the inactivation and structure of horseradish peroxidase. Food Chem. 2022, 398, 133875. [Google Scholar] [CrossRef]
- Xu, J.; Wang, B.; Wang, Y.; Zhang, M.; Chitrakar, B. Effect of Applied Voltage on the Aggregation and Conformational Changes in Peroxidase Under Electrospray. Food Bioprocess. Technol. 2020, 13, 245–255. [Google Scholar] [CrossRef]
- Guionet, A.; Fujiwara, T.; Sato, H.; Takahashi, K.; Takaki, K.; Matsui, M.; Tanino, T.; Ohshima, T. Pulsed electric fields act on tryptophan to inactivate α-amylase. J. Electrostat. 2021, 112, 103597. [Google Scholar] [CrossRef]
- Gu, Y.; Shi, W.; Liu, R.; Xing, Y.; Yu, X.; Jiang, H. Cold plasma enzyme inactivation on dielectric properties and freshness quality in bananas. Innov. Food Sci. Emerg. Technol. 2021, 69, 102649. [Google Scholar] [CrossRef]
- Marszałek, K.; Doesburg, P.; Starzonek, S.; Szczepańska, J.; Woźniak, Ł.; Lorenzo, J.M.; Skaopska, S.; Rzoska, S.; Barba, F.J. Comparative effect of supercritical carbon dioxide and high pressure processing on structural changes and activity loss of oxidoreductive enzymes. J. CO2 Util. 2019, 29, 46–56. [Google Scholar] [CrossRef]
- Marszałek, K.; Kruszewski, B.; Woźniak, Ł.; Skąpska, S. The application of supercritical carbon dioxide for the stabilization of native and commercial polyphenol oxidases and peroxidases in cloudy apple juice (cv. Golden Delicious). Innov. Food Sci. Emerg. Technol. 2017, 39, 42–48. [Google Scholar] [CrossRef]
- Monhemi, H.; Jalali, B. Changing the residues interaction pattern as a universal mechanism for enzyme inactivation and denaturation in supercritical CO2. J. Mol. Liq. 2021, 325, 114884. [Google Scholar] [CrossRef]
- Zambon, A.; Facco, P.; Morbiato, G.; Toffoletto, M.; Poloniato, G.; Sut, S.; Andrigo, P.; Dall’Acqua, S.; de Bernard, M.; Spilimbergo, S. Promoting the preservation of strawberry by supercritical CO2 drying. Food Chem. 2022, 397, 133789. [Google Scholar] [CrossRef]
- Suo, T.; Guo, X.N.; Zhu, K.X. Effects of tempering with plasma-activated water on total plate count and quality properties of wheat flour. J. Cereal Sci. 2022, 105, 103468. [Google Scholar] [CrossRef]
- Zeng, Z.; Wang, Y.; Xu, G.; Zhou, L.; Liu, C.; Luo, S. Peroxidase inactivation by cold plasma and its effects on the storage, physicochemical and bioactive properties of brown rice. Food Biosci. 2023, 52, 102383. [Google Scholar] [CrossRef]
- Podrepšek, G.H.; Knez, Ž.; Leitgeb, M. Development of chitosan functionalized magnetic nanoparticles with bioactive compounds. Nanomaterials 2020, 10, 1913. [Google Scholar] [CrossRef]
- Leitgeb, M.; Knez, Ž.; Podrepšek, G.H. Enzyme Activity and Physiochemical Properties of Flour after Supercritical Carbon Dioxide Processing. Foods 2022, 11, 1826. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, F.M.; Morbiato, G.; Facco, P.; Marszałek, K.; Pérez-Esteve, É.; Benedito, J.; Zambon, A.; Spilimbergo, S. Optimization of the supercritical CO2 pasteurization process for the preservation of high nutritional value of pomegranate juice. J. Supercrit. Fluids 2020, 164, 104914. [Google Scholar] [CrossRef]
- Machado, N.D.; Mosquera, J.E.; Martini, R.E.; Goñi, M.L.; Gañán, N.A. Supercritical CO2-assisted impregnation/deposition of polymeric materials with pharmaceutical, nutraceutical, and biomedical applications: A review (2015–2021). J. Supercrit. Fluids 2022, 191, 105763. [Google Scholar] [CrossRef]
- Pravallika, K.; Chakraborty, S.; Singhal, R.S. Supercritical drying of food products: An insightful review. J. Food Eng. 2023, 343, 111375. [Google Scholar] [CrossRef]
- Zorić, M.; Banožić, M.; Aladić, K.; Vladimir-Knežević, S.; Jokić, S. Supercritical CO2 extracts in cosmetic industry: Current status and future perspectives. Sustain. Chem. Pharm. 2022, 27. [Google Scholar] [CrossRef]
- Monhemi, H. Protein simulation in supercritical CO2: The challenge of force field. J. Mol. Liq. 2021, 343, 117662. [Google Scholar] [CrossRef]
- Sheikh, M.A.; Saini, C.S.; Sharma, H.K. Structural modification of plum (Prunus domestica L) kernel protein isolate by supercritical carbon-dioxide treatment: Functional properties and in-vitro protein digestibility. Int. J. Biol. Macromol. 2023, 230, 123128. [Google Scholar] [CrossRef]
- Gui, F.; Chen, F.; Wu, J.; Wang, Z.; Liao, X.; Hu, X. Inactivation and structural change of horseradish peroxidase treated with supercritical carbon dioxide. Food Chem. 2006, 97, 480–489. [Google Scholar] [CrossRef]
- Marszałek, K.; Krzyżanowska, J.; Woźniak, Ł.; Skąpska, S. Kinetic modelling of polyphenol oxidase, peroxidase, pectin esterase, polygalacturonase, degradation of the main pigments and polyphenols in beetroot juice during high pressure carbon dioxide treatment. LWT 2017, 85, 412–417. [Google Scholar] [CrossRef]
- Marszałek, K.; Skąpska, S.; Woźniak, Ł.; Sokołowska, B. Application of supercritical carbon dioxide for the preservation of strawberry juice: Microbial and physicochemical quality, enzymatic activity and the degradation kinetics of anthocyanins during storage. Innov. Food Sci. Emerg. Technol. 2015, 32, 101–109. [Google Scholar] [CrossRef]
- Leitgeb, M.; Čolnik, M.; Primožič, M.; Zalar, P.; Cimerman, N.G.; Knez, Ž. Activity of cellulase and α-amylase from Hortaea werneckii after cell treatment with supercritical carbon dioxide. J. Supercrit. Fluids 2013, 78, 143–148. [Google Scholar] [CrossRef]
- Senyay-Oncel, D.; Yesil-Celiktas, O. Activity and stability enhancement of α-amylase treated with sub- and supercritical carbon dioxide. J. Biosci. Bioeng. 2011, 112, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, P.; Rezende, C.A.; Martínez, J. Activity of immobilized lipase from Candida antarctica (Lipozyme 435) and its performance on the esterification of oleic acid in supercritical carbon dioxide. J. Supercrit. Fluids 2016, 107, 170–178. [Google Scholar] [CrossRef]
- Podrepšek, G.H.; Knez, Ž.; Leitgeb, M. The Influence of Supercritical Carbon Dioxide on Graham Flour Enzyme Polyphenol Oxidase Activity. Molecules 2020, 25, 5981. [Google Scholar] [CrossRef] [PubMed]
- Pliego, J.; Mateos, J.C.; Rodriguez, J.; Valero, F.; Baeza, M.; Femat, R.; Camacho, R.; Sandoval, G.; Herrera-López, E.J. Monitoring lipase/esterase activity by stopped flow in a sequential injection analysis system using p-nitrophenyl butyrate. Sensors 2015, 15, 2798–2811. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, L.; Murtaza, A.; Iqbal, A.; Zhang, J.; Xu, X.; Pan, S.; Hu, W. The effect of high pressure carbon dioxide on the inactivation kinetics and structural alteration of phenylalanine ammonia-lyase from Chinese water chestnut: An investigation using multi-spectroscopy and molecular docking methods. Innov. Food Sci. Emerg. Technol. 2022, 77, 102970. [Google Scholar] [CrossRef]
- Allai, F.M.; Azad, Z.R.A.A.; Mir, N.A.; Gul, K. Recent advances in non-thermal processing technologies for enhancing shelf life and improving food safety. Appl. Food Res. 2023, 3, 100258. [Google Scholar] [CrossRef]
- Feng, J.; Zheng, Y.; Zhang, X.; Zhou, R.; Ma, M. Effect of supercritical carbon dioxide on bacterial community, volatile profiles and quality changes during storage of Mongolian cheese. Food Control. 2023, 143, 109225. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, S.; Ji, H.; Zhang, C.; Deng, C.; Cao, W.; Mao, W.; Gao, J. Inactivation of polyphenol oxidase from Pacific white shrimp by dense phase carbon dioxide. Innov. Food Sci. Emerg. Technol. 2011, 12, 635–641. [Google Scholar] [CrossRef]
- Bahrami, N.; Bayliss, D.; Chope, G.; Penson, S.; Perehinec, T.; Fisk, I.D. Cold plasma: A new technology to modify wheat flour functionality. Food Chem. 2016, 202, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Yadav, J.K.; Prakash, V. Thermal stability of α-amylase in aqueous cosolvent systems. J. Biosci. 2009, 34, 377–387. [Google Scholar] [CrossRef] [PubMed]
- De Schepper, C.F.; Buvé, C.; Van Loey, A.M.; Courtin, C.M. A kinetic study on the thermal inactivation of barley malt α-amylase and β-amylase during the mashing process. Food Res. Int. 2022, 157, 111201. [Google Scholar] [CrossRef] [PubMed]
- Mathias, O.K.; Michael, D.; Forbes, L.; Joseph, A. Lipase inactivation by pressurized CO2 in presence of sensitizing additives. Eur. J. Sci. Res. 2010, 43, 272–282. [Google Scholar]
- Ling, B.; Lyng, J.G.; Wang, S. Effects of hot air-assisted radio frequency heating on enzyme inactivation, lipid stability and product quality of rice bran. LWT 2018, 91, 453–459. [Google Scholar] [CrossRef]
- Skřivan, P.; Sluková, M.; Jurkaninová, L.; Švec, I. Preliminary investigations on the use of a new milling technology for obtaining wholemeal flours. Appl. Sci. 2021, 11, 6138. [Google Scholar] [CrossRef]
- Leitgeb, M.; Knez, Z. The influence of water on the synthesis of n-butyl oleate by immobilized Mucor miehei lipase. J. Am. Oil Chem. Soc. 1990, 67, 775–778. [Google Scholar] [CrossRef]
- Hu, W.; Zhou, L.; Xu, Z.; Zhang, Y.; Liao, X. Enzyme Inactivation in Food Processing using High Pressure Carbon Dioxide Technology. Crit. Rev. Food Sci. Nutr. 2013, 53, 145–161. [Google Scholar] [CrossRef]
- Amaral, G.V.; Silva, E.K.; Cavalcanti, R.N.; Cappato, L.P.; Guimaraes, J.T.; Alvarenga, V.O.; Esmerino, E.A.; Portela, J.B.; Sant’ Ana, A.S.; Freitas, M.Q.; et al. Dairy processing using supercritical carbon dioxide technology: Theoretical fundamentals, quality and safety aspects. Trends Food Sci. Technol. 2017, 64, 94–101. [Google Scholar] [CrossRef]
- Kamat, S.; Critchley, G.; Beckman, E.J.; Russell, A.J. Biocatalytic Synthesis of Acrylates in Organic Solvents and Supercritical Fluids: 111. Does Carbon Dioxide Covalently Modify Enzymes? yanjay. Biotechnol. Bioeng. 1995, 46, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Habulin, M.; Knez, Ž. Influence of Reaction Parameters on Synthesis of n-Butyl Oleate by. Eur. J. Lipid Sci. Technol. 1993, 95, 249–252. [Google Scholar]
- Wimmer, Z.; Zarevúcka, M. A review on the effects of supercritical carbon dioxide on enzyme activity. Int. J. Mol. Sci. 2010, 11, 233–253. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.X.; Cheng, J.H.; Sun, D.W. Changes in activity, structure and morphology of horseradish peroxidase induced by cold plasma. Food Chem. 2019, 301, 125240. [Google Scholar] [CrossRef] [PubMed]
- Miles, A.J.; Janes, R.W.; Wallace, B.A. Tools and methods for circular dichroism spectroscopy of proteins: A tutorial review. Chem. Soc. Rev. 2021, 50, 8400–8413. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, N.J. Using circular dichroism spectra to estimate protein secondary structure. Nat. Protoc. 2007, 1, 2876–2890. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Li, C.; Huang, W.; Guo, C.; Jin, W.; Shen, W. Structural Transitions of Alpha-Amylase Treated with Pulsed Electric Fields: Effect of Coexisting Carrageenan. Foods 2022, 11, 4112. [Google Scholar] [CrossRef]
- Tian, M.L.; Fang, T.; Du, M.Y.; Zhang, F.S. Effects of Pulsed Electric Field (PEF) Treatment on Enhancing Activity and Conformation of α-Amylase. Protein J. 2016, 35, 154–162. [Google Scholar] [CrossRef]
- Esmaeilnejad-Ahranjani, P.; Kazemeini, M.; Singh, G.; Arpanaei, A. Amine-functionalized magnetic nanocomposite particles for efficient immobilization of lipase: Effects of functional molecule size on properties of the immobilized lipase. RSC Adv. 2015, 5, 33313–33327. [Google Scholar] [CrossRef]
- Kumar, V.; Yedavalli, P.; Gupta, V.; Rao, N.M. Engineering lipase A from mesophilic Bacillus subtilis for activity at low temperatures. Protein Eng. Des. Sel. 2014, 27, 73–82. [Google Scholar] [CrossRef]
- Shikha, S.; Thakur, K.G.; Bhattacharyya, M.S. Facile fabrication of lipase to amine functionalized gold nanoparticles to enhance stability and activity. RSC Adv. 2017, 7, 42845–42855. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, B.; Guo, X.; Liu, D.; Qiu, C.; Ma, H. Thermosonication inactivation of horseradish peroxidase with different frequency modes: Effect on activity, structure, morphology and mechanisms. Food Chem. 2022, 384, 132537. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Tang, Y. The activation mechanism of peroxidase by ultrasound. Ultrason. Sonochem. 2021, 71, 105362. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhang, Y.; Wang, Y.; Zhou, L.; Leng, X.; Liao, X.; Hu, X. Aggregation and homogenization, surface charge and structural change, and inactivation of mushroom tyrosinase in an aqueous system by subcritical/supercritical carbon dioxide. Langmuir 2011, 27, 909–916. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rathod, V.K. Sonochemical Effect on Activity and Conformation of Commercial Lipases. Appl. Biochem. Biotechnol. 2017, 181, 1435–1453. [Google Scholar] [CrossRef]
- Jachimska, B.; Wasilewska, M.; Adamczyk, Z. Characterization of globular protein solutions by dynamic light scattering, electrophoretic mobility, and viscosity measurements. Langmuir 2008, 24, 6867–6872. [Google Scholar] [CrossRef]


| Determined Values | Control Sample (Untreated) | Temperature | ||||||
|---|---|---|---|---|---|---|---|---|
| 35 °C | 42.5 °C | 50 °C | ||||||
| Pressure (Bar) | ||||||||
| 200 | 300 | 200 | 300 | 200 | 300 | |||
| Total protein content (g/100g) | 1.23 ± 0.02 a | 1.23 ± 0.01 a | 1.22 ± 0.04 a | 1.24 ± 0.03 a | 1.22 ± 0.03 a | 1.33 ± 0.00 a | 1.28 ± 0.08 a | |
| Residual activity (%) | α-amylase | 100 a | 115.4 ± 10.9 a | 115.2 ± 10.2 a | 125.6 ± 16.6 a | 116.0 ± 8.2 a | 114.1 ± 13.0 a | 112.1 ± 4.6 a |
| lipase | 100 e | 38.5 ± 2.7 b,c | 60.5 ± 3.0 d | 93.5 ± 8.6 e | 42.5 ± 3.9 c | 29.7 ± 2.7 b | 18.4 ± 1.2 a | |
| POD | 100 a | 124.5 ± 1.0 b | 135.0 ± 2.5 c | 105.6 ± 4.8 a | 101.6 ± 5.2 a | 105.9 ± 2.4 a | 103.0 ± 1.7 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanović, M.; Knez, Ž.; Leitgeb, M. Influence of Supercritical Carbon Dioxide on the Activity and Conformational Changes of α-Amylase, Lipase, and Peroxidase in the Solid State Using White Wheat Flour as an Example. Foods 2023, 12, 4499. https://doi.org/10.3390/foods12244499
Ivanović M, Knez Ž, Leitgeb M. Influence of Supercritical Carbon Dioxide on the Activity and Conformational Changes of α-Amylase, Lipase, and Peroxidase in the Solid State Using White Wheat Flour as an Example. Foods. 2023; 12(24):4499. https://doi.org/10.3390/foods12244499
Chicago/Turabian StyleIvanović, Milena, Željko Knez, and Maja Leitgeb. 2023. "Influence of Supercritical Carbon Dioxide on the Activity and Conformational Changes of α-Amylase, Lipase, and Peroxidase in the Solid State Using White Wheat Flour as an Example" Foods 12, no. 24: 4499. https://doi.org/10.3390/foods12244499






