Prevalence of Campylobacter spp., Salmonella spp., and Listeria monocytogenes, and Population Levels of Food Safety Indicator Microorganisms in Retail Raw Chicken Meat and Ready-To-Eat Fresh Leafy Greens Salads Sold in Greece
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Information on Sampling
2.2. Microbiological Analyses
2.2.1. Detection and Identification of Pathogens
2.2.2. Enumeration of Hygiene (Safety) Indicator Organisms
2.3. Campylobacter Species Identification and Subtyping
2.3.1. Preparation of Bacterial Cultures and Genomic DNA (gDNA) Isolation
2.3.2. Multiplex PCR (m-PCR)
2.3.3. Rep-PCR and Phylogenetic Discrimination of the Isolates
2.4. Statistics
3. Results
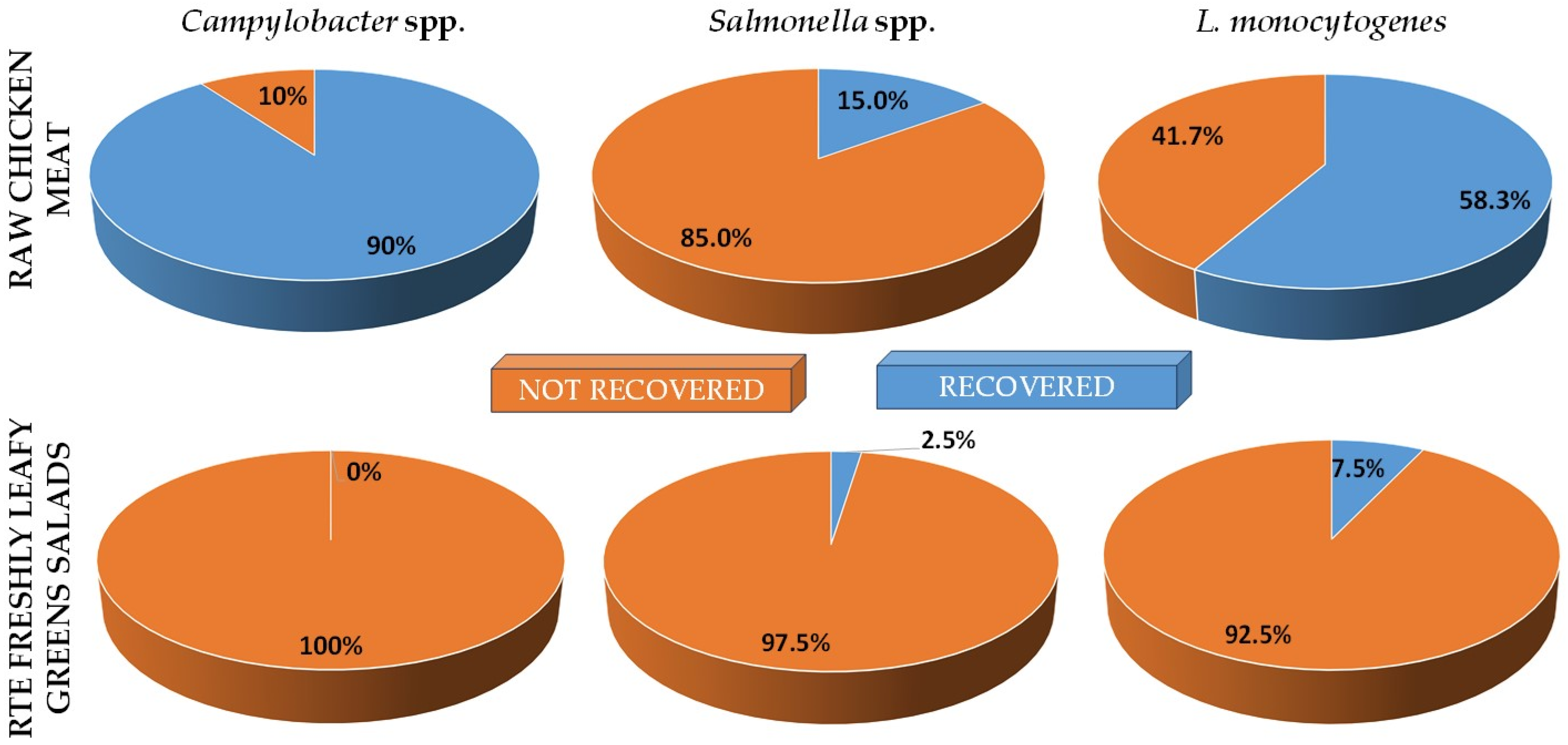
3.1. Prevalence of Pathogenic Bacteria in Raw Chicken Meat and RTE Fresh Leafy Greens Salads
3.2. Presence, Population Levels, and Frequencies of Hygiene (Safety) Indicator Organisms in Raw Chicken Meat and RTE Fresh Leafy Greens Salads
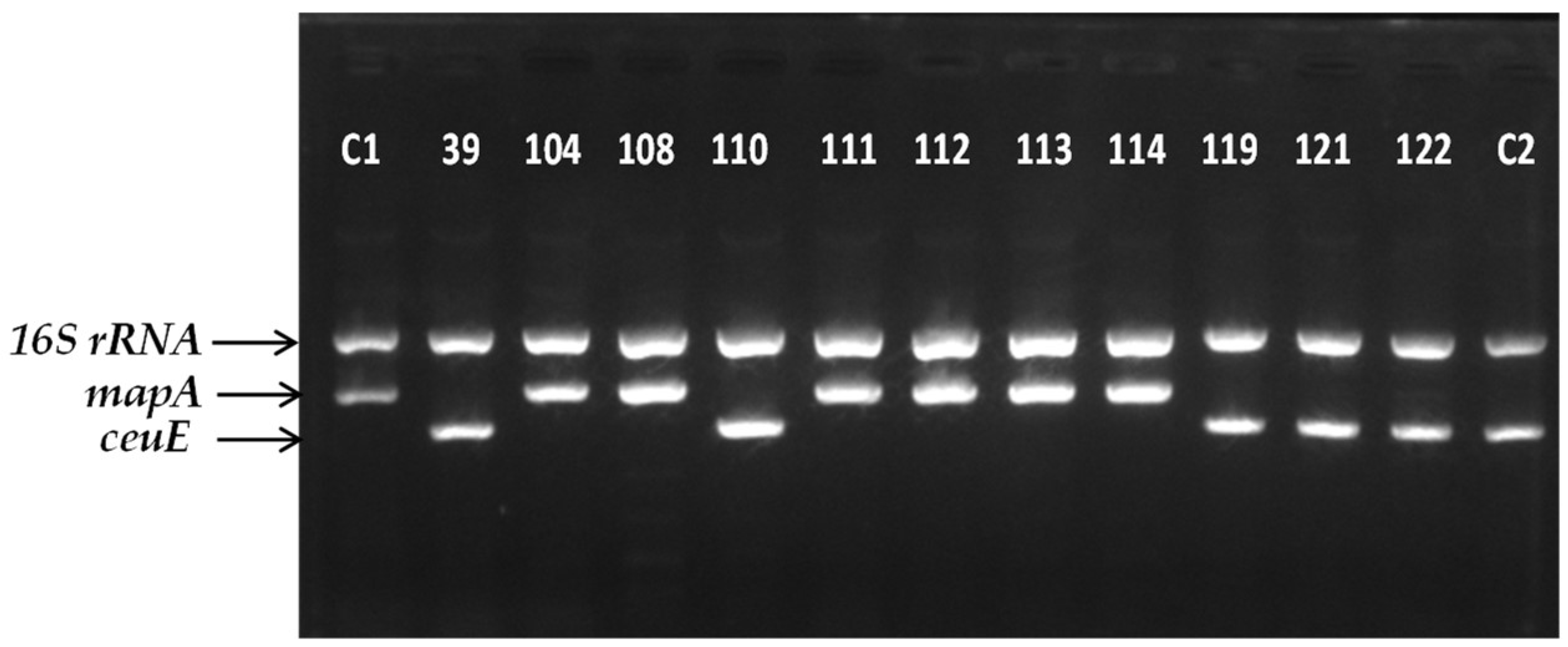
3.3. Molecular Identification of Campylobacter Isolates and Rep-PCR Phylogenetic Analyses
4. Discussion
5. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chlebicz, A.; Śliżewska, K. Campylobacteriosis, salmonellosis, yersiniosis, and listeriosis as zoonotic foodborne diseases: A review. Int. J. Environ. Res. Public Health 2018, 15, 863. [Google Scholar] [CrossRef] [PubMed]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control). The European Union One Health 2021 Zoonoses Report. EFSA J. 2022, 20, 7666. [Google Scholar] [CrossRef]
- Mota-Gutierrez, J.; Lis, L.; Lasagabaster, A.; Nafarrate, I.; Ferrocino, I.; Cocolin, L.; Rantsiou, K. Campylobacter spp. prevalence and mitigation strategies in the broiler production chain. Food Microbiol. 2022, 104, 103998. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Liu, Y.; Qin, X.; Aspridou, Z.; Zheng, J.; Wang, X.; Li, Z.; Dong, Q. The prevalence and epidemiology of Salmonella in retail raw poultry meat in China: A systematic review and meta-analysis. Foods 2021, 10, 2757. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.S.; Ebel, E.D.; Nyirabahizi, E. Comparative history of Campylobacter contamination on chicken meat and campylobacteriosis cases in the United States: 1994–2018. Int. J. Food Microbiol. 2021, 342, 109075. [Google Scholar] [CrossRef] [PubMed]
- Soro, A.B.; Whyte, P.; Bolton, D.J.; Tiwari, B.K. Strategies and novel technologies to control Campylobacter in the poultry chain: A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1353–1377. [Google Scholar] [CrossRef] [PubMed]
- Boysen, L.; Vigre, H.; Rosenquist, H. Seasonal influence on the prevalence of thermotolerant Campylobacter in retail broiler meat in Denmark. Food Microbiol. 2011, 28, 1028–1032. [Google Scholar] [CrossRef]
- Skarp, C.P.A.; Hänninen, M.L.; Rautelin, H.I.K. Campylobacteriosis: The role of poultry meat. Clin. Microbiol. Infect. 2016, 22, 103–109. [Google Scholar] [CrossRef]
- List of Prokaryotic Names with Standing in Nomenclature (LPSN). Leibniz Institute DSMZ-German Collection of Microorganisms and Cell Cultures GmbH. Genus Campylobacter. Available online: https://lpsn.dsmz.de/genus/campylobacter (accessed on 14 December 2023).
- Hansson, I.; Sandberg, M.; Habib, I.; Lowman, R.; Engvall, E.O. Knowledge gaps in control of Campylobacter for prevention of campylobacteriosis. Transbound. Emerg. Dis. 2018, 65 (Suppl. S1), 30–48. [Google Scholar] [CrossRef]
- Teklemariam, A.D.; Al-Hindi, R.R.; Albiheyri, R.S.; Alharbi, M.G.; Alghamdi, M.A.; Filimban, A.A.R.; Al Mutiri, A.S.; Al-Alyani, A.M.; Alseghayer, M.S.; Almaneea, A.M.; et al. Human salmonellosis: A continuous global threat in the farm-to-fork food safety continuum. Foods 2023, 12, 1756. [Google Scholar] [CrossRef]
- Jajere, S.M. A review of Salmonella enterica with particular focus on the pathogenicity and virulence factors, host specificity and antimicrobial resistance including multidrug resistance. Vet. World. 2019, 12, 504–521. [Google Scholar] [CrossRef] [PubMed]
- Garner, D.; Kathariou, S. Fresh produce-associated listeriosis outbreaks, sources of concern, teachable moments, and insights. J. Food Prot. 2016, 79, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Townsend, A.; Strawn, L.K.; Chapman, B.J.; Dunn, L.L. A systematic review of Listeria species and Listeria monocytogenes prevalence, persistence, and diversity throughout the fresh produce supply chain. Foods 2021, 10, 1427. [Google Scholar] [CrossRef] [PubMed]
- Finn, L.; Onyeaka, H.; O’Neill, S. Listeria monocytogenes biofilms in food-associated environments: A persistent enigma. Foods 2023, 12, 3339. [Google Scholar] [CrossRef]
- Tuytschaever, T.; Raes, K.; Sampers, I. Listeria monocytogenes in food businesses: From persistence strategies to intervention/prevention strategies-A review. Compr. Rev. Food Sci. Food Saf. 2023, 22, 3910–3950. [Google Scholar] [CrossRef]
- Callejón, R.M.; Rodríguez-Naranjo, M.I.; Ubeda, C.; Hornedo-Ortega, R.; Garcia-Parrilla, M.C.; Troncoso, A.M. Reported foodborne outbreaks due to fresh produce in the United States and European Union: Trends and causes. Foodborne Pathog. Dis. 2015, 12, 32–38. [Google Scholar] [CrossRef]
- Wadamori, Y.; Gooneratne, R.; Hussain, M.A. Outbreaks and factors influencing microbiological contamination of fresh produce. J. Sci. Food Agric. 2017, 97, 1396–1403. [Google Scholar] [CrossRef]
- Yeni, F.; Yavaş, S.; Alpas, H.; Soyer, Y. Most common foodborne pathogens and mycotoxins on fresh produce: A review of recent outbreaks. Crit. Rev. Food Sci. Nutr. 2016, 56, 1532–1544. [Google Scholar] [CrossRef]
- Mohammadpour, H.; Berizi, E.; Hosseinzadeh, S.; Majlesi, M.; Zare, M. The prevalence of Campylobacter spp. in vegetables, fruits, and fresh produce: A systematic review and meta-analysis. Gut Pathog. 2018, 10, 41. [Google Scholar] [CrossRef]
- Little, C.L.; Gillespie, I.A. Prepared salads and public health. J. Appl. Microbiol. 2008, 105, 1729–1743. [Google Scholar] [CrossRef]
- Söderqvist, K. Is your lunch salad safe to eat? Occurrence of bacterial pathogens and potential for pathogen growth in pre-packed ready-to-eat mixed-ingredient salads. Infect. Ecol. Epidemiol. 2017, 7, 1407216. [Google Scholar] [CrossRef] [PubMed]
- Holcomb, D.A.; Stewart, J.R. Microbial indicators of fecal pollution: Recent progress and challenges in assessing water quality. Curr. Environ. Health Rep. 2020, 7, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Savichtcheva, O.; Okabe, S. Alternative indicators of fecal pollution: Relations with pathogens and conventional indicators, current methodologies for direct pathogen monitoring and future application perspectives. Water Res. 2006, 40, 2463–2476. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.S.; Ebel, E.D. Estimating the correlation between concentrations of two species of bacteria with censored microbial testing data. Int. J. Food Microbiol. 2014, 175, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Churchill, K.J.; Sargeant, J.M.; Farber, J.M.; O’Connor, A.M. Prevalence of Listeria monocytogenes in select ready-to-eat foods-deli meat, soft cheese, and packaged salad: A systematic review and meta-analysis. J. Food Prot. 2019, 82, 344–357. [Google Scholar] [CrossRef]
- Osimani, A.; Aquilanti, L.; Pasquini, M.; Clementi, F. Prevalence and risk factors for thermotolerant species of Campylobacter in poultry meat at retail in Europe. Poult. Sci. 2017, 96, 3382–3391. [Google Scholar] [CrossRef]
- ISO 10272-1:2017; Microbiology of the Food Chain—Horizontal Method for Detection and Enumeration of Campylobacter spp.—Part 1: Detection Method. International Organization for Standardization (ISO): Geneva, Switzerland, 2017.
- ISO 6579-1:2017; Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella—Part 1: Detection of Salmonella spp. International Organization for Standardization (ISO): Geneva, Switzerland, 2017.
- ISO 11290-1:2017; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Listeria monocytogenes and of Listeria spp.—Part 1: Detection Method. International Organization for Standardization (ISO): Geneva, Switzerland, 2017.
- ISO 21528-2:2017; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Enterobacteriaceae—Part 2: Colony-Count Technique. International Organization for Standardization (ISO): Geneva, Switzerland, 2017.
- ISO 4832:2006; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Coliforms—Colony-Count Technique. International Organization for Standardization (ISO): Geneva, Switzerland, 2006.
- ISO 6888-1:2021; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Coagulase-Positive Staphylococci (Staphylococcus aureus and Other Species)—Part 1: Method Using Baird-Parker Agar Medium. International Organization for Standardization (ISO): Geneva, Switzerland, 2021.
- Martineau, F.; Picard, F.J.; Ke, D.; Paradis, S.; Roy, P.H.; Ouellette, M.; Bergeron, M.G. Development of a PCR assay for identification of staphylococci at genus and species levels. J. Clin. Microbiol. 2001, 39, 2541–2547. [Google Scholar] [CrossRef]
- Denis, M.; Soumet, C.; Rivoal, K.; Ermel, G.; Blivet, D.; Salvat, G.; Colin, P. Development of a m-PCR assay for simultaneous identification of Campylobacter jejuni and C. coli. Lett. Appl. Microbiol. 1999, 29, 406–410. [Google Scholar] [CrossRef]
- Kostoglou, D.; Tsaklidou, P.; Iliadis, I.; Garoufallidou, N.; Skarmoutsou, G.; Koulouris, I.; Giaouris, E. Advanced killing potential of thymol against a time and temperature optimized attached Listeria monocytogenes population in lettuce broth. Biomolecules 2021, 11, 397. [Google Scholar] [CrossRef]
- Heras, J.; Domínguez, C.; Mata, E.; Pascual, V.; Lozano, C.; Torres, C.; Zarazaga, M. GelJ—A tool for analyzing DNA fingerprint gel images. BMC Bioinform. 2015, 16, 270. [Google Scholar] [CrossRef]
- Malakauskas, M.; Malakauskas, A.; Christensen, H.; Olsen, J.E.; Brogren, C.H. Repetitive element sequence-based pcr typing for improved discrimination of Campylobacter jejuni. Vet. Med. Zoot. 2017, 75, 43–53. Available online: https://vetzoo.lsmuni.lt/data/vols/2017/75/pdf/malakauskas.pdf (accessed on 14 December 2023).
- COMMISSION REGULATION (EC) No 1441/2007 of 5 December 2007 Amending Regulation (EC) No 2073/2005 on Microbiological Criteria for Foodstuffs. Official Journal of the European Union 2007, L 322/12. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32007R1441&qid=1702720611550 (accessed on 14 December 2023).
- COMMISSION REGULATION (EU) No 1086/2011 amending Annex II to Regulation (EC) No 2160/2003 of the European Parliament and of the Council and Annex I to Commission Regulation (EC) No 2073/2005 as Regards Salmonella in Fresh Poultry Meat. Official Journal of the European Union 2011, L 281/7. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32011R1086 (accessed on 14 December 2023).
- COMMISSION REGULATION (EU) 2017/1495 amending Regulation (EC) No 2073/2005 as Regards Campylobacter in Broiler carcases. Official Journal of the European Union 2017, L 218/1. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32017R1495 (accessed on 14 December 2023).
- Lytou, A.E.; Renieri, C.T.; Doulgeraki, A.I.; Nychas, G.E.; Panagou, E.Z. Assessment of the microbiological quality and safety of marinated chicken products from Greek retail outlets. Int. J. Food Microbiol. 2020, 320, 108506. [Google Scholar] [CrossRef] [PubMed]
- Lopes, S.M.; da Silva, D.C.; Tondo, E.C. Bactericidal effect of marinades on meats against different pathogens: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 7650–7658. [Google Scholar] [CrossRef]
- Hakeem, M.J.; Lu, X. Survival and control of Campylobacter in poultry production environment. Front. Cell. Infect. Microbiol. 2021, 10, 615049. [Google Scholar] [CrossRef] [PubMed]
- Di Giannatale, E.; Calistri, P.; Di Donato, G.; Decastelli, L.; Goffredo, E.; Adriano, D.; Mancini, M.E.; Galleggiante, A.; Neri, D.; Antoci, S.; et al. Thermotolerant Campylobacter spp. in chicken and bovine meat in Italy: Prevalence, level of contamination and molecular characterization of isolates. PLoS ONE 2019, 14, e0225957. [Google Scholar] [CrossRef] [PubMed]
- Mikulić, M.; Humski, A.; Njari, B.; Ostović, M.; Duvnjak, S.; Cvetnić, Ž. Prevalence of thermotolerant Campylobacter spp. in chicken meat in Croatia and multilocus sequence typing of a small subset of Campylobacter jejuni and Campylobacter coli isolates. Food Technol. Biotechnol. 2016, 54, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Guyard-Nicodème, M.; Rivoal, K.; Houard, E.; Rose, V.; Quesne, S.; Mourand, G.; Rouxel, S.; Kempf, I.; Guillier, L.; Gauchard, F.; et al. Prevalence and characterization of Campylobacter jejuni from chicken meat sold in French retail outlets. Int. J. Food Microbiol. 2015, 203, 8–14. [Google Scholar] [CrossRef]
- Tedersoo, T.; Roasto, M.; Mäesaar, M.; Kisand, V.; Ivanova, M.; Meremäe, K. The prevalence, counts, and MLST genotypes of Campylobacter in poultry meat and genomic comparison with clinical isolates. Poult. Sci. 2022, 101, 101703. [Google Scholar] [CrossRef]
- Bort, B.; Martí, P.; Mormeneo, S.; Mormeneo, M.; Iranzo, M. Prevalence and antimicrobial resistance of Campylobacter spp. isolated from broilers throughout the supply chain in Valencia, Spain. Foodborne Pathog. Dis. 2022, 19, 717–724. [Google Scholar] [CrossRef]
- García-Sánchez, L.; Melero, B.; Diez, A.M.; Jaime, I.; Rovira, J. Characterization of Campylobacter species in Spanish retail from different fresh chicken products and their antimicrobial resistance. Food Microbiol. 2018, 76, 457–465. [Google Scholar] [CrossRef]
- Madden, R.H.; Moran, L.; Scates, P.; McBride, J.; Kelly, C. Prevalence of Campylobacter and Salmonella in raw chicken on retail sale in the republic of Ireland. J. Food Prot. 2011, 74, 1912–1916. [Google Scholar] [CrossRef] [PubMed]
- Poudel, S.; Li, T.; Chen, S.; Zhang, X.; Cheng, W.H.; Sukumaran, A.T.; Kiess, A.S.; Zhang, L. Prevalence, antimicrobial resistance, and molecular characterization of Campylobacter Isolated from broilers and broiler meat raised without antibiotics. Microbiol. Spectr. 2022, 10, e0025122. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.; Oyarzabal, O.A. Prevalence of Campylobacter spp. in skinless, boneless retail broiler meat from 2005 through 2011 in Alabama, USA. BMC Microbiol. 2012, 12, 184. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.J.; Wallace, R.L.; Smith, J.J.; Graham, T.; Saputra, T.; Symes, S.; Stylianopoulos, A.; Polkinghorne, B.G.; Kirk, M.D.; Glass, K. Prevalence of Campylobacter coli and Campylobacter jejuni in retail chicken, beef, lamb, and pork products in three Australian States. J. Food Prot. 2019, 82, 2126–2134. [Google Scholar] [CrossRef]
- Bai, Y.; Cui, S.; Xu, X.; Li, F. Enumeration and characterization of campylobacter species from retail chicken carcasses in Beijing, China. Foodborne Pathog. Dis. 2014, 11, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Zdragas, A.; Mazaraki, K.; Vafeas, G.; Giantzi, V.; Papadopoulos, T.; Ekateriniadou, L. Prevalence, seasonal occurrence and antimicrobial resistance of Salmonella in poultry retail products in Greece. Lett. Appl. Microbiol. 2012, 55, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, C.; Gómez, I.; Zumalacárregui, J. Prevalence of Salmonella and Campylobacter in retail chicken meat in Spain. Int. J. Food Microbiol. 2002, 72, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Alali, W.Q.; Gaydashov, R.; Petrova, E.; Panin, A.; Tugarinov, O.; Kulikovskii, A.; Mamleeva, D.; Walls, I.; Doyle, M.P. Prevalence of Salmonella on retail chicken meat in Russian Federation. J. Food Prot. 2012, 75, 1469–1473. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Q.; Cao, C.; Cui, S.; Wu, Y.; Yang, H.; Xiao, Y.; Yang, B. Prevalence and characteristics of Salmonella isolates recovered from retail raw chickens in Shaanxi Province, China. Poult. Sci. 2020, 99, 6031–6044. [Google Scholar] [CrossRef]
- Lunara Santos Pavelquesi, S.; Carolina Almeida de Oliveira Ferreira, A.; Fernandes Silva Rodrigues, L.; Maria de Souza Silva, C.; Cristina Rodrigues da Silva, I.; Castilho Orsi, D. Prevalence and antimicrobial resistance of Salmonella spp. isolated from chilled chicken meat commercialized at retail in Federal District, Brazil. J. Food Prot. 2023, 86, 100130. [Google Scholar] [CrossRef]
- Regalado-Pineda, I.D.; Rodarte-Medina, R.; Resendiz-Nava, C.N.; Saenz-Garcia, C.E.; Castañeda-Serrano, P.; Nava, G.M. Three-year longitudinal study: Prevalence of Salmonella enterica in chicken meat is higher in supermarkets than wet markets from Mexico. Foods 2020, 9, 264. [Google Scholar] [CrossRef] [PubMed]
- Schlech, W.F. Epidemiology and clinical manifestations of Listeria monocytogenes infection. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Praakle-Amin, K.; Hänninen, M.L.; Korkeala, H. Prevalence and genetic characterization of Listeria monocytogenes in retail broiler meat in Estonia. J. Food Prot. 2006, 69, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Morshdy, A.E.M.A.; Al-Mogbel, M.S.; Mohamed, M.E.M.; Elabbasy, M.T.; Elshafee, A.K.; Hussein, M.A. Bioactivity of essential oils for mitigation of Listeria monocytogenes isolated from fresh retail chicken meat. Foods 2021, 10, 3006. [Google Scholar] [CrossRef] [PubMed]
- Ristori, C.A.; Rowlands, R.E.; Martins, C.G.; Barbosa, M.L.; Yoshida, J.T.; Franco, B.D. Prevalence and populations of Listeria monocytogenes in meat products retailed in Sao Paulo, Brazil. Foodborne Pathog. Dis. 2014, 11, 969–973. [Google Scholar] [CrossRef]
- Goh, S.G.; Kuan, C.H.; Loo, Y.Y.; Chang, W.S.; Lye, Y.L.; Soopna, P.; Tang, J.Y.; Nakaguchi, Y.; Nishibuchi, M.; Afsah-Hejri, L.; et al. Listeria monocytogenes in retailed raw chicken meat in Malaysia. Poult. Sci. 2012, 91, 2686–2690. [Google Scholar] [CrossRef]
- Arienzo, A.; Murgia, L.; Fraudentali, I.; Gallo, V.; Angelini, R.; Antonini, G. Microbiological quality of ready-to-eat leafy green salads during shelf-life and home-refrigeration. Foods 2020, 9, 1421. [Google Scholar] [CrossRef]
- Wilson, M.D.; Stanley, R.A.; Eyles, A.; Ross, T. Innovative processes and technologies for modified atmosphere packaging of fresh and fresh-cut fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2019, 59, 411–422. [Google Scholar] [CrossRef]
- Wijnands, L.M.; Delfgou-van Asch, E.H.; Beerepoot-Mensink, M.E.; van der Meij-Florijn, A.; Fitz-James, I.; van Leusden, F.M.; Pielaat, A. Prevalence and concentration of bacterial pathogens in raw produce and minimally processed packaged salads produced in and for the Netherlands. J. Food Prot. 2014, 77, 388–394. [Google Scholar] [CrossRef]
- Whyte, P.; McGill, K.; Cowley, D.; Madden, R.H.; Moran, L.; Scates, P.; Carroll, C.; O’Leary, A.; Fanning, S.; Collins, J.D.; et al. Occurrence of Campylobacter in retail foods in Ireland. Int. J. Food Microbiol. 2004, 95, 111–118. [Google Scholar] [CrossRef]
- Kovačević, M.; Burazin, J.; Pavlović, H.; Kopjar, M.; Piližota, V. Prevalence and level of Listeria monocytogenes and other Listeria sp. in ready-to-eat minimally processed and refrigerated vegetables. World J. Microbiol. Biotechnol. 2013, 29, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Parker, E.; Albers, A.; Mollenkopf, D.; Korec, D.; Mathys, D.; Stuever, D.; Wittum, T. AmpC- and extended-spectrum β-lactamase-producing Enterobacteriaceae detected in fresh produce in Central Ohio. J. Food Prot. 2021, 84, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Food Safety Authority of Ireland (FSAI). Guidelines for the Interpretation of Results of Microbiological Testing of Ready-to-Eat Foods Placed on the Market (Revision 4); FSAI: Dublin, Ireland, 2020; ISBN 0-9539183-5-1. [Google Scholar]
- Health Protection Agency. Guidelines for Assessing the Microbiological Safety of Ready-to-Eat Foods; Health Protection Agency: London, UK, 2009.
- Health Canada. Microbial Guidelines for Ready-to-Eat Foods—A Guide for the Conveyances Industry and Environmental Health Officers (EHO). Ottawa, Ontario, Canada. 2013. Available online: https://publications.gc.ca/collections/collection_2014/sc-hc/H164-167-2013-eng.pdf (accessed on 9 December 2023).
- Khare, N.; Kaushik, M.; Martin, J.P.; Mohanty, A.; Gulati, P. Genotypic diversity in multi-drug-resistant E. coli isolated from animal feces and Yamuna River water, India, using rep-PCR fingerprinting. Environ. Monit. Assess. 2020, 192, 681. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Amadoro, C.; Conficoni, D.; Giaccone, V.; Colavita, G. Occurrence, diversity of Listeria spp. isolates from food and food-contact surfaces and the presence of virulence genes. Microorganisms 2020, 8, 294. [Google Scholar] [CrossRef]
- Patchanee, P.; Chokboonmongkol, C.; Zessin, K.H.; Alter, T.; Pornaem, S.; Chokesajjawatee, N. Comparison of multilocus sequence typing (MLST) and repetitive sequence-based PCR (rep-PCR) fingerprinting for differentiation of Campylobacter jejuni isolated from broiler in Chiang Mai, Thailand. J. Microbiol. Biotechnol. 2012, 22, 1467–1470. [Google Scholar] [CrossRef]
- Behringer, M.; Miller, W.G.; Oyarzabal, O.A. Typing of Campylobacter jejuni and Campylobacter coli isolated from live broilers and retail broiler meat by flaA-RFLP, MLST, PFGE and REP-PCR. J. Microbiol. Methods 2011, 84, 194–201. [Google Scholar] [CrossRef]
| Type of Retail Store | Campylobacter spp. | Salmonella spp. | L. monocytogenes | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Samples | Positive Samples | Positive Samples | Positive Samples | ||||||||
| n | % | n | % | n | % | n | % | ||||
| In Each Type of Retail Store | Of Total | In Each Type of Retail Store | Of Total | In Each Type of Retail Store | Of Total | ||||||
| Butcher shops | 20 | 33.3% | 17 | 85.0% | 31.5% | 1 | 5.0% | 11.1% | 13 | 65.0% | 37.1% |
| Supermarkets | 40 | 66.7% | 37 | 92.5% | 68.5% | 8 | 20.0% | 88.9% | 22 | 55.0% | 62.9% |
| Sum | 60 | 100.0% | 54 | 90.0% | 100.0% | 9 | 15.0% | 100.0% | 35 | 58.3% | 100.0% |
| Sample Disposal Method | Campylobacter spp. | Salmonella spp. | L. monocytogenes | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Samples | Positive Samples | Positive Samples | Positive Samples | ||||||||
| n | % | n | % | n | % | n | % | ||||
| In Each Disposal Method | Of Total | In Each Disposal Method | Of Total | In Each Disposal Method | Of Total | ||||||
| Bulk | 42 | 70.0% | 37 | 88.1% | 68.5% | 4 | 9.5% | 44.4% | 25 | 59.5% | 71.4% |
| Prepackaged | 18 | 30.0% | 17 | 94.4% | 31.5% | 5 | 27.8% | 55.6% | 10 | 55.6% | 28.6% |
| Sum | 60 | 100.0% | 54 | 90.0% | 100.0% | 9 | 15.0% | 100.0% | 35 | 58.3% | 100.0% |
| Hygiene (Safety) Indicator Organism | Population Level (CFU/g) and Frequencies (%) | |
|---|---|---|
| Raw Chicken Meat (n = 60) | RTE Freshly Leafy Greens Salads (n = 40) | |
| APC | ≥106 CFU/g in 32 samples (53.3%) | ≥106 CFU/g in 38 samples (95.0%) |
| Enterobacteriaceae | ≥104 CFU/g in 19 samples (31.7%) | ≥104 CFU/g in 4 samples (10.0%) |
| Total coliforms | ≥102 CFU/g in 54 samples (90.0%) | ≥102 CFU/g in 0 sample (0.0%) |
| E. coli | ≥102 CFU/g in 25 samples (41.7%) | ≥102 CFU/g in 0 sample (0.0%) |
| Staphylococci | ≥102 CFU/g in 6 samples (10.0%) | ≥102 CFU/g in 14 samples (35.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostoglou, D.; Simoni, M.; Vafeiadis, G.; Kaftantzis, N.-M.; Giaouris, E. Prevalence of Campylobacter spp., Salmonella spp., and Listeria monocytogenes, and Population Levels of Food Safety Indicator Microorganisms in Retail Raw Chicken Meat and Ready-To-Eat Fresh Leafy Greens Salads Sold in Greece. Foods 2023, 12, 4502. https://doi.org/10.3390/foods12244502
Kostoglou D, Simoni M, Vafeiadis G, Kaftantzis N-M, Giaouris E. Prevalence of Campylobacter spp., Salmonella spp., and Listeria monocytogenes, and Population Levels of Food Safety Indicator Microorganisms in Retail Raw Chicken Meat and Ready-To-Eat Fresh Leafy Greens Salads Sold in Greece. Foods. 2023; 12(24):4502. https://doi.org/10.3390/foods12244502
Chicago/Turabian StyleKostoglou, Dimitra, Maria Simoni, Georgios Vafeiadis, Nikolaos-Marios Kaftantzis, and Efstathios Giaouris. 2023. "Prevalence of Campylobacter spp., Salmonella spp., and Listeria monocytogenes, and Population Levels of Food Safety Indicator Microorganisms in Retail Raw Chicken Meat and Ready-To-Eat Fresh Leafy Greens Salads Sold in Greece" Foods 12, no. 24: 4502. https://doi.org/10.3390/foods12244502








