Small Peptides Isolated from Enzymatic Hydrolyzate of Pneumatophorus japonicus Bone Promote Sleep by Regulating Circadian Rhythms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Zebrafish Husbandry
2.3. Drug Treatment and Grouping
2.4. Behavior Analysis
2.5. Preparation Method of PBPs
2.6. Targeted Isolation and Identification of Sleep-Promoting Peptides from PBPs
2.7. Zebrafish Infrared (IR) Spectra Data Acquisition
2.8. Transcriptomic Analysis
2.8.1. RNA-seq Extraction
2.8.2. Statistics of Differentially Expressed Genes (DEGs)
2.8.3. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Enrichment Analysis of DEGs
2.9. Statistical Analysis
3. Results
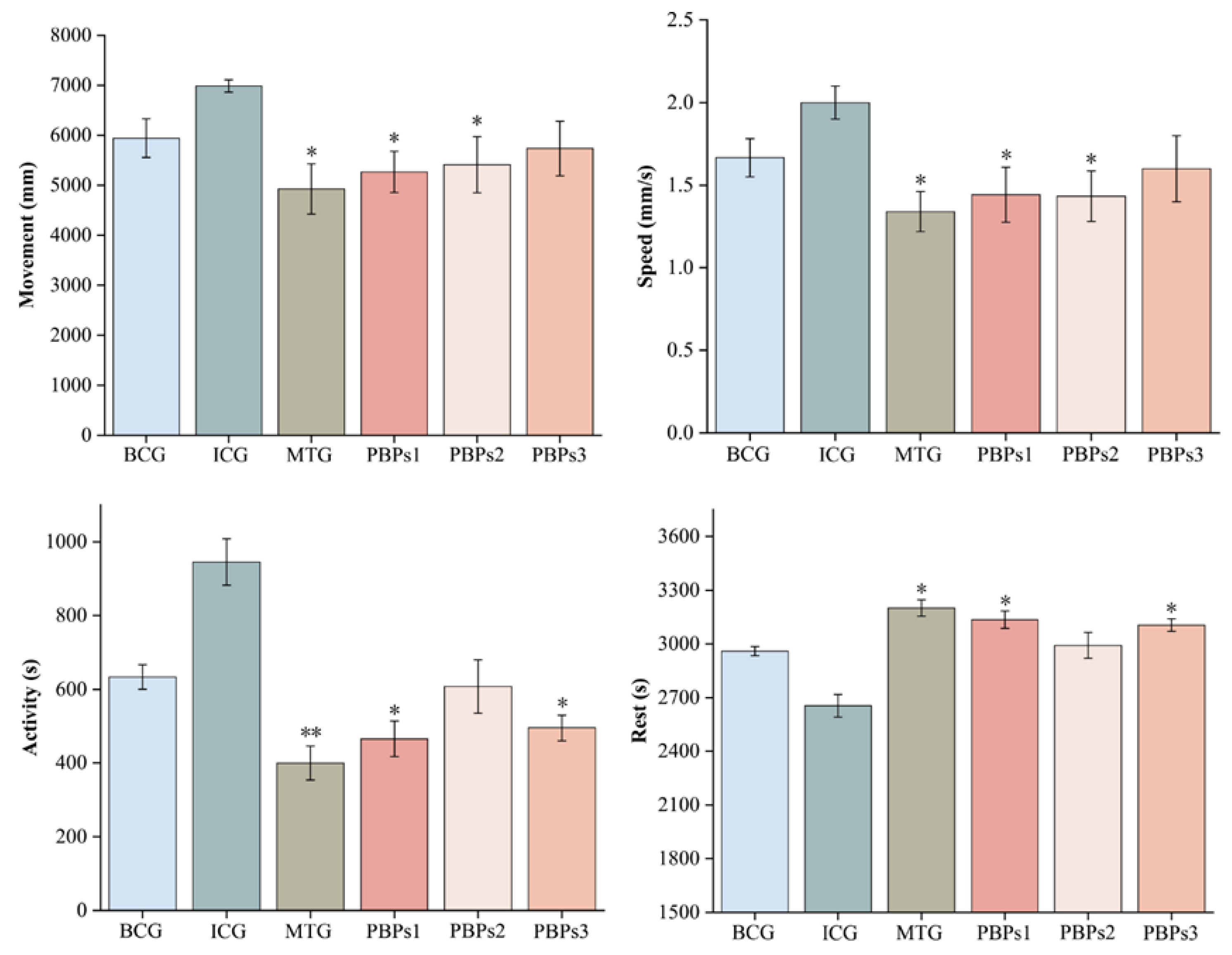
3.1. Targeted Isolation of Sleep-Promoting Peptides from PBPs
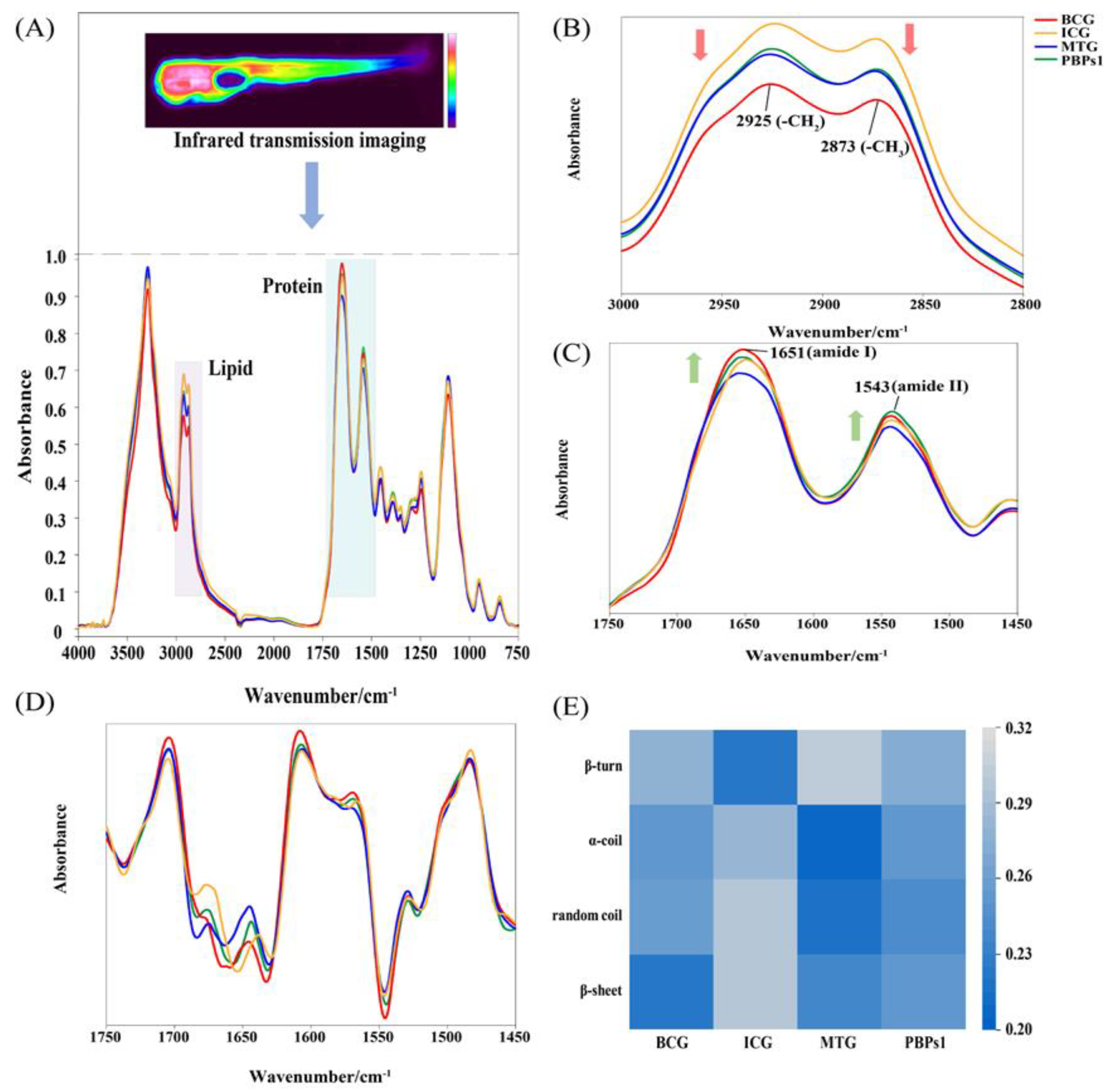
3.2. Analysis of IR Spectra of Zebrafish Treated with PBPs1
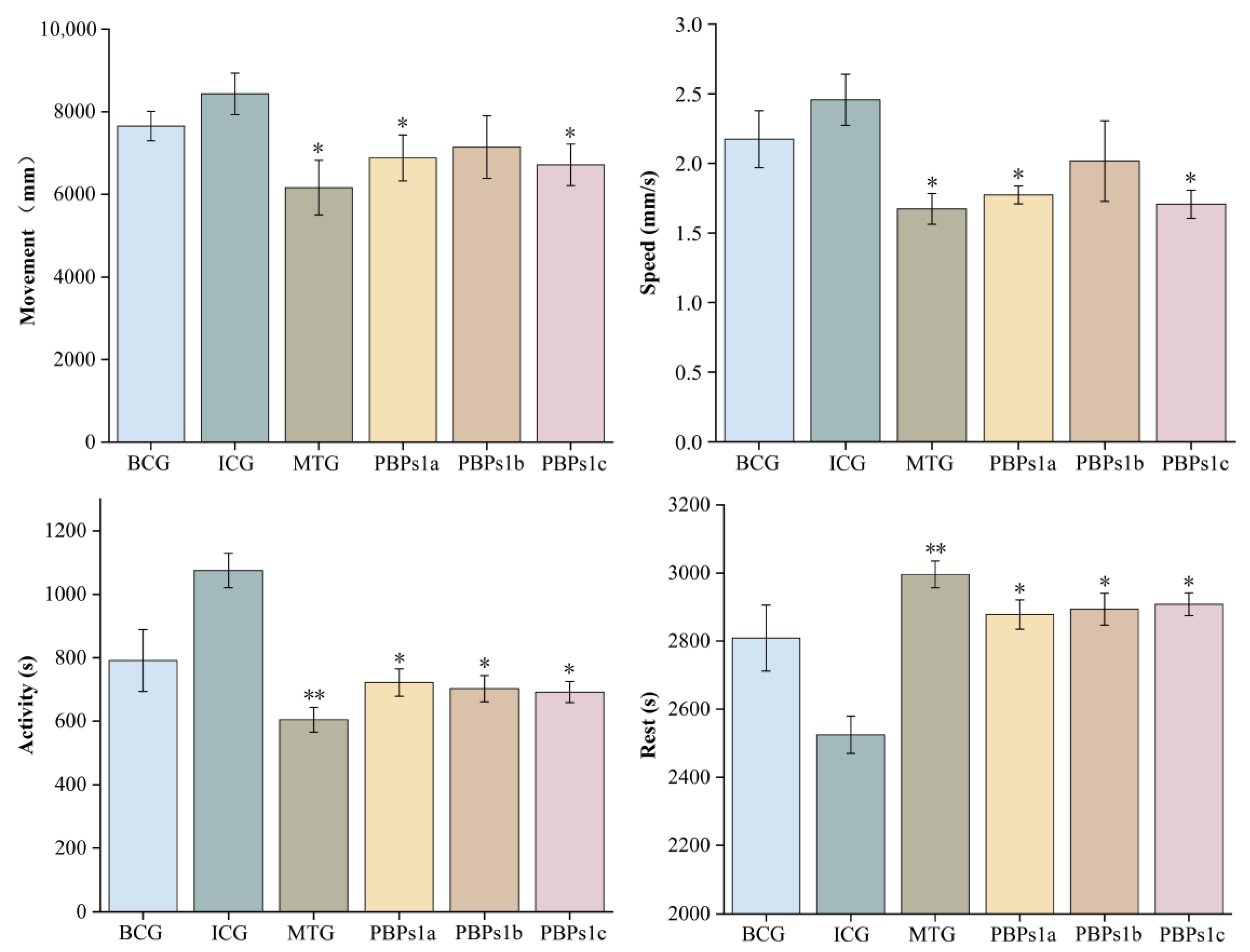
3.3. Evaluation of the Sleep-Promoting Activity of PBPs1
3.4. Sequence Identification of Sleep-Promoting Peptides in PBPs1c
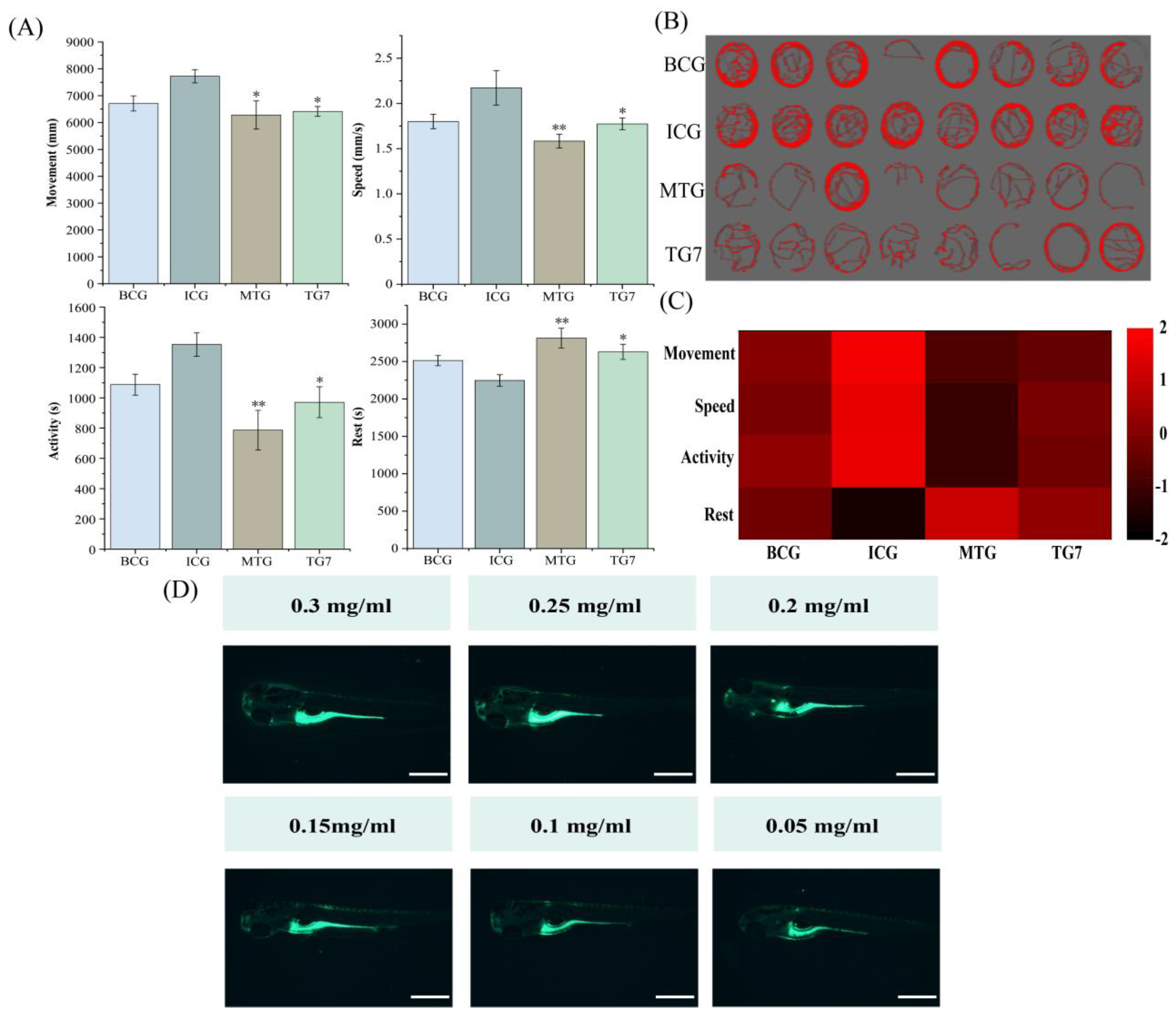
3.5. Evaluation of the Sleep-Promoting Activity of TG7
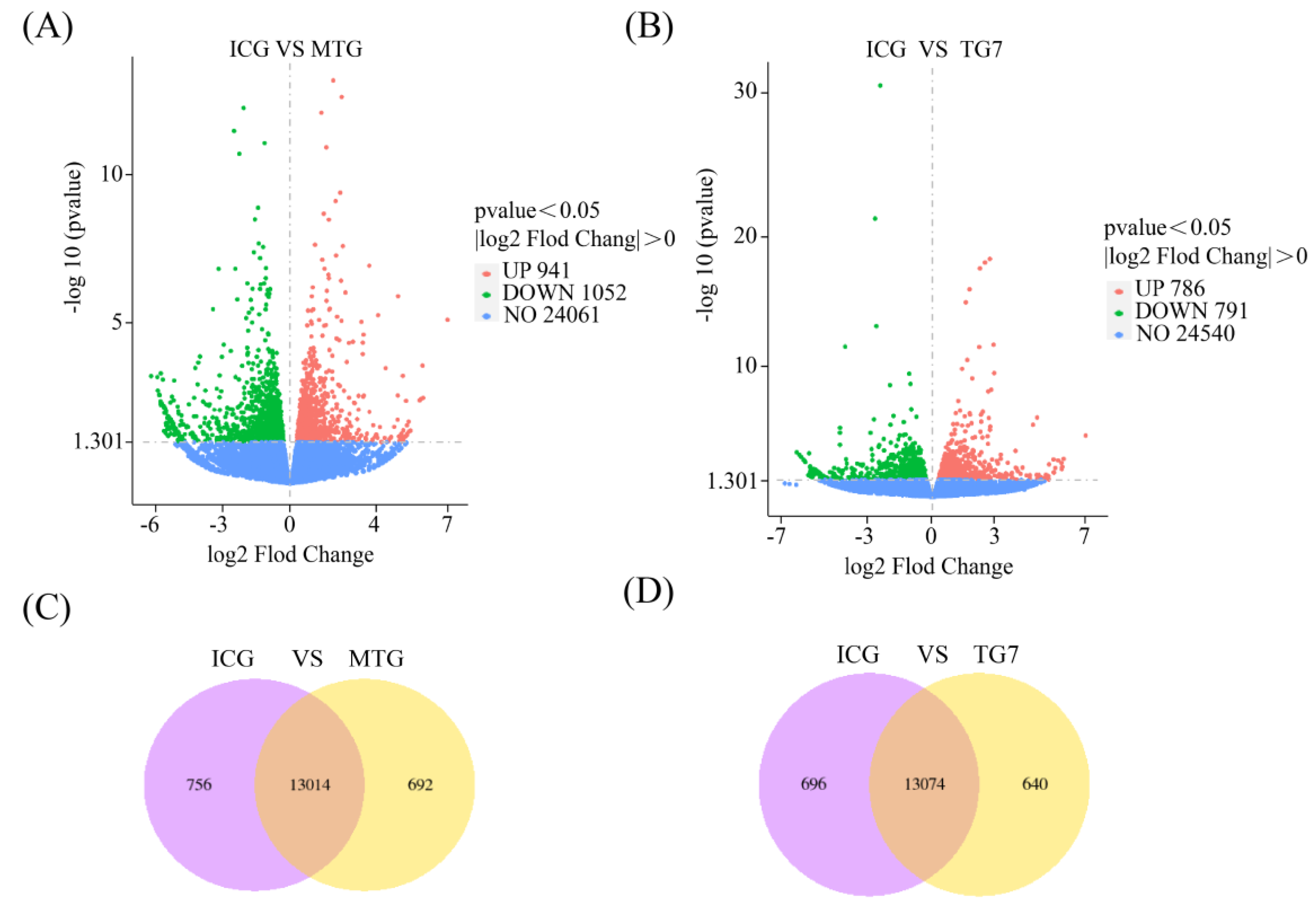
3.6. Statistics of DEGs
3.7. Screening of DEGs
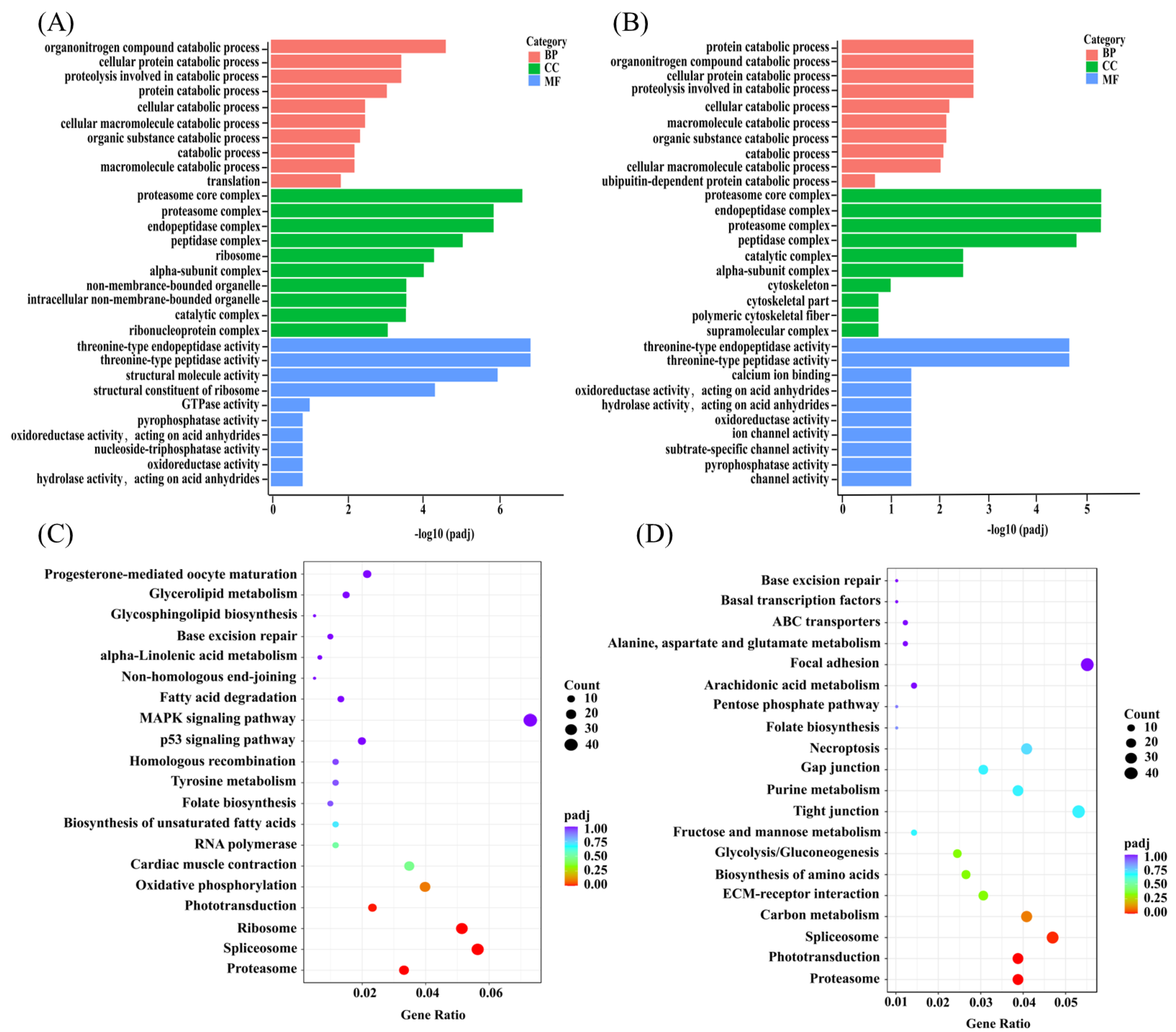
3.8. GO and KEGG Enrichment Analysis of DEGs
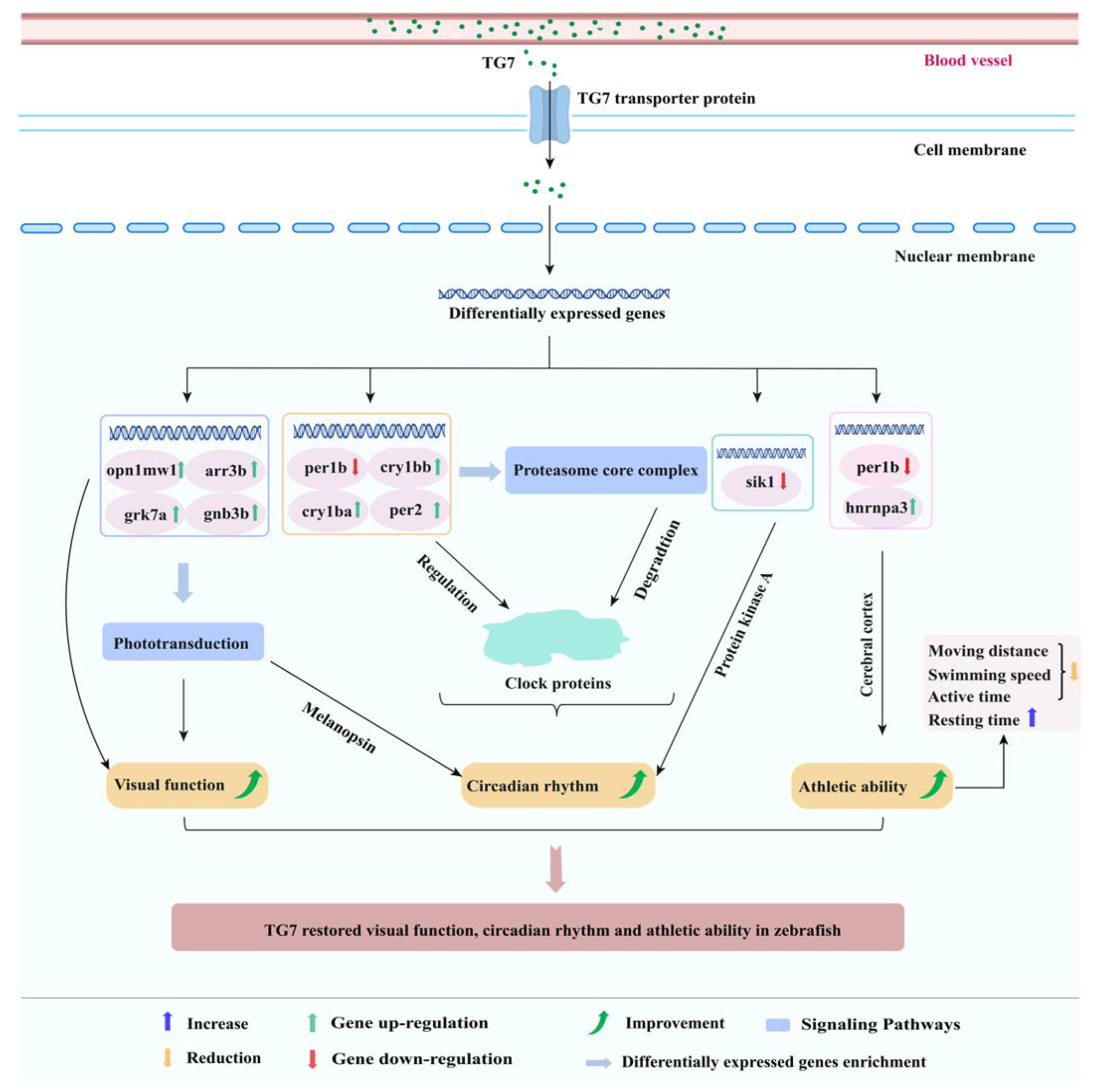
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Putilov, A.A. Physiological Sleep Propensity Might Be Unaffected by Significant Variations in Self-Reported Well-Being, Activity, and Mood. Sleep Disord. 2015, 2015, 532831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lassmann, L.; Pollis, M.; Zoltowska, A.; Manfredini, D. Gut Bless Your Pain-Roles of the Gut Microbiota, Sleep, and Melatonin in Chronic Orofacial Pain and Depression. Biomedicines 2022, 10, 1528. [Google Scholar] [CrossRef] [PubMed]
- Dutil, C.; Walsh, J.J.; Featherstone, R.B.; Gunnell, K.E.; Tremblay, M.S.; Gruber, R.; Weiss, S.K.; Cote, K.A.; Sampson, M.; Chaput, J.P. Influence of sleep on developing brain functions and structures in children and adolescents: A systematic review. Sleep Med. Rev. 2018, 42, 184–201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Whitehurst, L.N.; Mednick, S.C. The role of sleep for episodic memory consolidation: Stabilizing or rescuing? Neurobiol. Learn. Mem. 2022, 191, 107621. [Google Scholar] [CrossRef]
- Li, M.; Zhang, X.-W.; Hou, W.-S.; Tang, Z.-Y. Insomnia and risk of cardiovascular disease: A meta-analysis of cohort studies. Int. J. Cardiol. 2014, 176, 1044–1047. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.; Anderson, W.M.; Cole, S.R.; Cornoni-Huntley, J.; Hays, J.C.; Blazer, D. Insomnia and heart disease: A review of epidemiologic studies. J. Psychosom. Res. 1999, 47, 313–333. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ren, R.; Sanford, L.D.; Yang, L.; Tang, X. Sleep in Parkinson’s disease: A systematic review and meta-analysis of polysomnographic findings. Sleep Med. Rev. 2020, 51, 101281. [Google Scholar] [CrossRef]
- Riemann, D.; Perlis, M.L. The treatments of chronic insomnia: A review of benzodiazepine receptor agonists and psychological and behavioral therapies. Sleep Med. Rev. 2009, 13, 205–214. [Google Scholar] [CrossRef]
- Yan, Y.H.; Li, Y.Z.; Zhang, Z.W.; Wang, X.H.; Niu, Y.Z.; Zhang, S.H.; Xu, W.L.; Ren, C.G. Advances of peptides for antibacterial applications. Colloids Surf. B-Biointerfaces 2021, 202, 111682. [Google Scholar] [CrossRef]
- Zheng, L.H.; Wang, Y.J.; Sheng, J.; Wang, F.; Zheng, Y.; Lin, X.K.; Sun, M. Antitumor Peptides from Marine Organisms. Mar. Drugs 2011, 9, 1840–1859. [Google Scholar] [CrossRef]
- Lee, S.Y.; Hur, S.J. Mechanisms of Neuroprotective Effects of Peptides Derived from Natural Materials and Their Production and Assessment. Compr. Rev. Food Sci. Food Saf. 2019, 18, 923–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Su, G.; Zhang, Q.; Zhao, T.; Zhao, M. Walnut (Juglans regia) Peptides Reverse Sleep Deprivation-Induced Memory Impairment in Rat via Alleviating Oxidative Stress. J. Agric. Food Chem. 2018, 66, 10617–10627. [Google Scholar] [CrossRef]
- Yayeh, T.; Leem, Y.H.; Kim, K.M.; Jung, J.C.; Oh, S. Administration of Alpha(s1)-Casein Hydrolysate Increases Sleep and Modulates GABA(A) Receptor Subunit Expression. Biomol. Ther. 2018, 26, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.F.; Safdar, B.; Zhang, Y.H.; Li, Y.; Liu, X.Q. A study of the mechanism of small-molecule soybean-protein-derived peptide supplement to promote sleep in a mouse model. RSC Adv. 2020, 10, 11264–11273. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.B.; Zhou, Q.; Yan, J.X.; Luo, L.S.; Zhang, J.L. Enzymolysis peptides from Mauremys mutica plastron improve the disorder of neurotransmitter system and facilitate sleep-promoting in the PCPA-induced insomnia mice. J. Ethnopharmacol. 2021, 274, 114047. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Kobayashi, S.; Yamashita, M. Comparison of connective tissue structure and muscle toughness of spotted mackerel Scomber australasicus and Pacific mackerel S-japonicus during chilled and frozen storage. Fish. Sci. 2017, 83, 133–139. [Google Scholar] [CrossRef]
- Cahill, G.M. Clock mechanisms in zebrafish. Cell Tissue Res. 2002, 309, 27–34. [Google Scholar] [CrossRef]
- Li, L. Circadian Vision in Zebrafish: From Molecule to Cell and from Neural Network to Behavior. J. Biol. Rhythm. 2019, 34, 451–462. [Google Scholar] [CrossRef]
- Elbaz, I.; Foulkes, N.S.; Gothilf, Y.; Appelbaum, L. Circadian clocks, rhythmic synaptic plasticity and the sleep-wake cycle in zebrafish. Front. Neural Circuits 2013, 7, 9. [Google Scholar] [CrossRef] [Green Version]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Monti, J.M.; Jantos, H. Effects of L-arginine and SIN-1 on sleep and waking in the rat during both phases of the light-dark cycle. Life Sci. 2004, 75, 2027–2034. [Google Scholar] [CrossRef] [PubMed]
- Ki, Y.; Lim, C. Sleep-promoting effects of threonine link amino acid metabolism in Drosophila neuron to GABAergic control of sleep drive. eLife 2019, 8, e40593. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Mallick, B.N. Glutamic acid stimulation of the perifornical-lateral hypothalamic area promotes arousal and inhibits non-REM/REM sleep. Neurosci. Lett. 2008, 439, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Aaba, B.; Knz, C.; Adva, B.; Asba, B.; Kadcd, E.; Msda, F.; Vyba, B.; Kab, A.; Kbyi, J.; Mata, B. Modeling neurodegenerative disorders in zebrafish. Neurosci. Biobehav. Rev. 2022, 138, 104679. [Google Scholar] [CrossRef]
- Hong, H.; Fan, H.; Chalamaiah, M.; Wu, J. Preparation of low-molecular-weight, collagen hydrolysates (peptides): Current progress, challenges, and future perspectives. Food Chem. 2019, 301, 125222. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; He, S.; Xie, J.; Li, M.; Hong, M.; Guan, F.; Hu, Y.; Huang, Y.; Xu, C. Integral characterization of normal and alopecic hair at different degeneration stages by in-situ visible and chemical imaging. Spectrochim. Acta. Part A Mol. Biomol. Spectrosc. 2020, 235, 118315. [Google Scholar] [CrossRef]
- Cai, G.H.; Theorell-Haglow, J.; Janson, C.; Svartengren, M.; Elmstahl, S.; Lind, L.; Lindberg, E. Insomnia symptoms and sleep duration and their combined effects in relation to associations with obesity and central obesity. Sleep Med. 2018, 46, 81–87. [Google Scholar] [CrossRef]
- Vitaterna, M.H.; Shimomura, K.; Jiang, P. Genetics of Circadian Rhythms. Neurol. Clin. 2019, 37, 487. [Google Scholar] [CrossRef]
- Chrispell, J.D.; Dong, E.H.; Osawa, S.; Liu, J.D.; Cameron, D.J.; Weiss, E.R. Grk1b and Grk7a Both Contribute to the Recovery of the Isolated Cone Photoresponse in Larval Zebrafish. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5116–5124. [Google Scholar] [CrossRef] [Green Version]
- Fidelin, K.; Djenoune, L.; Stokes, C.; Prendergast, A.; Gomez, J.; Baradel, A.; Del Bene, F.; Wyart, C. State-Dependent Modulation of Locomotion by GABAergic Spinal Sensory Neurons. Curr. Biol. 2015, 25, 3035–3047. [Google Scholar] [CrossRef]
- Ou, M.Y.; Ju, X.C.; Cai, Y.J.; Sun, X.Y.; Wang, J.F.; Fu, X.Q.; Sun, Q.; Luo, Z.G. Heterogeneous nuclear ribonucleoprotein A3 controls mitotic progression of neural progenitors via interaction with cohesin. Development 2020, 147, dev185132. [Google Scholar] [CrossRef] [PubMed]
- Katoh, Y.; Takemori, H.; Min, L.; Muraoka, M.; Doi, J.; Horike, N.; Okamoto, M. Salt-inducible kinase-1 represses cAMP response element-binding protein activity both in the nucleus and in the cytoplasm. FEBS J. 2010, 271, 4307–4319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Y.Y.; Tao, N.P.; Wang, X.C.; Deng, S.; Lu, Y.; Xu, C.H. Simultaneous detection of mixed foodborne pathogens by multi-molecular infrared spectroscopy identification system. Food Control. 2022, 136, 108861. [Google Scholar] [CrossRef]
- Bolsius, Y.G.; Zurbriggen, M.D.; Kim, J.K.; Kas, M.J.; Meerlo, P.; Aton, S.J.; Havekes, R. The role of clock genes in sleep, stress and memory. Biochem. Pharmacol. 2021, 191, 114493. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhong, Z.M.; Wang, M.Y.; Chen, X.F.; Tan, Y.C.; Zhang, S.Q.; He, W.; He, X.; Huang, G.D.; Lu, H.P.; et al. Circadian Modulation of Dopamine Levels and Dopaminergic Neuron Development Contributes to Attention Deficiency and Hyperactive Behavior. J. Neurosci. 2015, 35, 2572–2587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez-Cruz, I.; Sanchez-Diaz, I.; Narvaez-Padilla, V.; Reynaud, E. Rpt2 proteasome subunit reduction causes Parkinson’s disease like symptoms in Drosophila. IBRO Rep. 2020, 9, 65–77. [Google Scholar] [CrossRef]
- Freeman, A.A.H.; Mandilaras, K.; Missirlis, F.; Sanyal, S. An emerging role for Cullin-3 mediated ubiquitination in sleep and circadian rhythm: Insights from Drosophila. Fly 2013, 7, 39–43. [Google Scholar] [CrossRef] [Green Version]
- David, L.; Amalia, C.P.; Franzén, I.; Dan, L.; Abalo, X.M.; Neuhauss, S. Transducin Duplicates in the Zebrafish Retina and Pineal Complex: Differential Specialisation after the Teleost Tetraploidisation. PLoS ONE 2015, 10, e0121330. [Google Scholar] [CrossRef] [Green Version]
- Shi, W.J.; Jiang, Y.X.; Ma, D.D.; Huang, G.Y.; Ying, G.G. Dydrogesterone affects the transcription of genes in visual cycle and circadian rhythm network in the eye of zebrafish. Ecotoxicol. Environ. Saf. 2019, 183, 109556. [Google Scholar] [CrossRef]
- Rea, M.S.; Figueiro, M.G.; Bullough, J.D.; Bierman, A. A model of phototransduction by the human circadian system. Brain Res. Rev. 2005, 50, 213–228. [Google Scholar] [CrossRef]
- Ogueta, M.; Hardie, R.C.; Stanewsky, R. Non-canonical Phototransduction Mediates Synchronization of the Drosophila melanogaster Circadian Clock and Retinal Light Responses. Elsevier Spons. Doc. 2018, 28, 1725–1735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jagannath, A.; Butler, R.; Godinho, S.I.H.; Couch, Y.; Brown, L.A.; Vasudevan, S.R.; Flanagan, K.C.; Anthony, D.; Churchill, G.C.; Wood, M.J.A.; et al. The CRTC1-SIK1 Pathway Regulates Entrainment of the Circadian Clock. Cell 2013, 154, 1100–1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, M.; Miyoshi, C.; Fujiyama, T.; Kakizaki, M.; Funato, H. Loss of the conserved PKA sites of SIK1 and SIK2 increases sleep need. Sci. Rep. 2020, 10, 8676. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.S.; Feng, Y.; Hu, L.F.; Lin, C.L.; Ye, R.; Yuan, Z.Z. Jiaotaiwan increased GABA level in brain and serum, improved sleep via increasing NREM sleep and REM sleep, and its component identification. J. Ethnopharmacol. 2022, 285, 114866. [Google Scholar] [CrossRef]
| Gene Name | Gene Description | MTG vs. ICG | TG7 vs. ICG | ||
|---|---|---|---|---|---|
| log2 FC | padj | log2 FC | padj | ||
| per2 | period circadian clock 2 | +1.88 | 3.23 × 10−9 | +2.10 | 6.51 × 10−15 |
| gnat2 | guanine nucleotide-binding protein alpha transducing activity polypeptide 2 | +2.25 | 5.34 × 10−9 | ||
| per1b | period circadian clock 1b | −2.03 | 7.90 × 10−9 | −2.54 | 2.25 × 10−18 |
| cry1bb | cryptochrome circadian clock 1bb | +1.36 | 8.31 × 10−9 | +1.48 | 1.99 × 10−12 |
| dmtn | dematin actin-binding protein | −2.45 | 2.49 × 10−8 | ||
| h3f3d | H3 histone%2C family 3D | −1.11 | 4.98 × 10−8 | ||
| cry1ba | cryptochrome circadian clock 1ba | +1.58 | 5.83 × 10−8 | +1.54 | 3.36 × 10−9 |
| Si: dkey-33c14.3 | Si: dkey-33c14.3 | −2.22 | 8.09 × 10−8 | ||
| arr3b | arrestin 3b | +2.19 | 1.23 × 10−6 | +2.70 | 2.95 × 10−9 |
| opn1mw1 | opsin 1 medium-wave-sensitive 1 | +1.98 | 2.03 × 10−6 | +2.54 | 1.98 × 10−15 |
| crygmx | crystallin%2C gamma MX | −1.40 | 3.00 × 10−6 | ||
| opn1sw1 | opsin 1 short-wave-sensitive 1 | +1.47 | 4.22 × 10−6 | ||
| grk7a | G protein-coupled receptor kinase 7a | +2.01 | 5.56 × 10−6 | +2.47 | 1.22 × 10−10 |
| gnb3b | guanine nucleotide-binding protein beta polypeptide 3b | +1.69 | 5.56 × 10−6 | +2.32 | 2.81 × 10−15 |
| mipb | The major intrinsic protein of lens fiber b | −1.37 | 2.91 × 10−5 | ||
| fkbp5 | FK506 binding protein 5 | −2.31 | 2.00 × 10−28 | ||
| gngt2b | guanine nucleotide-binding protein gamma transducing activity polypeptide 2b | +1.64 | 2.28 × 10−13 | ||
| gabra6a | gamma-aminobutyric acid A receptor%2C alpha 6a | +2.22 | 2.15 × 10−9 | ||
| hnrnpa3 | heterogeneous nuclear ribonucleoprotein A3 | +1.77 | 4.22 × 10−9 | ||
| nadk2 | NAD kinase 2 | −3.88 | 3.67 × 10−9 | ||
| pde6c | phosphodiesterase 6C | +2.07 | 3.67 × 10−9 | ||
| sik1 | salt-inducible kinase 1 | −1.62 | 1.52 × 10−7 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhang, L.; Tao, N.; Wang, X.; Deng, S.; Li, M.; Zu, Y.; Xu, C. Small Peptides Isolated from Enzymatic Hydrolyzate of Pneumatophorus japonicus Bone Promote Sleep by Regulating Circadian Rhythms. Foods 2023, 12, 464. https://doi.org/10.3390/foods12030464
Wang J, Zhang L, Tao N, Wang X, Deng S, Li M, Zu Y, Xu C. Small Peptides Isolated from Enzymatic Hydrolyzate of Pneumatophorus japonicus Bone Promote Sleep by Regulating Circadian Rhythms. Foods. 2023; 12(3):464. https://doi.org/10.3390/foods12030464
Chicago/Turabian StyleWang, Junbao, Lu Zhang, Ningping Tao, Xichang Wang, Shanggui Deng, Mingyou Li, Yao Zu, and Changhua Xu. 2023. "Small Peptides Isolated from Enzymatic Hydrolyzate of Pneumatophorus japonicus Bone Promote Sleep by Regulating Circadian Rhythms" Foods 12, no. 3: 464. https://doi.org/10.3390/foods12030464






