Preparation and Characterization of Intracellular and Exopolysaccharides during Cycle Cultivation of Spirulina platensis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cultivation of S. platensis
2.2. Evaluation of S. platensis Growth
2.3. Experimental Design of RSM for Extraction of IPS from S. platensis
2.4. Extraction of Extracellular Polysaccharide (EPS)
2.5. Chemical Composition Analysis of IPS and EPS
2.6. Fourier-Transform Infrared (FTIR) Spectroscopy and Scanning Electron Microscopy (SEM)
2.7. Zeta Potentials and Thermal-Gravimetric (TG) Analysis
2.8. In Vitro Antioxidant Activity Measurements
2.8.1. DPPH Radical Scavenging Assay
2.8.2. ABTS Radical Scavenging Assay
2.8.3. HO• Radical Scavenging Assay
2.8.4. Fe2+ Chelating Ability
2.8.5. EC50 Calculation
2.9. Statistical Analysis
3. Results and Discussion
3.1. Change of S. platensis and Polysaccharide Content during Cycle Cultivation
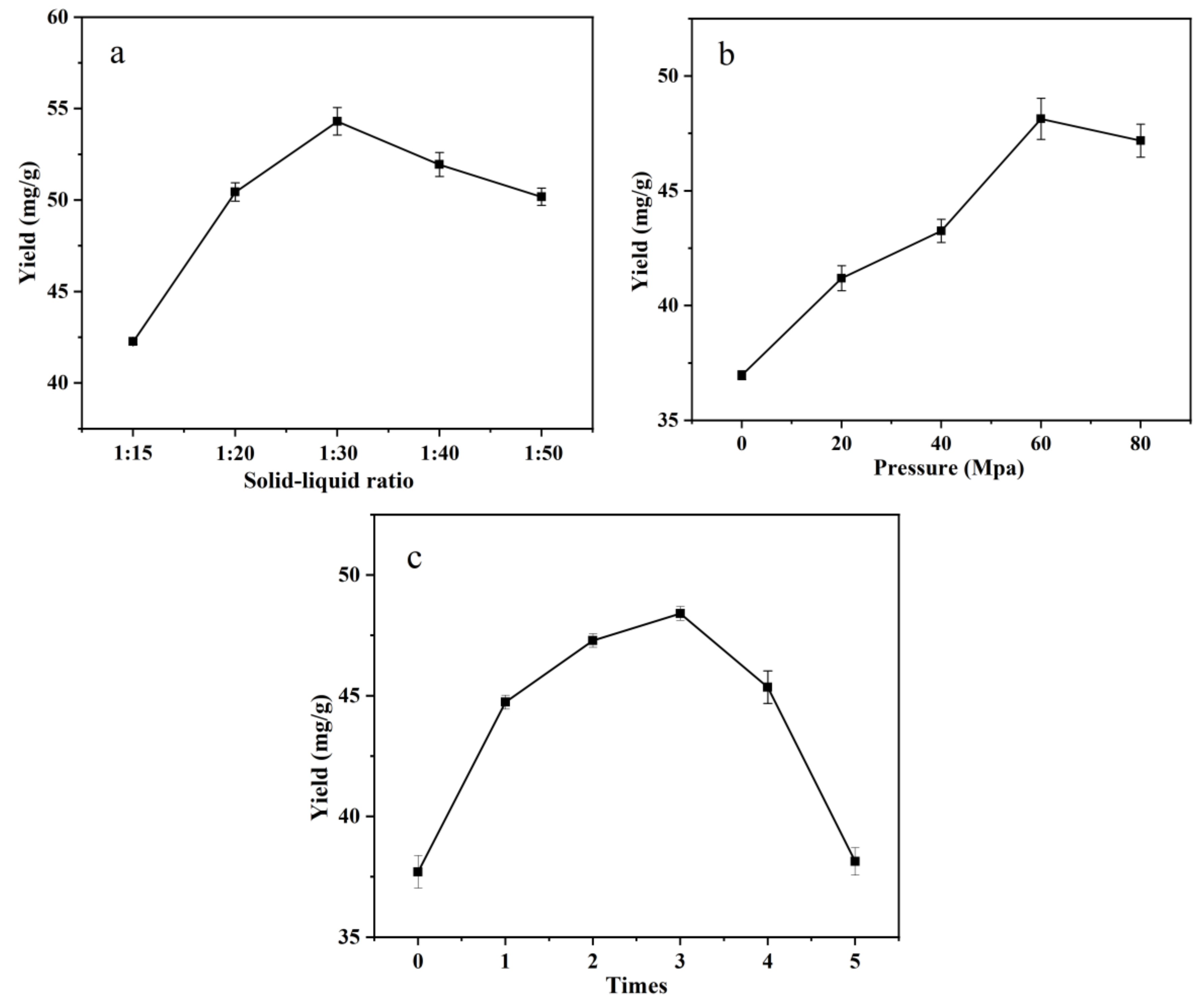
3.2. Single-Factor Test of IPS Extraction
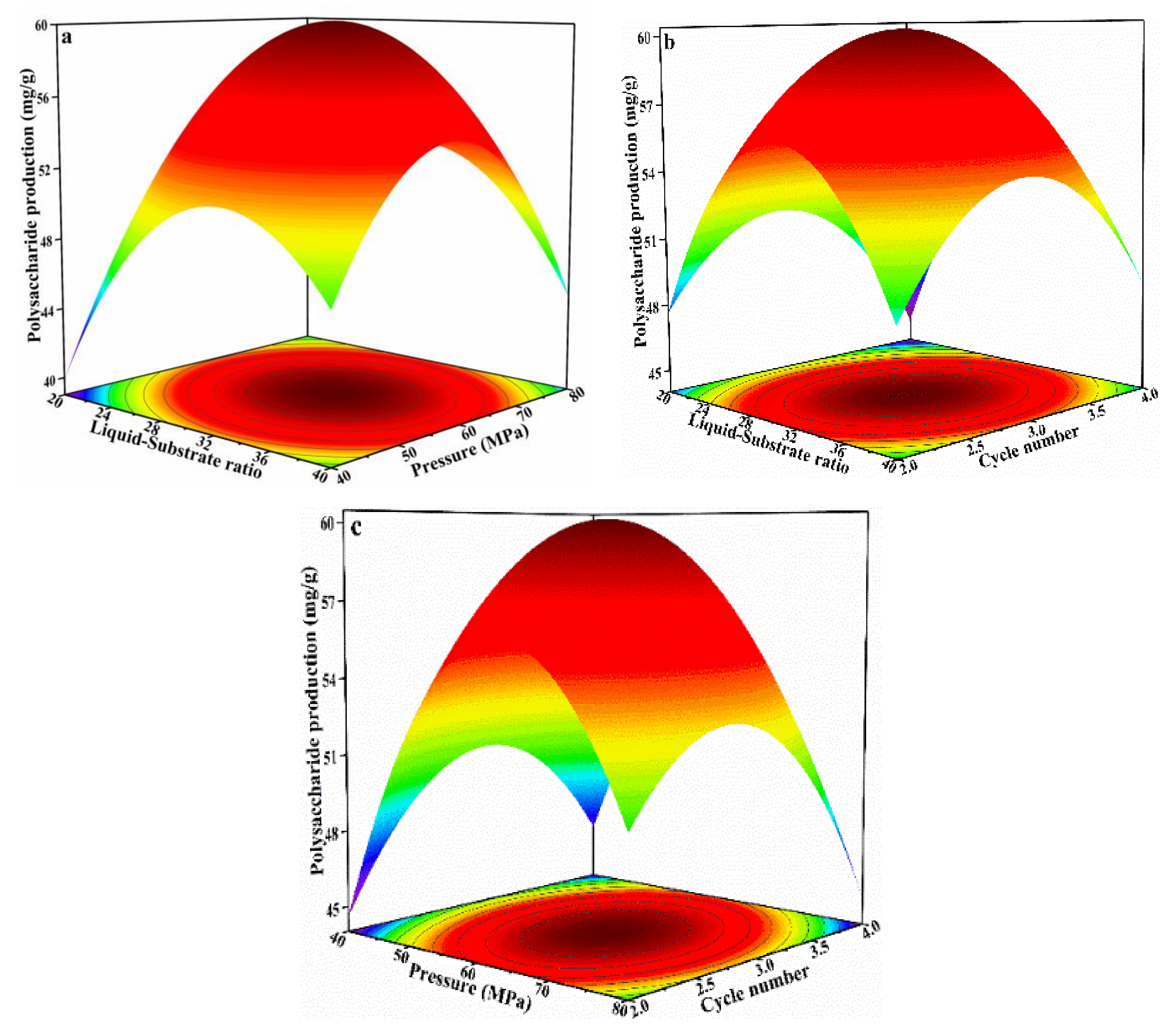
3.3. Optimization of IPS Extraction
3.4. IPS and EPS Composition
3.5. FTIR Spectrum Analysis and SEM Imaging
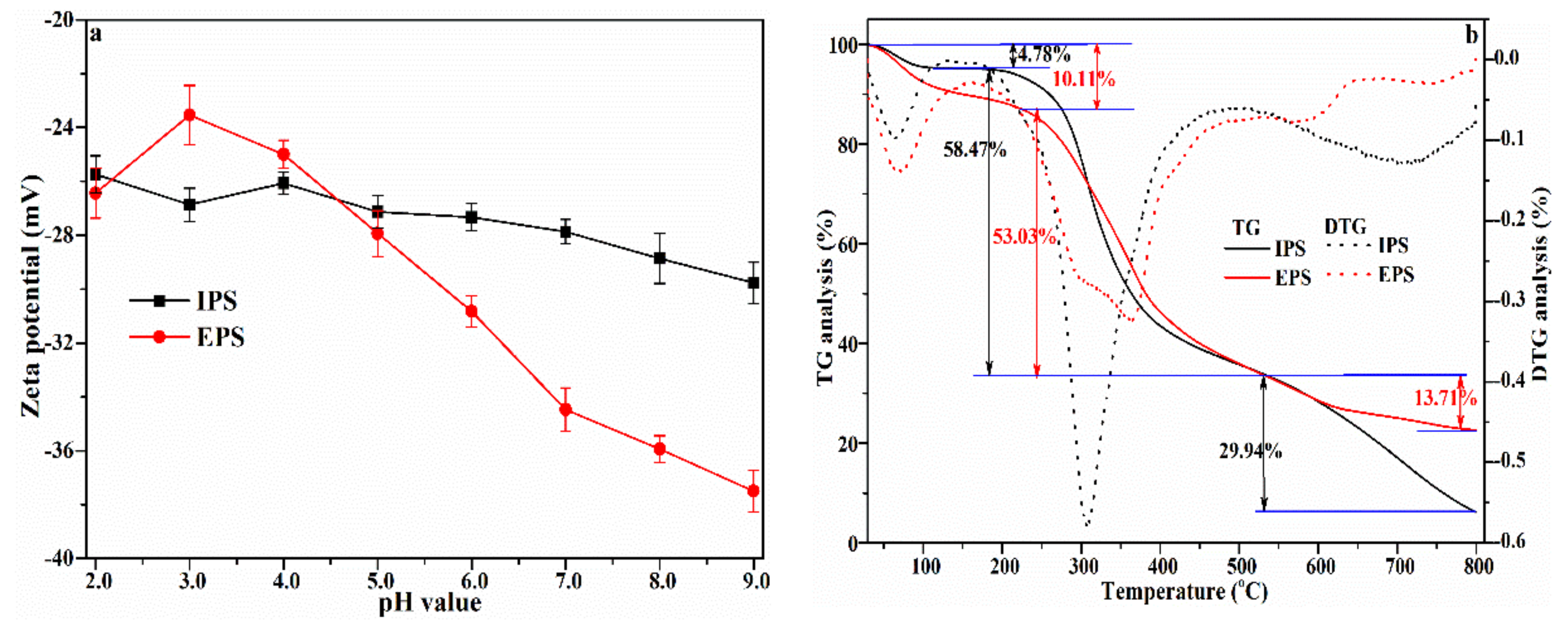
3.6. Zeta Potential and TG Analysis
3.7. Antioxidant Capacity Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jin, S.-E.; Lee, S.J.; Park, C.-Y. Mass-production and biomarker-based characterization of high-value Spirulina powder for nutritional supplements. Food Chem. 2020, 325, 126751. [Google Scholar] [CrossRef] [PubMed]
- Soni, R.A.; Sudhakar, K.; Rana, R. Spirulina–From growth to nutritional product: A review. Trends Food Sci. Technol. 2017, 69, 157–171. [Google Scholar] [CrossRef] [Green Version]
- Lafarga, T.; Sevilla, J.M.F.; González-López, C.; Fernandez, F.G.A. Spirulina for the food and functional food industries. Food Res. Int. 2020, 137, 109356. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Du, L.; Hosokawa, M.; Miyashita, K. Effect of Spirulina lipids on high-fat and high-sucrose diet induced obesity and hepatic lipid accumulation in C57BL/6J mice. J. Funct. Foods 2019, 65, 103741. [Google Scholar] [CrossRef]
- Cai, B.; Zhao, X.; Luo, L.; Wan, P.; Chen, H.; Pan, J. Structural characterization, and in vitro immunostimula-tory and antitumor activity of an acid polysaccharide from Spirulina platensis. Int. J. Biol. Macromol. 2022, 196, 46–53. [Google Scholar] [CrossRef]
- Ma, H.; Xiong, H.; Zhu, X.; Ji, C.; Xue, J.; Li, R.; Ge, B.; Cui, H. Polysaccharide from Spirulina platensis ameliorates diphenoxylate-induced constipation symptoms in mice. Int. J. Biol. Macromol. 2019, 133, 1090–1101. [Google Scholar] [CrossRef]
- Tian, H.; Liu, H.; Song, W.; Zhu, L.; Zhang, T.; Li, R.; Yin, X. Structure, antioxidant and immunostimulatory activities of the polysaccharides from Sargassum carpophyllum. Algal Res. 2020, 49, 101853. [Google Scholar] [CrossRef]
- Bernaerts, T.; Gheysen, L.; Kyomugasho, C.; Kermani, Z.; Vandionant, S.; Foubert, I.; Hendrickx, M.; Lo-ey, A. Comparison of microalgal biomasses as functional food ingredients: Focus on the composition of cell wall re-lated polysaccharides. Algal Res. 2018, 32, 150–161. [Google Scholar] [CrossRef]
- Chaiklahan, R.; Chirasuwan, N.; Triratana, P.; Loha, V.; Tia, S.; Bunnag, B. Polysaccharide extraction from Spirulina sp. and its antioxidant capacity. Int. J. Biol. Macromol. 2013, 58, 73–78. [Google Scholar] [CrossRef]
- Silva, A.; Magalhaes, W.; Moreira, L.; Rocha, M.; Bastos, A. Microwave-assisted extraction of poly-saccharides from Arthrospira (Spirulina) platensis using the concept of green chemistry. Algal Res. 2018, 35, 178–184. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Ren, Y.; Chen, F. Characterization of exopolysaccharides produced by microalgae with anti-tumor activity on human colon cancer cells. Int. J. Biol. Macromol. 2019, 128, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Chentir, I.; Hamdi, M.; Doumandji, A.; HadjSadok, A.; Ouada, H.B.; Nasri, M.; Jridi, M. Enhancement of extracellular polymeric substances (EPS) production in Spirulina (Arthrospira sp.) by two-step cultivation process and partial characterization of their polysaccharidic moiety. Int. J. Biol. Macromol. 2017, 105, 1412–1420. [Google Scholar] [CrossRef] [PubMed]
- Willis, A.; Chiovitti, A.; Dugdale, T.; Wetherbee, R.; Mock, T. Characterization of the Extracellular Matrix of Phaeo-dactylum Tricornutum (bacillariophyceae): Structure, Composition, and Adhesive Characteristics. J. Phycol. 2013, 49, 49–937. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, Y.; Zhou, T.; Cao, L.; Cai, Y.; Wang, Y.; Cui, X.; Yan, H.; Ruan, R.; Zhang, Q. Effects of Culture Conditions on the Performance of Arthrospira platensis and Its Production of Exopolysaccharides. Foods 2022, 11, 2020. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wassie, T.; Gan, R.; Wu, X. Structural characteristics, and immunomodulatory effects of sulfated poly-saccharides derived from marine algae. Crit. Rev. Food Sci. Nutr. 2022, 1–17. [Google Scholar]
- Tang, Q.; Huang, G. Preparation and antioxidant activities of cuaurbit polysaccharide. Int. J. Biol. Macromol. 2018, 117, 362–365. [Google Scholar] [CrossRef]
- Wang, G.; Liu, C.; Liu, J.; Liu, B.; Li, P.; Qin, G.; Xu, Y.; Chen, K.; Liu, H.; Chen, K. Exopolysaccharide from Trichoderma pseudokoningii induces the apoptosis of MCF-7 cells through an intrinsic mitochondrial pathway. Carbohydr. Polym. 2016, 136, 1065–1073. [Google Scholar] [CrossRef]
- Zhou, T.; Cao, L.; Zhang, Q.; Liu, Y.; Xiang, S.; Liu, T.; Ruan, R. Effect of chlortetracycline on the growth and intracellular components of Spirulina platensis and its biodegradation pathway. J. Hazard. Mater. 2021, 413, 125310. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, F.; Chen, X.; Yang, Z.; Cao, Y. Effects of the polysaccharide SPS-3-1 purified from Spirulina on bar-rier integrity and proliferation of Caco-2 cells. Int. J. Biol. Macromol. 2020, 163, 279–287. [Google Scholar] [CrossRef]
- Sasaki, M.; Takagi, A.; Sasaki, D.; Nakamura, A.; Asayama, M. Characteristics and function of an extracellular poly-saccharide from a green alga Parachlorella. Carbohydr. Polym. 2021, 254, 117252. [Google Scholar] [CrossRef]
- Su, Y.; Li, L. Structural characterization and antioxidant activity of polysaccharide from four auriculariales. Carbohydr. Polym. 2020, 229, 115407. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Liao, A.; Huang, J.; Thakur, K.; Li, X.; Wei, Z. Physicochemical and antioxidant potential of polysac-charides sequentially extracted from Amana edulis. Int. J. Biol. Macromol. 2019, 131, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Hsu, B.; Chen, B. Structural characterization of polysaccharides from Zizyphus jujuba and evalua-tion of antioxidant activity. Int. J. Biol. Macromol. 2010, 47, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Kanmani, P.; Kumar, R.; Yuvaraj, N.; Paari, K.; Pattukumar, V.; Arul, V. Production and purification of a novel ex-opolysaccharide from lactic acid bacterium Streptococcus phocae PI80 and its functional characteristics activity in vitro. Bioresour. Technol. 2011, 102, 4827–4833. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, X.; Sun, L.; Gao, Y. Characterization and anti-diabetic evaluation of sulfated polysaccharide from Spirulina platensis. J. Funct. Foods 2022, 95, 105155. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, S.; Zhu, P.; Nie, C.; Ma, S.; Wang, N.; Du, X.; Zhou, Y. Purification, characterization and immunomodulatory activity of water extractable polysaccharides from the swollen culms of Zizania latifolia. Int. J. Biol. Macromol. 2018, 107, 882–890. [Google Scholar] [CrossRef]
- Sirajunnisa, A.; Vijayagopal, V.; Sivaprakash, B.; Viruthagiri, T.; Surendhiran, D. Optimization, kinetics and anti-oxidant activity of exopolysaccharide produced from rhizosphere isolate, Pseudomonas fluorescens CrN6. Carbo-Hydrate Polym. 2016, 135, 35–43. [Google Scholar] [CrossRef]
- Wang, W.-N.; Li, T.; Li, Y.; Zhang, Y.; Wu, H.-L.; Xiang, W.-Z.; Li, A.-F. Exopolysaccharides from the Energy Microalga Strain Botryococcus braunii: Purification, Characterization, and Antioxidant Activity. Foods 2022, 11, 110. [Google Scholar] [CrossRef]




| Number | Factors | Yield (mg/g) | |||
|---|---|---|---|---|---|
| A: Solid–Liquid Ratio | B: Pressure (Mpa) | C: Times | Observed | Predicted | |
| 1 | 1:20 | 40 | 3 | 39.87 | 39.88 |
| 2 | 1:30 | 60 | 3 | 60.33 | 60.12 |
| 3 | 1:30 | 60 | 3 | 60.33 | 60.12 |
| 4 | 1:30 | 40 | 4 | 46.60 | 46.23 |
| 5 | 1:30 | 60 | 3 | 60.40 | 60.12 |
| 6 | 1:40 | 60 | 4 | 48.77 | 48.77 |
| 7 | 1:20 | 80 | 3 | 46.58 | 46.14 |
| 8 | 1:20 | 60 | 4 | 44.63 | 45.06 |
| 9 | 1:40 | 80 | 3 | 44.47 | 44.45 |
| 10 | 1:30 | 80 | 2 | 49.24 | 49.68 |
| 11 | 1:40 | 40 | 3 | 46.35 | 46.79 |
| 12 | 1:30 | 60 | 3 | 60.04 | 60.12 |
| 13 | 1:40 | 60 | 2 | 49.59 | 49.16 |
| 14 | 1:30 | 80 | 4 | 45.07 | 45.07 |
| 15 | 1:30 | 40 | 2 | 44.60 | 44.59 |
| 16 | 1:20 | 60 | 2 | 47.65 | 47.64 |
| 17 | 1:30 | 60 | 3 | 59.54 | 60.12 |
| Source | Sum of Square | df | Mean Square | F-Value | p-Value | Significant |
|---|---|---|---|---|---|---|
| Model | 770.00 | 9 | 85.56 | 363.16 | <0.0001 | ** |
| A | 13.66 | 1 | 13.66 | 57.97 | 0.0001 | ** |
| B | 7.70 | 1 | 7.70 | 32.67 | 0.0007 | ** |
| C | 4.40 | 1 | 4.40 | 18.67 | 0.0035 | ** |
| AB | 18.46 | 1 | 18.46 | 78.37 | <0.0001 | ** |
| AC | 1.21 | 1 | 1.21 | 5.51 | 0.0575 | |
| BC | 9.77 | 1 | 9.77 | 41.45 | 0.0004 | ** |
| A2 | 222.68 | 1 | 222.68 | 945.22 | <0.0001 | ** |
| B2 | 306.89 | 1 | 306.89 | 1302.68 | <0.0001 | ** |
| C2 | 113.59 | 1 | 113.59 | 482.15 | <0.0001 | ** |
| Residual | 1.65 | 7 | 0.2356 | |||
| Lack of Fit | 1.15 | 3 | 0.3834 | 3.07 | 0.1532 | |
| Pure Error | 0.4990 | 4 | 0.1248 | |||
| R2 | 0.9979 | |||||
| R2adj | 0.9951 | |||||
| Total variation | 771.65 | 16 | ||||
| Parameters | IPS | EPS | Parameters | IPS | EPS |
|---|---|---|---|---|---|
| Total sugar (%) | 71.95 | 65.38 | Glucuronic acid (%) | 2.39 | 7.65 |
| Protein (%) | 3.71 | 4.41 | Galacturonic acid (%) | 1.88 | 11.13 |
| Phenolic (%) | 7.3 | 6.5 | Glucose (%) | 83.62 | 14.75 |
| Ash (%) | 5.7 | 7.8 | Galactose (%) | 1.77 | 10.78 |
| Mannitol (%) | 0.91 | 5.29 | Xylose (%) | 1.07 | 4.91 |
| Ribose (%) | 0.095 | 3.94 | Arabinose (%) | 0.60 | 5.96 |
| Rhamnose (%) | 4.42 | 15.61 | Fucose (%) | 3.25 | 19.99 |
| Molecular weight (KDa) | 272.85 | 185.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Wang, Y.; Cao, L.; Huang, Z.; Zhou, Y.; Fan, R.; Li, C. Preparation and Characterization of Intracellular and Exopolysaccharides during Cycle Cultivation of Spirulina platensis. Foods 2023, 12, 1067. https://doi.org/10.3390/foods12051067
Liu Y, Wang Y, Cao L, Huang Z, Zhou Y, Fan R, Li C. Preparation and Characterization of Intracellular and Exopolysaccharides during Cycle Cultivation of Spirulina platensis. Foods. 2023; 12(5):1067. https://doi.org/10.3390/foods12051067
Chicago/Turabian StyleLiu, Yuhuan, Yu Wang, Leipeng Cao, Zhenghua Huang, Yue Zhou, Ruijuan Fan, and Congmiao Li. 2023. "Preparation and Characterization of Intracellular and Exopolysaccharides during Cycle Cultivation of Spirulina platensis" Foods 12, no. 5: 1067. https://doi.org/10.3390/foods12051067





