Effect of pH-Shifting Process on the Cathepsin Activity, Muddy Off-Odor Compounds’ Content and Gelling Properties of Isolated Protein from Silver Carp
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Mince Collection
2.2.2. Preparation of Conventional Water-Washed Surimi
2.2.3. The pH-Shifting Process
2.2.4. Protein Recovery Rate
2.2.5. Cathepsin Activity
2.2.6. Determination of GEO and MIB
2.2.7. Rheology Studies
2.2.8. Heat-Induced Gel
2.2.9. Determination of the Gel Strength: Hardness and Elasticity
2.2.10. TCA-Soluble Peptide Measurement
2.2.11. Determination of Disulfide Bond Content
2.2.12. SDS-Polyacrylamide Gel Electrophoresis
2.2.13. Statistical Analysis
3. Results and Discussion
3.1. Protein Recovery, Activity of Cathepsin, Content of GEO and MIB
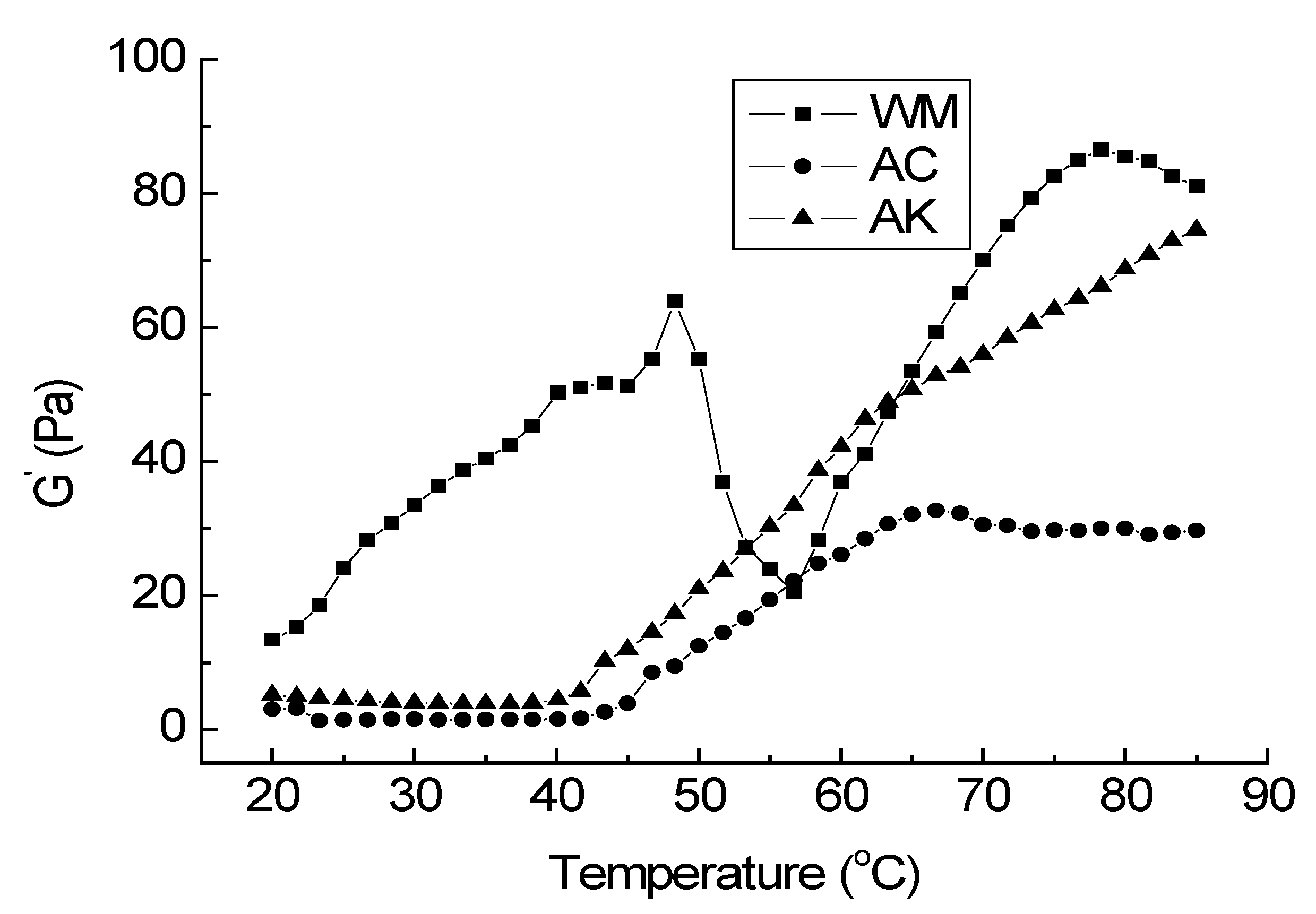
3.2. Rheology Properties
3.3. The Content of TCA-Soluble Peptide
3.4. The Content of Disulfide
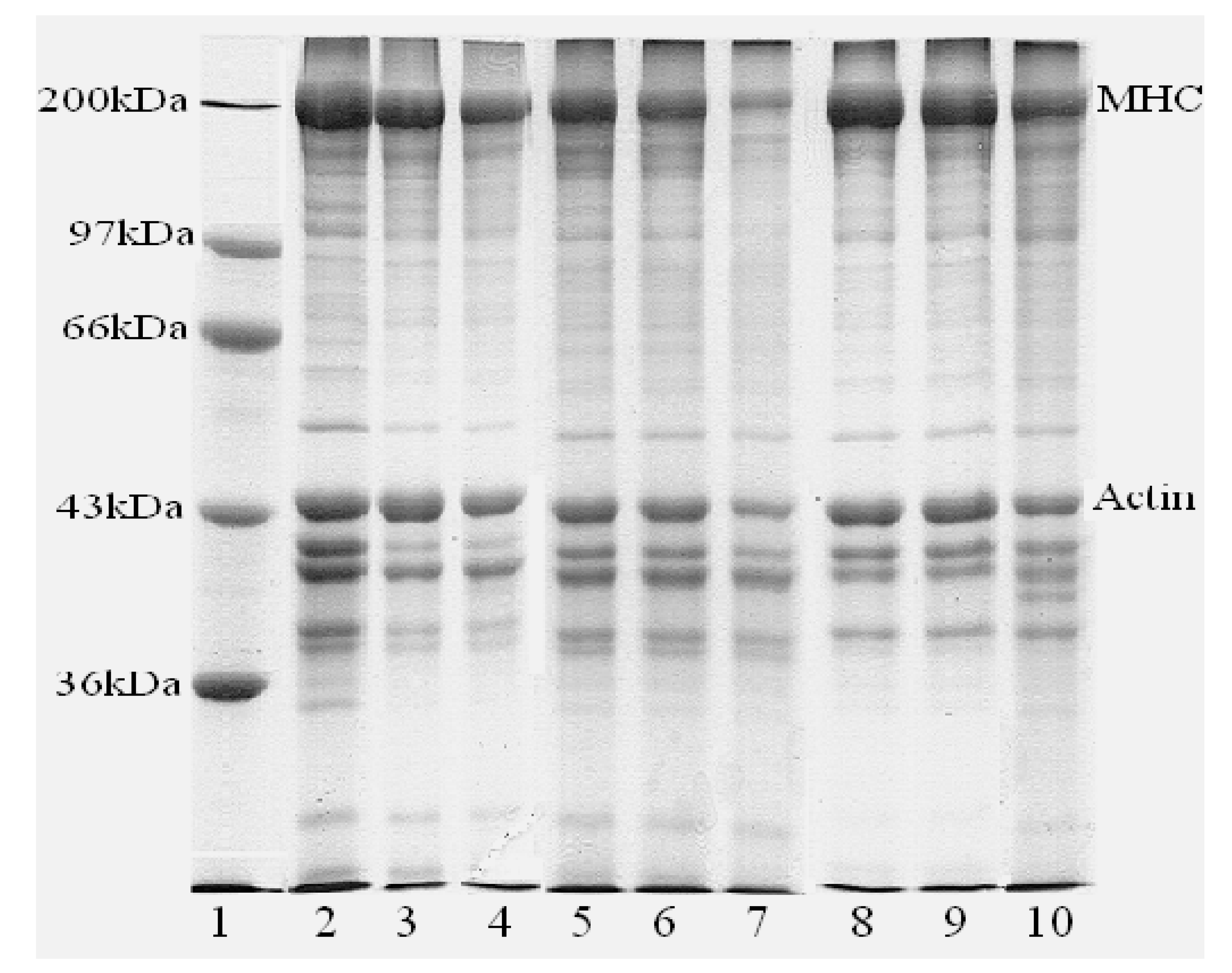
3.5. Protein Pattern of WM, IPs and Gels
3.6. Gel Strength
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taskaya, L.; Chen, Y.; Jaczynski, J. Functional properties of proteins recovered from silver carp (Hypophthalmichthys molitrix) by isoelectric solubilization/precipitation. LWT-Food Sci. Technol. 2009, 42, 1082–1089. [Google Scholar] [CrossRef]
- Paker, I.; Beamer, S.; Jaczynski, J.; Matak, K.E. The effect of organic acids on gelation characteristics of protein gels made from silver carp (Hypophthalmichthys molitrix) protein recovered by isoelectric solubilization and precipitation. LWT-Food Sci. Technol. 2013, 53, 37–43. [Google Scholar] [CrossRef]
- Paker, I.; Beamer, S.; Jaczynski, J.; Matak, K.E. Compositional Characteristics of Materials Recovered from Headed Gutted Silver Carp (Hypophthalmichthys molitrix) by Isoelectric Solubilization and Precipitation Using Organic Acids. J. Food Sci. 2013, 78, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Shen, H.; Pan, D.; Bu, G.H. Gel properties of surimi from silver carp (Hypophthalmichthys molitrix) as affected by heat treatment and soy protein isolates. Food Hydrocoll. 2008, 22, 1513–1519. [Google Scholar] [CrossRef]
- Hultin, H.O.; Kelleher, S.D. Process for Isolating a Protein Composition from a Muscle Source and Protein Composition. U.S. Patent 08/797,929, 12 February 1997. [Google Scholar]
- Phetsang, H.; Panpipat, W.; Undeland, I.; Panya, A.; Phonsatta, N.; Chaijan, M. Comparative quality and volatilomic characterisation of unwashed mince, surimi, and pH-shift-processed protein isolates from farm-raised hybrid catfish (Clarias macrocephalus × Clarias gariepinus). Food Chem. 2021, 364, 130365. [Google Scholar] [CrossRef]
- Yarnpakdee, S.; Benjakul, S.; Penjamras, P.; Kristinsson, H.G. Chemical compositions and muddy flavour/odour of protein hydrolysate from Nile tilapia and broadhead catfish mince and protein isolate. Food Chem. 2014, 142, 210–216. [Google Scholar] [CrossRef]
- Perez-Mateos, M.; Lanier, T.C. Comparison of Atlantic menhaden gels from surimi processed by acid or alkaline solubilization. Food Chem. 2006, 101, 1223–1229. [Google Scholar] [CrossRef] [Green Version]
- Palafox, H.; Cordova-Murueta, J.H.; Toro, M.A.N.D.; Garcia-Carreno, F.L. Protein isolates from jumbo squid (Dosidicus gigas) by pH-shift processing. Process Biochem. 2009, 44, 584–587. [Google Scholar] [CrossRef]
- Zhou, Y.G.; Yang, H.S. Effects of calcium ion on gel properties and gelation of tilapia (Oreochromis niloticus) protein isolates processed with pH shift method. Food Chem. 2019, 277, 327–335. [Google Scholar] [CrossRef]
- Zhou, Y.G.; Liu, J.J.H.; Kang, Y.; Cui, H.J.; Yang, H.S. Effects of acid and alkaline treatments on physicochemical and rheological properties of tilapia surimi prepared by pH shift method during cold storage. Food Res. Int. 2021, 145, 110424. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Park, J.W. Biochemical and physical characterizations of fish protein isolate and surimi prepared from fresh and frozen whole fish. LWT-Food Sci. Technol. 2017, 77, 200–207. [Google Scholar] [CrossRef]
- Tomé, A.S.; Pires, C.; Batista, I.; Sousa, I.; Raymundo, A. Protein gels and emulsions from mixtures of Cape hake and pea proteins. J. Sci. Food Agric. 2015, 95, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Arfat, Y.A.; Benjakul, S. Effect of zinc sulphate on gelling properties of phosphorylated protein isolate from yellow stripe trevally. Food Chem. 2013, 141, 2848–2857. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Ruiz, J.A.; Pacheco-Aguilar, R.; Ramírez-Suárez, C.J.; Lugo-Sánchez, M.E.; García-Orozco, K.D.; Sotelo-Mundo, R.R.; Peña-Ramos, A. Conformational changes in proteins recovered from jumbo squid (Dosidicus gigas) muscle through pH shift washing treatments. Food Chem. 2016, 196, 769–775. [Google Scholar] [CrossRef] [PubMed]
- González-González, D.C.; Lugo-Sánchez, M.E.; García-Sifuentes, C.O.; Ramírez-Suárez, J.C.; Pacheco-Aguilar, R. Influence of pH, ionic strength and isoascorbic acid on the gel-forming ability of Jumbo squid muscle (Dosidicus gigas). Food Chem. 2021, 337, 127993. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, M.; Rezaei, M.; Jafarpour, A.; Undeland, I. Dynamic rheological, microstructural and physicochemical properties of blend fish protein recovered from kilka (Clupeonella cultriventris) and silver carp (Hypophthalmichthys molitrix) by the pH-shift process or washing-based technology. Food Chem. 2017, 229, 695–709. [Google Scholar] [CrossRef] [PubMed]
- Taskaya, L.; Chen, Y.C.; Jaczynski, J. Color improvement by titanium dioxide and its effect on gelation and texture of proteins recovered from whole fish using isoelectric solubilization/ precipitation. LWT-Food Sci. Technol. 2010, 43, 401–408. [Google Scholar] [CrossRef]
- Liu, H.; Yin, L.J.; Zhang, N.; Li, S.H.; Ma, C.W. Purification and characterization of cathepsin L from the muscle of silver carp (Hypophthalmichthys molitrix). J. Agric. Food Chem. 2006, 54, 9584–9591. [Google Scholar] [CrossRef]
- Liu, H.; Yin, L.J.; Zhang, N.; Li, S.H.; Ma, C.W. Isolation of cathepsin B from the muscle of silver carp (Hypophthalmichthys molitrix) and comparison of cathepsins B and L actions on surimi gel softening. Food Chem. 2008, 110, 310–318. [Google Scholar] [CrossRef]
- Fang, M.X.; Luo, X.Y.; Xiong, S.B.; Yin, T.; Hu, Y.; Liu, R.; Du, H.Y.; Liu, Y.M.; You, J. In vitro trypsin digestion and identification of possible cross-linking sites induced by transglutaminase (TGase) of silver carp (Hypophthalmichthys molitrix) surimi gels with different degrees of cross-linking. Food Chem. 2021, 364, 130443. [Google Scholar] [CrossRef]
- Yamprayoon, J.; Noomhorm, A. Effects of preservation methods on geosmin content and off-flavor in Nile tilapia (Oreochromis niloticus). J. Aquat. Food Prod. Technol. 2000, 9, 95–107. [Google Scholar] [CrossRef]
- Kleinholz Christina, M.D.; Kleinholz, C.W.; Vann, D.G.; Bilby, C.A.; Schrader, K.K. Evaluation of Acid and Alkaline Processing to Remove Muddy Off-Flavors in Channel Catfish (Ictalurus punctatus). J. Aquat. Food Prod. Technol. 2007, 16, 77–90. [Google Scholar]
- Fu, X.; Lin, Q.; Xu, S. Effect of Drying methods and Antioxidants on the Flavor and Lipid Oxidation of Silver Carp Slices. LWT-Food Sci. Technol. 2015, 27, 251–257. [Google Scholar] [CrossRef]
- Fu, X.; Hayat, K.; Li, Z.H.; Lin, Q.L.; Xu, S.Y.; Wang, S.P. Effect of microwave heating on the low-salt gel from silver carp (Hypophthalmichthys molitrix) surimi. Food Hydrocoll. 2012, 27, 301–308. [Google Scholar] [CrossRef]
- Benjakul, S.; Visessanguan, W.; Chantarasuwan, C. Effect of high-temperature setting on gelling characteristic of surimi from some tropical fish. Int. J. Food Sci. Technol. 2004, 39, 671–680. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Heidtmann, H.H.; Salge, U.; Havemann, K.; Wiederanders, B. Secretion of a latent, acid activatable cathepsin l precursor by human non-small cell lung cancer cell lines. Oncol. Res. 1993, 5, 441–451. [Google Scholar]
- Egelandsdal, B.; Fretheim, K.; Samejima, K. Dynamic rheological measurements on heat-induced myosin gels: Effect of ionic strength, protein concentration and addition of adenosine triphosphate or pyrophosphate. J. Sci. Food Agr. 1986, 37, 915–926. [Google Scholar] [CrossRef]
- Fu, X.; Xu, S.; Wang, Z. Kinetics of lipid oxidation and off-odor formation in silver carp mince: The effect of lipoxygenase and hemoglobin. Food Res. Int. 2009, 42, 85–90. [Google Scholar] [CrossRef]
- Runglerdkriangkrai, J.; Itoh, Y.; Kishi, A.; Obatake, A. Responsibility of myosin S-1 and rod for the polymerization of myosin heavy chain through disulfide bonding upon heating of actomyosin. Fish. Sci. 2008, 65, 310–314. [Google Scholar] [CrossRef] [Green Version]
- Kristinsson, H.G.; Hultin, H.O. Changes in conformation and subunit assembly of cod myosin at low and high pH and after subsequent refolding. J. Agric. Food Chem. 2003, 51, 7187–7196. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.I.; Itoh, Y.; Morioka, K.; Obatake, A. Inhibiting effect of polymerization and degradation of myosin heavy chain during preheating at 30 °C and 50 °C on the gel-forming ability of walleye pollack surimi. Fish. Sci. 2010, 67, 718–725. [Google Scholar] [CrossRef] [Green Version]
- Tsukamasa, Y.; Sato, K.; Shimizu, Y.; Imai, C.; Sugiyam, M.; Minegishi, Y.; Kawabata, M. (γ-Glutamyl) lysine Cross link Formation in Sardine Myofibril Sol during Setting at 25 °C. J. Food Sci. 2004, 58, 785–787. [Google Scholar] [CrossRef]
- Visessanguan, W.; An, H. Effects of proteolysis and mechanism of gel weakening in heat-induced gelation of fish myosin. J. Agric. Food Chem. 2000, 48, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
| Protein Recovery (%) | Cathepsin B (U/g) | Cathepsin L (U/g) | GEO (ug/kg) | MIB (ug/kg) | |
|---|---|---|---|---|---|
| Muscle | _ | 152.33 ± 8.21 a | 92.51 ± 6.20 a | 1.51 ± 0.08 a | 2.06 ± 0.11 a |
| WM | 28.8 ± 0.87 b | 7.79 ± 2.13 c | 29.14 ± 1.59 c | 0.92 ± 0.05 b | 1.02 ± 0.05 b |
| AC | 38.5 ± 0.59 a | 25.58 ± 4.85 b | 65.43 ± 4.91 b | 0.35 ± 0.03 c | 0.33 ± 0.03 c |
| AK | 40.9 ± 1.24 a | 8.53 ± 2.60 c | 27.67 ± 2.38 c | 0.26 ± 0.04 d | 0.21 ± 0.03 d |
| Samples | Peptide Content (mg/g) | S-S Content (mol/107 g Protein) | ||||
|---|---|---|---|---|---|---|
| WM | AC | AK | WM | AC | AK | |
| Surimi/IPs | 31.71 ± 1.32 c | 46.85 ± 2.50 a | 36.37 ± 1.87 b | 2.49 ± 0.12 C | 3.97 ± 0.18 B | 4.46 ± 0.21 A |
| Kamaboko | 44.18 ± 2.41 b | 61.42 ± 3.08 a | 47.05 ± 1.35 b | 15.26 ± 1.10 A | 15.01 ± 0.81 A | 15.14 ± 1.25 A |
| Setting gel | 52.56 ± 2.64 c | 77.55 ± 2.52 a | 61.27 ± 3.99 b | 15.35 ± 0.96 A | 15.30 ± 0.64 A | 15.42 ± 0.77 A |
| Modori gel | 62.92 ± 2.86 c | 90.89 ± 4.65 a | 76.84 ± 2.15 b | 15.18 ± 1.05 A | 14.81 ± 1.32 A | 15.20 ± 1.19 A |
| Surimi/IPs | Breaking Force (g) | Deformation (mm) | |
|---|---|---|---|
| WM | Kamaboko gel | 467.2 ± 20.6 b | 12.1 ± 0.3 b |
| Setting gel | 489.1 ± 16.1 b | 12.3 ± 0.3 b | |
| Modori gel | 315.9 ± 18.8 e | 10.8 ± 0.5 de | |
| AC | Kamaboko gel | 347.5 ± 24.7 e | 11.7 ± 0.3 cd |
| Setting gel | 302.6 ± 20.4 e | 10.3 ± 0.4 e | |
| Modori gel | 226.2 ± 19.5 f | 8.3 ± 0.4 f | |
| AK | Kamaboko gel | 435.8± 24.6 c | 11.9 ± 0.4 bc |
| Setting gel | 518.9 ± 21.4 a | 12.8 ± 0.3 a | |
| Modori gel | 386.4 ± 15.7 d | 11.6 ± 0.2 cd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, W.; Zhan, M.; Liu, H.; Fu, X.; Wu, W. Effect of pH-Shifting Process on the Cathepsin Activity, Muddy Off-Odor Compounds’ Content and Gelling Properties of Isolated Protein from Silver Carp. Foods 2023, 12, 939. https://doi.org/10.3390/foods12050939
Guo W, Zhan M, Liu H, Fu X, Wu W. Effect of pH-Shifting Process on the Cathepsin Activity, Muddy Off-Odor Compounds’ Content and Gelling Properties of Isolated Protein from Silver Carp. Foods. 2023; 12(5):939. https://doi.org/10.3390/foods12050939
Chicago/Turabian StyleGuo, Weidan, Miao Zhan, Hui Liu, Xiangjin Fu, and Wei Wu. 2023. "Effect of pH-Shifting Process on the Cathepsin Activity, Muddy Off-Odor Compounds’ Content and Gelling Properties of Isolated Protein from Silver Carp" Foods 12, no. 5: 939. https://doi.org/10.3390/foods12050939






