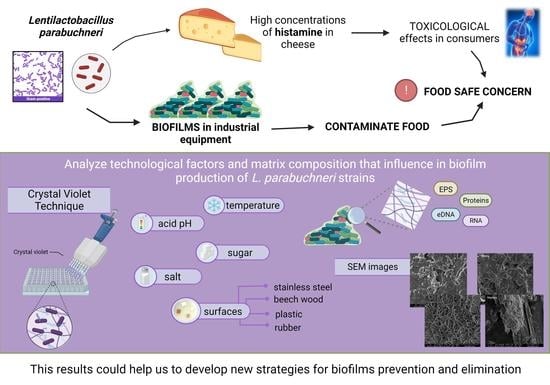
Characterization of the Biofilms Formed by Histamine-Producing Lentilactobacillus parabuchneri Strains in the Dairy Environment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
2.2. Biofilm Formation Capacity of L. parabuchneri Strains
- ODc < OD ≤ 2 × ODc = weak producer
- 2 × ODc < OD ≤ 4 × ODc = moderate producer
- OD > 4 × ODc = strong producer.
2.3. Biochemical Composition of Biofilm Matrices: Dispersal Assays
2.4. Bacterial Adherence to Different Surfaces
2.5. Scanning Electron Microscopy Images
2.6. Data Analysis
3. Results
3.1. Biofilm Formation by L. parabuchneri Strains
3.2. Biochemical Composition of Biofilms
3.3. Biofilm Formation after Different Incubation Times
3.4. Biofilm Formation at Colder Temperatures
3.5. Biofilm Formation in an Acidic Environment
3.6. Influence of Carbon Source on Biofilm Formation
3.7. Effect of NaCl on Biofilm Formation
3.8. Bacterial Adherence to Stainless Steel, Food-Grade Plastic, Beech Wood and Rubber
3.9. SEM Analysis of Biofilm Formation on Different Surfaces
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Govaert, M.; Smet, C.; Baka, M.; Janssens, T.; Van Impe, J. Influence of incubation conditions on the formation of model biofilms by Listeria monocytogenes and Salmonella Typhimurium on abiotic surfaces. J. Appl. Microbiol. 2018, 125, 1890–1900. [Google Scholar] [CrossRef]
- Sauer, K.; Stoodley, P.; Goeres, D.M.; Hall-Stoodley, L.; Burmølle, M.; Stewart, P.S.; Bjarnsholt, T. The biofilm life cycle: Expanding the conceptual model of biofilm formation. Nat. Rev. Microbiol. 2022, 20, 608–620. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Tolker-Nielsen, T. Biofilm development. Microbiol. Spectr. 2015, 3, 51–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Høiby, N. A short history of microbial biofilms and biofilm infections. Apmis 2017, 125, 272–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Zhao, F.; Wang, J.; Zhong, N. Biofilm formation and control strategies of foodborne pathogens: Food safety perspectives. RSC Adv. 2017, 7, 36670–36683. [Google Scholar] [CrossRef] [Green Version]
- Pang, X.; Song, X.; Chen, M.; Tian, S.; Lu, Z.; Sun, J.; Li, X.; Lu, Y.; Yuk, H. Combating biofilms of foodborne pathogens with bacteriocins by lactic acid bacteria in the food industry. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1657–1676. [Google Scholar] [CrossRef]
- Alvarez-Ordóñez, A.; Coughlan, L.M.; Briandet, R.; Cotter, P.D. Biofilms in food processing environments: Challenges and opportunities. Annu. Rev. Food Sci. Technol. 2019, 10, 173–195. [Google Scholar] [CrossRef]
- Karaca, B.; Buzrul, S.; Coleri Cihan, A. Anoxybacillus and Geobacillus biofilms in the dairy industry: Effects of surface material, incubation temperature and milk type. Biofouling 2019, 35, 551–560. [Google Scholar] [CrossRef]
- Faille, C.; Bénézech, T.; Midelet-Bourdin, G.; Lequette, Y.; Clarisse, M.; Ronse, G.; Ronse, A.; Slomianny, C. Sporulation of Bacillus spp. within biofilms: A potential source of contamination in food processing environments. Food Microbiol. 2014, 40, 64–74. [Google Scholar] [CrossRef]
- Lu, J.; Hu, X.; Ren, L. Biofilm control strategies in food industry: Inhibition and utilization. Trends Food Sci. Technol. 2022, 123, 103–113. [Google Scholar] [CrossRef]
- Vishwakarma, V. Impact of environmental biofilms: Industrial components and its remediation. J. Basic Microbiol. 2020, 60, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Johansen, E. Use of natural selection and evolution to develop new starter cultures for fermented foods. Annu. Rev. Food Sci. Technol. 2018, 9, 411–428. [Google Scholar] [CrossRef]
- WHO. The Burden of Foodborne Diseases in the WHO European Region; World Health Organization: Genève, Switzerland, 2017. [Google Scholar]
- Havelaar, A.H.; Kirk, M.D.; Torgerson, P.R.; Gibb, H.J.; Hald, T.; Lake, R.J.; Praet, N.; Bellinger, D.C.; de Silva, N.R.; Gargouri, N.; et al. World Health Organization global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med. 2015, 12, e1001923. [Google Scholar] [CrossRef] [Green Version]
- Makovcova, J.; Babak, V.; Kulich, P.; Masek, J.; Slany, M.; Cincarova, L. Dynamics of mono- and dual-species biofilm formation and interactions between Staphylococcus aureus and Gram-negative bacteria. Microb. Biotechnol. 2017, 10, 819–832. [Google Scholar] [CrossRef] [PubMed]
- Diaz, M.; Ladero, V.; del Rio, B.; Redruello, B.; Fernández, M.; Martin, M.C.; Alvarez, M.A. Biofilm-forming capacity in biogenic amine-producing bacteria isolated from dairy products. Front. Microbiol. 2016, 7, 591. [Google Scholar] [CrossRef] [PubMed]
- Ladero, V.; Linares, D.M.; Pérez, M.; del Rio, B.; Fernández, M.; Alvarez, M.A. Biogenic amines in dairy products. In Microbial Toxins in Dairy Products; Wiley Blackwell: Hoboken, NJ, USA, 2016; pp. 94–131. [Google Scholar]
- Barbieri, F.; Montanari, C.; Gardini, F.; Tabanelli, G. Biogenic amine production by lactic acid bacteria: A review. Foods 2019, 8, 17. [Google Scholar] [CrossRef] [Green Version]
- del Rio, B.; Redruello, B.; Linares, D.M.; Ladero, V.; Ruas-Madiedo, P.; Fernandez, M.; Martin, M.C.; Alvarez, M.A. The biogenic amines putrescine and cadaverine show in vitro cytotoxicity at concentrations that can be found in foods. Sci. Rep. 2019, 9, 120. [Google Scholar] [CrossRef] [Green Version]
- Linares, D.M.; del Rio, B.; Redruello, B.; Ladero, V.; Martin, M.C.; Fernandez, M.; Ruas-Madiedo, P.; Alvarez, M.A. Comparative analysis of the in vitro cytotoxicity of the dietary biogenic amines tyramine and histamine. Food Chem. 2016, 197, 658–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ladero, V.; Calles-Enríquez, M.; Fernández, M.; Alvarez, M.A. Toxicological effects of dietary biogenic amines. Curr. Nutr. Food Sci. 2010, 6, 145–156. [Google Scholar] [CrossRef]
- Ruiz-Capillas, C.; Herrero, A. Impact of biogenic amines on food quality and safety. Foods 2019, 8, 62. [Google Scholar] [CrossRef] [Green Version]
- Linares, D.M.; Martín, M.; Ladero, V.; Alvarez, M.A.; Fernández, M. Biogenic amines in dairy products. Crit. Rev. Food Sci. Nutr. 2011, 51, 691–703. [Google Scholar] [CrossRef] [PubMed]
- del Rio, B.; Redruello, B.; Linares, D.M.; Ladero, V.; Fernandez, M.; Martin, M.C.; Ruas-Madiedo, P.; Alvarez, M.A. The dietary biogenic amines tyramine and histamine show synergistic toxicity towards intestinal cells in culture. Food Chem. 2017, 218, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Maintz, L.; Novak, N. Histamine and histamine intolerance. Am. J. Clin. Nutr. 2007, 85, 1185–1196. [Google Scholar] [CrossRef] [Green Version]
- Şanlı, T.; Şenel, E. Formation of biogenic amines in cheese. In Processing and Impact on Active Components in Food; Elsevier: Amsterdam, The Netherlands, 2015; pp. 223–230. ISBN 9780124047099. [Google Scholar]
- Chaidoutis, E.; Migdanis, A.; Keramydas, D.; Papalexis, P. Biogenic amines in food as a public health concern an outline of histamine food poisoning. Arch. Hell. Med. 2019, 36, 419–425. [Google Scholar]
- Diaz, M.; Ladero, V.; Redruello, B.; Sanchez-Llana, E.; del Rio, B.; Fernandez, M.; Martin, M.C.; Alvarez, M.A. A PCR-DGGE method for the identification of histamine-producing bacteria in cheese. Food Control 2016, 63, 216–223. [Google Scholar] [CrossRef] [Green Version]
- Ascone, P.; Maurer, J.; Haldemann, J.; Irmler, S.; Berthoud, H.; Portmann, R.; Fröhlich-Wyder, M.-T.; Wechsler, D. Prevalence and diversity of histamine-forming Lactobacillus parabuchneri strains in raw milk and cheese—A case study. Int. Dairy J. 2017, 70, 26–33. [Google Scholar] [CrossRef]
- Fröhlich-Wyder, M.-T.; Guggisberg, D.; Badertscher, R.; Wechsler, D.; Wittwer, A.; Irmler, S. The effect of Lactobacillus buchneri and Lactobacillus parabuchneri on the eye formation of semi-hard cheese. Int. Dairy J. 2013, 33, 120–128. [Google Scholar] [CrossRef]
- Fröhlich-Wyder, M.-T.; Bisig, W.; Guggisberg, D.; Irmler, S.; Jakob, E.; Wechsler, D. Influence of low pH on the metabolic activity of Lactobacillus buchneri and Lactobacillus parabuchneri strains in Tilsit-type model cheese. Dairy Sci. Technol. 2015, 95, 569–585. [Google Scholar] [CrossRef] [Green Version]
- Berthoud, H.; Wüthrich, D.; Bruggmann, R.; Wechsler, D.; Fröhlich-Wyder, M.-T.; Irmler, S. Development of new methods for the quantitative detection and typing of Lactobacillus parabuchneri in dairy products. Int. Dairy J. 2017, 70, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Wüthrich, D.; Berthoud, H.; Wechsler, D.; Eugster, E.; Irmler, S.; Bruggmann, R. The histidine decarboxylase gene cluster of Lactobacillus parabuchneri was gained by horizontal gene transfer and is mobile within the species. Front. Microbiol. 2017, 8, 218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maurer, A.J.; Haldemann, J.; Ascone, P.; Wechsler, D. Recommandations destinées aux producteurs de lait de fromagerie pour éviter les contaminations par des bactéries propioniques et Lactobacillus parabuchneri dans les installations de traite. Denrées Aliment. 2016, 1, 1–4. [Google Scholar]
- Diaz, M.; del Rio, B.; Sanchez-Llana, E.; Ladero, V.; Redruello, B.; Fernandez, M.; Martin, M.C.; Alvarez, M.A.; Fernández, M.; Martin, M.C.; et al. Histamine-producing Lactobacillus parabuchneri strains isolated from grated cheese can form biofilms on stainless steel. Food Microbiol. 2016, 59, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Diaz, M.; del Rio, B.; Ladero, V.; Redruello, B.; Fernández, M.; Martin, M.C.; Alvarez, M.A.; Fernandez, M.; Martin, M.C.; Alvarez, M.A. Isolation and typification of histamine-producing Lactobacillus vaginalis strains from cheese. Int. J. Food Microbiol. 2015, 215, 117–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, M.C.; Fernandez, M.; Linares, D.M.; Alvarez, M.A. Sequencing, characterization and transcriptional analysis of the histidine decarboxylase operon of Lactobacillus buchneri. Microbiology 2005, 151, 1219–1228. [Google Scholar] [CrossRef] [Green Version]
- Molenaar, D.; Bosscher, J.S.; ten Brink, B.; Driessen, A.J.M.; Konings, W.N. Generation of a proton motive force by histidine decarboxylation and electrogenic histidine/histamine antiport in Lactobacillus buchneri. J. Bacteriol. 1993, 175, 2864–2870. [Google Scholar] [CrossRef] [Green Version]
- Kubota, H.; Senda, S.; Nomura, N.; Tokuda, H.; Uchiyama, H. Biofilm formation by lactic acid bacteria and resistance to environmental stress. J. Biosci. Bioeng. 2008, 106, 381–386. [Google Scholar] [CrossRef]
- Extremina, C.I.; Costa, L.; Aguiar, A.I.; Peixe, L.; Fonseca, A.P. Optimization of processing conditions for the quantification of enterococci biofilms using microtitre-plates. J. Microbiol. Methods 2011, 84, 167–173. [Google Scholar] [CrossRef]
- Bajrami, D.; Fischer, S.; Barth, H.; Sarquis, M.A.; Ladero, V.M.; Fernández, M.; Sportelli, M.C.; Cioffi, N.; Kranz, C.; Mizaikoff, B. In situ monitoring of Lentilactobacillus parabuchneri biofilm formation via real-time infrared spectroscopy. Npj Biofilms Microbiomes 2022, 8, 92. [Google Scholar] [CrossRef]
- Dakheel, K.H.; Abdul Rahim, R.; Neela, V.K.; Al-Obaidi, J.R.; Hun, T.G.; Yusoff, K. Methicillin-resistant Staphylococcus aureus biofilms and their influence on bacterial adhesion and cohesion. BioMed Res. Int. 2016, 2016, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, U.T.; Burrows, L.L. DNAse I and proteinase K impair Listeria monocytogenes biofilm formation and induce dispersal of pre-existing biofilms. Int. J. Food Microbiol. 2014, 187, 26–32. [Google Scholar] [CrossRef]
- Dewangan, A.K.; Patel, A.D.; Bhadania, A.G. Stainless steel for dairy and food industry: A review. J. Mater. Sci. Eng. 2015, 4, 10–13. [Google Scholar] [CrossRef]
- Aviat, F.; Gerhards, C.; Rodriguez-Jerez, J.; Michel, V.; Bayon, I.L.; Ismail, R.; Federighi, M. Microbial safety of wood in contact with food: A review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Ratha, P.; Noyonika; Chaurasiya, D.; Kaul, G. Can plastics be ever replaced in the dairy industry? Curr. Sci. 2020, 119, 1411–1416. [Google Scholar] [CrossRef]
- Pozzi, C.; Waters, E.M.; Rudkin, J.K.; Schaeffer, C.R.; Lohan, A.J.; Tong, P.; Loftus, B.J.; Pier, G.B.; Fey, P.D.; Massey, R.C.; et al. Methicillin resistance alters the biofilm phenotype and attenuates virulence in Staphylococcus aureus device-associated infections. PLoS Pathog. 2012, 8, e1002626. [Google Scholar] [CrossRef] [PubMed]
- Boles, B.R.; Horswill, A.R. Agr-mediated dispersal of Staphylococcus aureus biofilms. PLoS Pathog. 2008, 4, e1000052. [Google Scholar] [CrossRef] [PubMed]
- Schlei, K.P.; Angioletti, B.L.; Fernandes de Carvalho, L.; Bertoli, S.L.; Ratto Reiter, M.G.; Krebs de Souza, C. Influence of temperature on microbial growth during processing of kochkäse cheese made from raw and pasteurised milk. Int. Dairy J. 2020, 109, 104786. [Google Scholar] [CrossRef]
- Rezaei, A.; Alirezalu, K.; Damirchi, S.A.; Hesari, J.; Papademas, P.; Domínguez, R.; Lorenzo, J.M.; Yaghoubi, M. Effect of pasteurization and ripening temperature on chemical and sensory characteristics of traditional motal cheese. Fermentation 2020, 6, 95. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, Y.; Li, Z.; Wang, Y.; Liu, Y.; Dong, Q. Characterization of Listeria monocytogenes biofilm formation kinetics and biofilm transfer to cantaloupe surfaces. Food Res. Int. 2022, 161, 111839. [Google Scholar] [CrossRef]
- Furukawa, S. Studies on formation, control and application of biofilm formed by food related microorganisms. Biosci. Biotechnol. Biochem. 2015, 79, 1050–1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debeer, J.; Bell, J.W.; Nolte, F.; Arcieri, J.; Correa, G. Histamine limits by country: A survey and review. J. Food Prot. 2021, 84, 1610–1628. [Google Scholar] [CrossRef] [PubMed]
- EFSA Scientific opinion on risk based control of biogenic amine formation in fermented foods. EFSA Panel on Biological Hazards (BIOHAZ). EFSA J. 2011, 9, 2393–2486. [CrossRef] [Green Version]
- Galié, S.; García-Gutiérrez, C.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Biofilms in the food industry: Health aspects and control methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef] [PubMed]
- Ladero, V.; Fernández, M.; Alvarez, M.A. Effect of post-ripening processing on the histamine and histamine-producing bacteria contents of different cheeses. Int. Dairy J. 2009, 19, 759–762. [Google Scholar] [CrossRef]
- Sharan, M.; Vijay, D.; Dhaka, P.; Bedi, J.S.; Gill, J.P.S. Biofilms as a microbial hazard in the food industry: A scoping review. J. Appl. Microbiol. 2022, 133, 2210–2234. [Google Scholar] [CrossRef]
- Fernández Ramírez, M.D.; Smid, E.J.; Abee, T.; Nierop Groot, M.N. Characterisation of biofilms formed by Lactobacillus plantarum WCFS1 and food spoilage isolates. Int. J. Food Microbiol. 2015, 207, 23–29. [Google Scholar] [CrossRef]
- Spiliopoulou, A.I.; Krevvata, M.I.; Kolonitsiou, F.; Harris, L.G.; Wilkinson, T.S.; Davies, A.P.; Dimitracopoulos, G.O.; Karamanos, N.K.; Mack, D.; Anastassiou, E.D. An extracellular Staphylococcus epidermidis polysaccharide: Relation to polysaccharide intercellular adhesin and its implication in phagocytosis. BMC Microbiol. 2012, 12, 76. [Google Scholar] [CrossRef] [Green Version]
- Mack, D.; Fischer, W.; Krokotsch, A.; Leopold, K.; Hartmann, R.; Egge, H.; Laufs, R. The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear beta-1,6-linked glucosaminoglycan: Purification and structural analysis. J. Bacteriol. 1996, 178, 175–183. [Google Scholar] [CrossRef] [Green Version]
- Sutherland, I.W. Biofilm exopolysaccharides: A strong and sticky framework. Microbiology 2001, 147, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Sager, M.; Benten, W.P.M.; Engelhardt, E.; Gougoula, C.; Benga, L. Characterization of biofilm formation in Pasteurella pneumotropica and Actinobacillus muris isolates of mouse origin. PLoS ONE 2015, 10, e0138778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, N.T.; Nou, X.; Bauchan, G.R.; Murphy, C.; Lefcourt, A.M.; Shelton, D.R.; Lo, Y.M. Effects of environmental parameters on the dual-species biofilms formed by Escherichia coli O157:H7 and Ralstonia insidiosa, a strong biofilm producer isolated from a fresh-cut produce processing plant. J. Food Prot. 2015, 78, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Flint, S.; Bremer, P.; Brooks, J.; Palmer, J.; Sadiq, F.A.; Seale, B.; Teh, K.H.; Wu, S.; Md Zain, S.N. Bacterial fouling in dairy processing. Int. Dairy J. 2020, 101, 104593. [Google Scholar] [CrossRef]
- Marchianò, V.; Salvador, M.; Moyano, A.; Gutiérrez, G.; Matos, M.; Yáñez-Vilar, S.; Piñeiro, Y.; Rivas, J.; Martínez-García, J.C.; Peddis, D.; et al. Electrodecoration and characterization of superparamagnetic iron oxide nanoparticles with bioactive synergistic nanocopper: Magnetic hyperthermia-induced ionic release for anti-biofilm action. Antibiotics 2021, 10, 119. [Google Scholar] [CrossRef]
- Hossain, S.I.; Bajrami, D.; Sportelli, M.C.; Picca, R.A.; Volpe, A.; Gaudiuso, C.; Ancona, A.; Gentile, L.; Palazzo, G.; Ditaranto, N.; et al. Preparation of Laser-ablated Ag nanoparticle–MMT Clay-based beeswax antibiofilm coating. Antibiotics 2023, 12, 194. [Google Scholar] [CrossRef]
- Bajrami, D.; Fischer, S.; Barth, H.; Hossain, S.I.; Cioffi, N.; Mizaikoff, B. Antimicrobial efficiency of Chitosan and its methylated derivative against Lentilactobacillus parabuchneri biofilms. Molecules 2022, 27, 8647. [Google Scholar] [CrossRef]
- Dygico, L.K.; Gahan, C.G.M.; Grogan, H.; Burgess, C.M. The ability of Listeria monocytogenes to form biofilm on surfaces relevant to the mushroom production environment. Int. J. Food Microbiol. 2020, 317, 108385. [Google Scholar] [CrossRef]
- Bridier, A.; Dubois-Brissonnet, F.; Boubetra, A.; Thomas, V.; Briandet, R. The biofilm architecture of sixty opportunistic pathogens deciphered using a high throughput CLSM method. J. Microbiol. Methods 2010, 82, 64–70. [Google Scholar] [CrossRef]
- Olszewska, M.A.; Diez-Gonzalez, F. Characterization of binary biofilms of Listeria monocytogenes and Lactobacillus and their response to chlorine treatment. Front. Microbiol. 2021, 12, e638933. [Google Scholar] [CrossRef]
- Ma, Z.; Bumunang, E.W.; Stanford, K.; Bie, X.; Niu, Y.D.; McAllister, T.A. Biofilm formation by Shiga toxin-producing Escherichia coli on stainless steel coupons as affected by temperature and incubation time. Microorganisms 2019, 7, 95. [Google Scholar] [CrossRef] [Green Version]
- Kadam, S.R.; den Besten, H.M.W.; van der Veen, S.; Zwietering, M.H.; Moezelaar, R.; Abee, T. Diversity assessment of Listeria monocytogenes biofilm formation: Impact of growth condition, serotype and strain origin. Int. J. Food Microbiol. 2013, 165, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Diaz, M.; del Rio, B.; Sanchez-Llana, E.; Ladero, V.; Redruello, B.; Fernández, M.; Martin, M.C.; Alvarez, M.A. Lactobacillus parabuchneri produces histamine in refrigerated cheese at a temperature-dependent rate. Int. J. Food Sci. Technol. 2018, 53, 2342–2348. [Google Scholar] [CrossRef] [Green Version]
- Mathlouthi, A.; Pennacchietti, E.; Biase, D. De effect of temperature, pH and plasmids on in vitro biofilm formation in Escherichia coli. Acta Nat. 2018, 10, 129–132. [Google Scholar] [CrossRef] [Green Version]
- Yin, W.; Wang, Y.; Liu, L.; He, J. Biofilms: The microbial “Protective Clothing” in extreme environments. Int. J. Mol. Sci. 2019, 20, 3423. [Google Scholar] [CrossRef] [Green Version]
- del Rio, B.; Linares, D.M.; Ladero, V.; Redruello, B.; Fernandez, M.; Martin, M.C.; Alvarez, M.A.; Fernández, M.; Martin, M.C.; Alvarez, M.A. Putrescine production via the agmatine deiminase pathway increases the growth of Lactococcus lactis and causes the alkalinization of the culture medium. Appl. Microbiol. Biotechnol. 2015, 99, 897–905. [Google Scholar] [CrossRef] [Green Version]
- del Rio, B.; Ladero, V.; Redruello, B.; Linares, D.M.; Fernandez, M.; Martin, M.C.; Alvarez, M.A.; Fernández, M.; Martín, M.C.; Alvarez, M.A. Lactose-mediated carbon catabolite repression of putrescine production in dairy Lactococcus lactis is strain dependent. Food Microbiol. 2015, 48, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Yuksekdag, Z.N.; Aslim, B. Influence of different carbon sources on exopolysaccharide production by Lactobacillus delbrueckii subsp. bulgaricus (B3, G12) and Streptococcus thermophilus (W22). Braz. Arch. Biol. Technol. 2008, 51, 581–585. [Google Scholar] [CrossRef]
- Shi, Y.; Huang, J.; Zeng, G.; Gu, Y.; Chen, Y.; Hu, Y.; Tang, B.; Zhou, J.; Yang, Y.; Shi, L. Exploiting extracellular polymeric substances (EPS) controlling strategies for performance enhancement of biological wastewater treatments: An overview. Chemosphere 2017, 180, 396–411. [Google Scholar] [CrossRef]
- Grimes, C.A.; Riddell, L.J.; Nowson, C.A. Consumer knowledge and attitudes to salt intake and labelled salt information. Appetite 2009, 53, 189–194. [Google Scholar] [CrossRef]
- Kim, M.K.; Lopetcharat, K.; Gerard, P.D.; Drake, M.A. Consumer awareness of salt and sodium reduction and sodium labeling. J. Food Sci. 2012, 77, S307–S313. [Google Scholar] [CrossRef]
- Møller, C.O.D.A.; Castro-Mejía, J.L.; Krych, L.; Rattray, F.P. Histamine-forming ability of Lentilactobacillus parabuchneri in reduced salt Cheddar cheese. Food Microbiol. 2021, 98, 103789. [Google Scholar] [CrossRef] [PubMed]








| Strain | Origin | Reference |
|---|---|---|
| B301 | Emmental | [39] |
| DSM 5987 | Cheese | DSMZ-German Collection of Microorganisms |
| IPLA11117 | Zamorano | [18] |
| IPLA11122 | Emmental | [37] |
| IPLA11123 | Emmental | Molecular Microbiology Group IPLA-CSIC |
| IPLA11125 | Emmental | [37] |
| IPLA11126 | Emmental | [37] |
| IPLA11129 | Emmental | [37] |
| IPLA11131 | Emmental | [37] |
| IPLA11132 | Emmental | [37] |
| IPLA11133 | Emmental | Molecular Microbiology Group IPLA-CSIC |
| IPLA11137 | Emmental | Molecular Microbiology Group IPLA-CSIC |
| IPLA11150 | Cabrales | [18] |
| IPLA11151 | Cabrales | [18] |
| IPLA11152 | Zamorano | [18] |
| IPLA15003 | Mozzarella | Molecular Microbiology Group IPLA-CSIC |
| IPLA15005 | Mozzarella | [18] |
| IPLA15006 | Mozzarella | Molecular Microbiology Group IPLA-CSIC |
| IPLA15007 | Mozzarella | Molecular Microbiology Group IPLA-CSIC |
| IPLA15008 | Mozzarella | Molecular Microbiology Group IPLA-CSIC |
| IPLA15009 | Mozzarella | [18] |
| IPLA15010 | Mozzarella | Molecular Microbiology Group IPLA-CSIC |
| IPLA15012 | Mozzarella | [18] |
| St2A | Zamorano | [40] |
| DSM 5707T | Human saliva (type strain) | DSMZ-German Collection of Microorganisms |
| L. parabuchneri IPLA | ||||||
|---|---|---|---|---|---|---|
| Material/Strain | 11151 | 11150 | 11129 | 11125 | 11122 | 11117 |
| Polystyrene | 6.79 ± 0.74 | 6.68 ± 1.12 | 6.45 ± 1.18 | 6.93 ± 0.74 | 7.08 ± 0.76 | 6.90 ± 0.87 |
| Stainless Steel | 6.22 ± 1.03 ac | 4.07 ± 0.99 bc* | 4.29 ± 0.83 bc* | 4.6 ± 0.32 abc* | 5.05 ± 1.15 ab* | 3.18 ± 1.29 bc* |
| Plastic | 5.37 ± 0.93 a | 4.10 ± 0.68 ab* | 5.17 ± 0.69 a | 4.85 ± 0.64 ab* | 4.97 ± 0.65 ab* | 3.52 ± 1.24 b* |
| Beech Wood | 6.11 ± 0.37 | 5.53 ± 0.93 | 6.40 ± 0.55 | 6.01 ± 0.48 | 6.13 ± 0.20 | 5.49 ± 1.39 |
| Rubber | 5.58 ± 0.71 a | 4.32 ± 1.45 ab* | 4.53 ± 0.43 ab* | 5.21 ± 0.43 a* | 4.46 ± 0.86 ab* | 3.40 ± 1.12 b* |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarquis, A.; Bajrami, D.; Mizaikoff, B.; Ladero, V.; Alvarez, M.A.; Fernandez, M. Characterization of the Biofilms Formed by Histamine-Producing Lentilactobacillus parabuchneri Strains in the Dairy Environment. Foods 2023, 12, 1503. https://doi.org/10.3390/foods12071503
Sarquis A, Bajrami D, Mizaikoff B, Ladero V, Alvarez MA, Fernandez M. Characterization of the Biofilms Formed by Histamine-Producing Lentilactobacillus parabuchneri Strains in the Dairy Environment. Foods. 2023; 12(7):1503. https://doi.org/10.3390/foods12071503
Chicago/Turabian StyleSarquis, Agustina, Diellza Bajrami, Boris Mizaikoff, Victor Ladero, Miguel A. Alvarez, and Maria Fernandez. 2023. "Characterization of the Biofilms Formed by Histamine-Producing Lentilactobacillus parabuchneri Strains in the Dairy Environment" Foods 12, no. 7: 1503. https://doi.org/10.3390/foods12071503