Investigating the Effect of Rosemary Essential Oil, Supercritical CO2 Processing and Their Synergism on the Quality and Microbial Inactivation of Chicken Breast Meat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culture and Cell Suspension
2.2. Sample Preparation and Microbial Inoculation
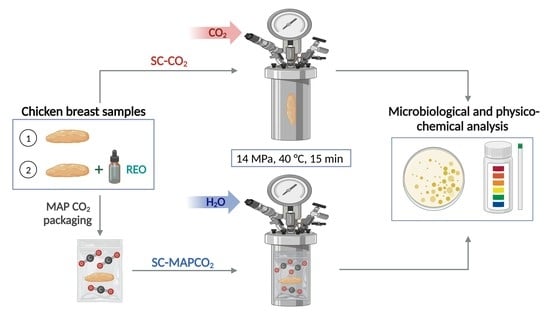
2.3. High-Pressure Processes
2.4. Microbial Enumeration
2.5. Physicochemical Analysis: Color, pH and aw
2.6. Design of Experiment and Statistical Analysis
3. Results
3.1. Comparison between SC-CO2 and SC-MAPCO2 without REO
3.2. Effect of REO Alone and with CO2 Treatments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Uzundumlu, A.S.; Dilli, M. Estimating Chicken Meat Productions of Leader Countries for 2019–2025 Years. Cienc. Rural 2023, 53, 2. [Google Scholar] [CrossRef]
- Tong, H.; Cao, C.; Du, Y.; Liu, Y.; Huang, W. Ultrasonic-Assisted Phosphate Curing: A Novel Approach to Improve Curing Rate and Chicken Meat Quality. Int. J. Food Sci. Technol. 2022, 57, 2906–2917. [Google Scholar] [CrossRef]
- González-Alonso, V.; Cappelletti, M.; Bertolini, F.M.; Lomolino, G.; Zambon, A.; Spilimbergo, S. Research Note: Microbial Inactivation of Raw Chicken Meat by Supercritical Carbon Dioxide Treatment Alone and in Combination with Fresh Culinary Herbs. Poult. Sci. 2020, 99, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Yucel, B.; Taskin, T. Animal Husbandry and Nutrition; BoD—Books on Demand: Burlington, ON, Canada, 2018. [Google Scholar]
- Katiyo, W.; de Kock, H.L.; Coorey, R.; Buys, E.M. Sensory Implications of Chicken Meat Spoilage in Relation to Microbial and Physicochemical Characteristics during Refrigerated Storage. LWT 2020, 128, 109468. [Google Scholar] [CrossRef]
- Chun, H.H.; Kim, J.Y.; Lee, B.D.; Yu, D.J.; Song, K.B. Effect of UV-C Irradiation on the Inactivation of Inoculated Pathogens and Quality of Chicken Breasts during Storage. Food Control 2010, 21, 276–280. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control, European Food Safety Authority. Multi-Country Outbreak of Salmonella Mbandaka ST413, Possibly Linked to Consumption of Chicken Meat in the EU/EEA, Israel and the UK. EFSA Support. Publ. 2022, 19, 7707E. [Google Scholar]
- Piñon, M.; Alarcon-Rojo, A.; Paniwnyk, L.; Mason, T.; Luna, L.; Renteria, A. Ultrasound for Improving the Preservation of Chicken Meat. Food Sci. Technol. 2018, 39, 129–135. [Google Scholar] [CrossRef]
- Chouliara, E.; Badeka, A.; Savvaidis, I.; Kontominas, M.G. Combined Effect of Irradiation and Modified Atmosphere Packaging on Shelf-Life Extension of Chicken Breast Meat: Microbiological, Chemical and Sensory Changes. Eur. Food Res. Technol. 2008, 226, 877–888. [Google Scholar] [CrossRef]
- D’Souza, C.; Apaolaza, V.; Hartmann, P.; Brouwer, A.R.; Nguyen, N. Consumer Acceptance of Irradiated Food and Information Disclosure—A Retail Imperative. J. Retail. Consum. Serv. 2021, 63, 102699. [Google Scholar] [CrossRef]
- Režek Jambrak, A. Nonthermal Processing in Agri-Food-Bio Sciences: Sustainability and Future Goals; Springer Nature: Berlin, Germany, 2022. [Google Scholar]
- Kruk, Z.A.; Yun, H.; Rutley, D.L.; Lee, E.J.; Kim, Y.J.; Jo, C. The Effect of High Pressure on Microbial Population, Meat Quality and Sensory Characteristics of Chicken Breast Fillet. Food Control 2011, 22, 6–12. [Google Scholar] [CrossRef]
- Chen, H.; Guan, Y.; Wang, A.; Zhong, Q. Inactivation of Escherichia Coli K12 on Raw Almonds Using Supercritical Carbon Dioxide and Thyme Oil. Food Microbiol. 2022, 103, 103955. [Google Scholar] [CrossRef]
- Ferrentino, G.; Belscak-Cvitanovic, A.; Komes, D.; Spilimbergo, S. Quality Attributes of Fresh-Cut Coconut after Supercritical Carbon Dioxide Pasteurization. J. Chem. 2013, 2013, 703057. [Google Scholar] [CrossRef]
- Spilimbergo, S.; Komes, D.; Vojvodic, A.; Levaj, B.; Ferrentino, G. High Pressure Carbon Dioxide Pasteurization of Fresh-Cut Carrot. J. Supercrit. Fluids 2013, 79, 92–100. [Google Scholar] [CrossRef]
- Spilimbergo, S.; Zambon, A.; Michelino, F.; Polato, S. Method for Food Pasteurization. Patent no. WO2019043442A1, 25 June 2020. [Google Scholar]
- Barberi, G.; González-Alonso, V.; Spilimbergo, S.; Barolo, M.; Zambon, A.; Facco, P. Optimization of the Appearance Quality in CO2 Processed Ready-to-Eat Carrots through Image Analysis. Foods 2021, 10, 2999. [Google Scholar] [CrossRef]
- Zambon, A.; González-Alonso, V.; Lomolino, G.; Zulli, R.; Rajkovic, A.; Spilimbergo, S. Increasing the Safety and Storage of Pre-Packed Fresh-Cut Fruits and Vegetables by Supercritical CO2 Process. Foods 2022, 12, 21. [Google Scholar] [CrossRef]
- Bae, Y.Y.; Choi, Y.M.; Kim, M.J.; Kim, K.H.; Kim, B.C.; Rhee, M.S. Application of Supercritical Carbon Dioxide for Microorganism Reductions in Fresh Pork. J. Food Saf. 2011, 31, 511–517. [Google Scholar] [CrossRef]
- De Souza Pedrosa, G.T.; Pimentel, T.C.; Gavahian, M.; de Medeiros, L.L.; Pagán, R.; Magnani, M. The Combined Effect of Essential Oils and Emerging Technologies on Food Safety and Quality. LWT 2021, 147, 111593. [Google Scholar] [CrossRef]
- Sivarajan, M.; Lalithapriya, U.; Mariajenita, P.; Vajiha, B.A.; Harini, K.; Madhushalini, D.; Sukumar, M. Synergistic Effect of Spice Extracts and Modified Atmospheric Packaging towards Non-Thermal Preservation of Chicken Meat under Refrigerated Storage. Poult. Sci. 2017, 96, 2839–2844. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Nanda, P.K.; Pateiro, M.; Lorenzo, J.M.; Dhar, P.; Das, A.K. Lactic Acid Bacteria and Bacteriocins: Novel Biotechnological Approach for Biopreservation of Meat and Meat Products. Microorganisms 2022, 10, 2058. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, M.; Yang, C. Application of Ultrasound Technology in Processing of Ready-to-Eat Fresh Food: A Review. Ultras. Sonochem. 2020, 63, 104953. [Google Scholar] [CrossRef]
- Stratakos, A.C.; Delgado-Pando, G.; Linton, M.; Patterson, M.F.; Koidis, A. Synergism between High-Pressure Processing and Active Packaging against Listeria Monocytogenes in Ready-to-Eat Chicken Breast. Innov. Food Sci. Emerg. Technol. 2015, 27, 41–47. [Google Scholar] [CrossRef]
- Bozin, B.; Mimica-Dukic, N.; Samojlik, I.; Jovin, E. Antimicrobial and Antioxidant Properties of Rosemary and Sage (Rosmarinus Officinalis L. and Salvia Officinalis L., Lamiaceae) Essential Oils. J. Agric. Food Chem. 2007, 55, 7879–7885. [Google Scholar] [CrossRef] [PubMed]
- Hamedo, H.A.; Abdelmigid, H.M. Use of Antimicrobial and Genotoxicity Potentiality for Evaluation of Essential Oils as Food Preservatives. Open Biotechnol. J. 2009, 3, 50–56. [Google Scholar] [CrossRef]
- Alvarez, M.V.; Ortega-Ramirez, L.A.; Silva-Espinoza, B.A.; Gonzalez-Aguilar, G.A.; Ayala-Zavala, J.F. Antimicrobial, Antioxidant, and Sensorial Impacts of Oregano and Rosemary Essential Oils over Broccoli Florets. J. Food Process. Preserv. 2019, 43, e13889. [Google Scholar] [CrossRef]
- Vrinda Menon, K.; Garg, S.R. Inhibitory Effect of Clove Oil on Listeria Monocytogenes in Meat and Cheese. Food Microbiol. 2001, 18, 647–650. [Google Scholar] [CrossRef]
- Garcia-Sotelo, D.; Silva-Espinoza, B.; Perez-Tello, M.; Olivas, I.; Alvarez-Parrilla, E.; González-Aguilar, G.A.; Ayala-Zavala, J.F. Antimicrobial Activity and Thermal Stability of Rosemary Essential Oil:Β−cyclodextrin Capsules Applied in Tomato Juice. LWT 2019, 111, 837–845. [Google Scholar] [CrossRef]
- Yang, H.-J.; Lee, J.-H.; Lee, K.-Y.; Song, K.B. Antimicrobial Effect of an Undaria Pinnatifida Composite Film Containing Vanillin against Escherichia Coli and Its Application in the Packaging of Smoked Chicken Breast. Int. J. Food Sci. Technol. 2017, 52, 398–403. [Google Scholar] [CrossRef]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.-J. Colour Measurement and Analysis in Fresh and Processed Foods: A Review. Food Bioprocess Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Chen, M.; Sui, X.; Ma, X.; Feng, X.; Han, Y. Application of Response Surface Methodology to Optimise Microbial Inactivation of Shrimp and Conch by Supercritical Carbon Dioxide. J. Sci. Food Agric. 2015, 95, 1016–1023. [Google Scholar] [CrossRef]
- Sirisee, U.; Hsieh, F.; Huff, H.E. Microbial Safety of Supercritical Carbon Dioxide Processes1. J. Food Proc. Preserv. 1998, 22, 387–403. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, Y.; Hu, X.; Wu, J.; Liao, X. Effect of High Pressure Carbon Dioxide on the Quality of Carrot Juice. Inn. Food Sci. Emerg. Technol. 2009, 10, 321–327. [Google Scholar] [CrossRef]
- Bak, K.H.; Bolumar, T.; Karlsson, A.H.; Lindahl, G.; Orlien, V. Effect of High Pressure Treatment on the Color of Fresh and Processed Meats: A Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 228–252. [Google Scholar] [CrossRef]
- Monhemi, H.; Housaindokht, M.R. The Molecular Mechanism of Protein Denaturation in Supercritical CO2: The Role of Exposed Lysine Residues Is Explored. J. Supercrit. Fluids 2019, 147, 222–230. [Google Scholar] [CrossRef]
- Hu, M.; Gurtler, J.B. Selection of Surrogate Bacteria for Use in Food Safety Challenge Studies: A Review. J Food Prot. 2017, 80, 1506–1536. [Google Scholar] [CrossRef]
- Morbiato, G.; Zambon, A.; Toffoletto, M.; Poloniato, G.; Dall’Acqua, S.; de Bernard, M.; Spilimbergo, S. Supercritical Carbon Dioxide Combined with High Power Ultrasound as Innovate Drying Process for Chicken Breast. J. Supercrti. Fluids 2019, 147, 24–32. [Google Scholar] [CrossRef]
- Wei, C.I.; Balaban, M.O.; Fernando, S.Y.; Peplow, A.J. Bacterial Effect of High Pressure CO2 Treatment on Foods Spiked with Listeria or Salmonella. J. Food Prot. 1991, 54, 189–193. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, L.; Geeraerd, A.H.; Spilimbergo, S.; Elst, K.; Van Ginneken, L.; Debevere, J.; Van Impe, J.F.; Devlieghere, F. High Pressure Carbon Dioxide Inactivation of Microorganisms in Foods: The Past, the Present and the Future. Int. J. Food Microbiol. 2007, 117, 1–28. [Google Scholar] [CrossRef]
- Sievers, U. Die Thermodynamischen Eigenschaften von Kohlendioxid; Springer Nature: Berlin, Germany, 1984; Volume 50. [Google Scholar]
- Ji, J.; Shankar, S.; Royon, F.; Salmieri, S.; Lacroix, M. Essential Oils as Natural Antimicrobials Applied in Meat and Meat Products-a Review. Crit. Rev. Food Sci. Nutr. 2021, 63, 993–1009. [Google Scholar] [CrossRef]
- Sarïcaoglu, F.T.; Turhan, S. Antimicrobial Activity and Antioxidant Capacity of Thyme, Rosemary and Clove Essential Oils and Their Mixtures. J. Inn. Sci. Eng. 2018, 2, 25–33. [Google Scholar]
- Zhang, Y.; Ma, Q.; Critzer, F.; Davidson, P.M.; Zhong, Q. Organic Thyme Oil Emulsion as an Alternative Washing Solution to Enhance the Microbial Safety of Organic Cantaloupes. Food Control 2016, 67, 31–38. [Google Scholar] [CrossRef]
- Teixeira, A.A. Thermal Food Preservation Techniques (Pasteurization, Sterilization, Canning and Blanching). In Conventional and Advanced Food Processing Technologies; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 115–128. [Google Scholar]

| Sample | pH | aw |
|---|---|---|
| Control | 5.85 ± 0.05 a | 0.971 ± 0.002 a |
| SC-CO2 | 5.90 ± 0.09 a | 0.951 ± 0.003 b |
| SC-MAPCO2 | 5.72 ± 0.07 a | 0.954 ± 0.007 b |
| Sample | E. coli (log CFU/g) | L. innocua (log CFU/g) |
|---|---|---|
| Control | 7.03 ± 0.06 a | 7.41 ± 0.14 a |
| SC-CO2 | 5.74 ± 0.62 b | 5.99 ± 0.11 b |
| SC-MAPCO2 | 6.40 ± 0.21 ab | 7.25 ± 0.06 a |
| REO (%) | L* | a* | b* | ∆E | |
|---|---|---|---|---|---|
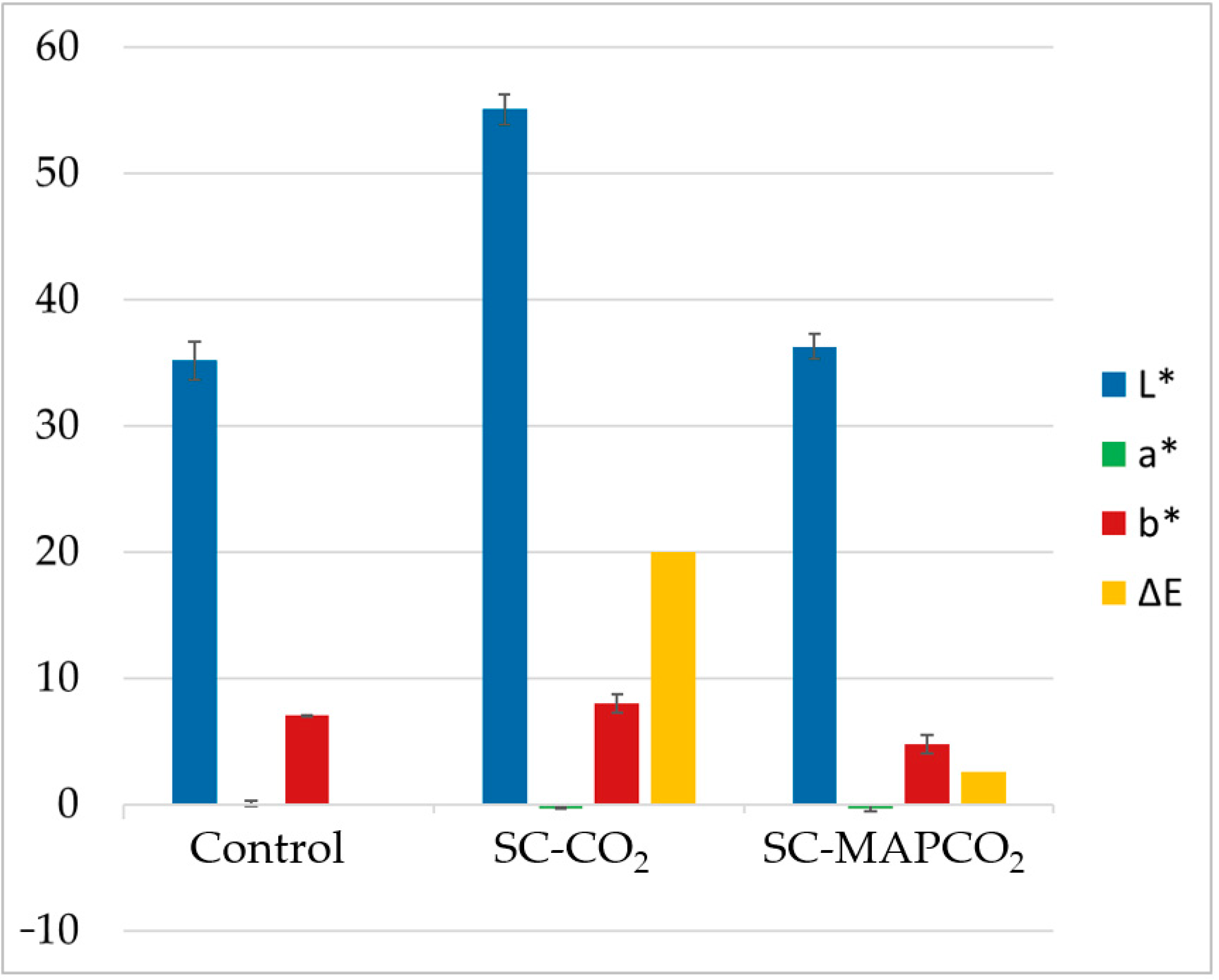
| Control | 0.0 | 35.15 ± 2.98 c | 0.06 ± 0.33 a | 7.03 ± 0.06 a | / |
| 0.1 | 33.93 ± 0.53 c | −0.02 ± 0.48 a | 6.50 ± 0.26 abc | 1.33 | |
| 0.5 | 33.93 ± 1.06 c | 0.14 ± 0.26 a | 6.97 ± 0.14 ab | 1.22 | |
| 1.0 | 33.72 ± 1.49 c | 0.29 ± 0.50 a | 6.93 ± 0.01 ab | 1.45 | |
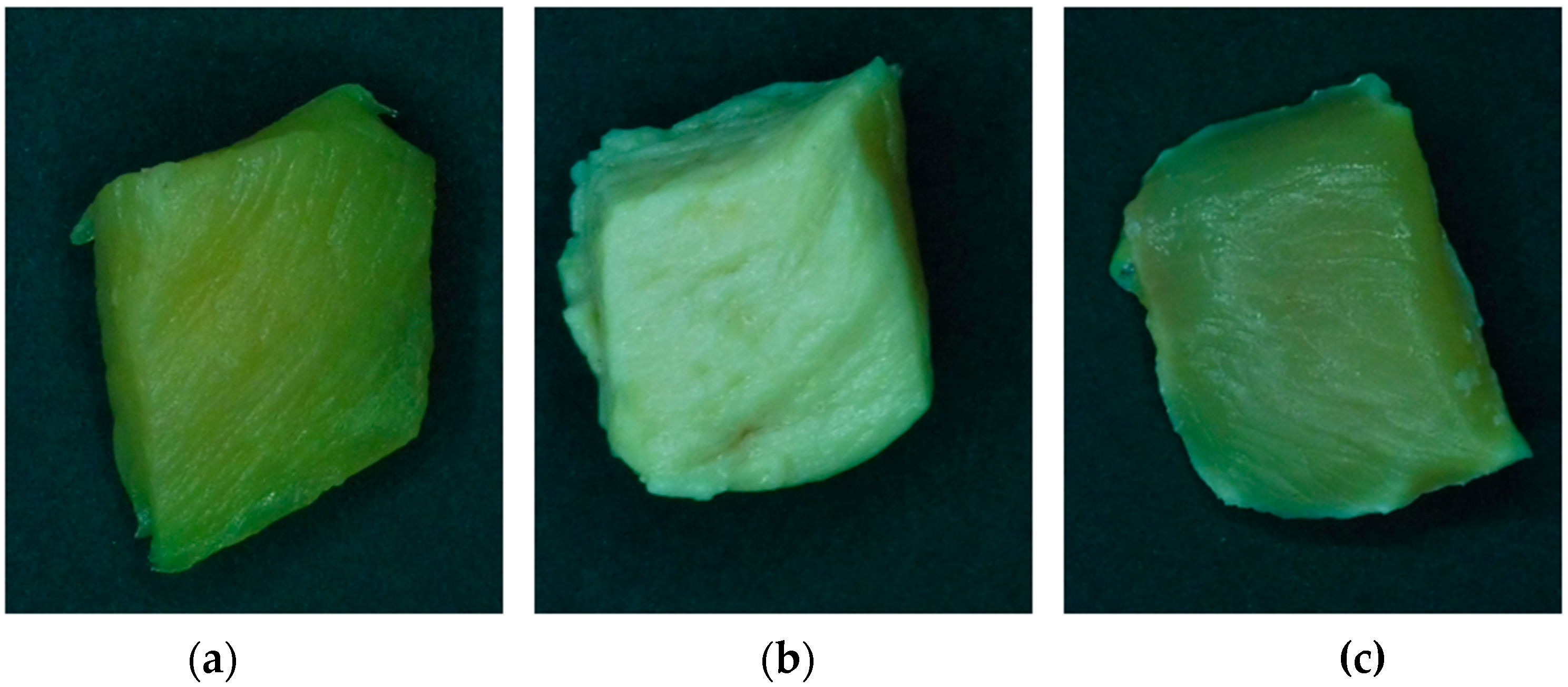
| SC-CO2 | 0.0 | 55.07 ± 2.36 a | −0.31± 0.16 a | 7.96 ± 1.49 a | 19.95 |
| 0.1 | 52. 29 ± 1.02 a | −0.08 ± 0.78 a | 6.26 ± 2.53 ab | 17.16 | |
| 0.5 | 53.43 ± 2.15 a | 0.29 ± 0.51 a | 7.02 ± 0.20 ab | 18.28 | |
| 1.0 | 53.11 ± 0.81 a | −0.30 ± 0.17 a | 5.79 ± 1.28 abc | 18.01 | |
| SC-MAPCO2 | 0.0 | 36.27 ± 2.06 c | −0.33 ± 0.50 a | 4.74 ± 1.42 abc | 2.58 |
| 0.1 | 42.84 ± 2.97 b | −0.59 ± 0.70 a | 2.53 ± 0.77 c | 8.83 | |
| 0.5 | 42.56 ± 0.26 b | −0.99 ± 0.35 a | 3.94 ± 1.05 bc | 8.10 | |
| 1.0 | 43.26± 2.13 b | −0.99 ± 0.31 a | 3.66 ± 0.74 bc | 8.84 |
| REO (%) | E. coli (log CFU/g) | L. innocua (log CFU/g) | |
|---|---|---|---|
| Control | - | 7.03 ± 0.06 a | 7.41 ± 0.14 a |
| 0.1 | 6.50 ± 0.26 abc | 7.50 ± 0.11 a | |
| 0.5 | 6.97 ± 0.14 ab | 7.30 ± 0.06 a | |
| 1.0 | 6.93 ± 0.01 ab | 7.48 ± 0.25 a | |
| SC-CO2 | - | 5.74 ± 0.62 cde | 5.99 ± 0.11 c |
| 0.1 | 5.02 ± 0.08 de | 6.44 ± 0.13 bc | |
| 0.5 | 4.58 ± 0.25 ef | 6.31 ± 0.01 c | |
| 1.0 | 3.62 ± 0.26 f | 6.30 ± 0.62 c | |
| SC-MAPCO2 | - | 6.40 ± 0,21 abc | 7.25 ± 0.06 a |
| 0.1 | 6.56 ± 0.36 abc | 6.96 ± 0.06 ab | |
| 0.5 | 5.98 ± 0.10 bc | 7.02 ± 0.05 ab | |
| 1.0 | 5.73 ± 0.74 cd | 7.19 ± 0.22 a |
| Sample | Mesophilic Bacteria | Psychrophilic Bacteria |
|---|---|---|
| Control | 5.38 ± 0.04 a | 5.24 ± 0.30 a |
| Control + 1% REO | 5.12 ± 0.17 ab | 5.52 ± 0.47 a |
| SC-MAPCO2 | 4.10 ± 0.66 bc | 4.56 ± 0.15 a |
| SC-MAPCO2 + 1% REO | 3.27 ± 0.62 c | 3.19 ± 0.70 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santi, F.; Zulli, R.; Lincetti, E.; Zambon, A.; Spilimbergo, S. Investigating the Effect of Rosemary Essential Oil, Supercritical CO2 Processing and Their Synergism on the Quality and Microbial Inactivation of Chicken Breast Meat. Foods 2023, 12, 1786. https://doi.org/10.3390/foods12091786
Santi F, Zulli R, Lincetti E, Zambon A, Spilimbergo S. Investigating the Effect of Rosemary Essential Oil, Supercritical CO2 Processing and Their Synergism on the Quality and Microbial Inactivation of Chicken Breast Meat. Foods. 2023; 12(9):1786. https://doi.org/10.3390/foods12091786
Chicago/Turabian StyleSanti, Fabio, Riccardo Zulli, Elisa Lincetti, Alessandro Zambon, and Sara Spilimbergo. 2023. "Investigating the Effect of Rosemary Essential Oil, Supercritical CO2 Processing and Their Synergism on the Quality and Microbial Inactivation of Chicken Breast Meat" Foods 12, no. 9: 1786. https://doi.org/10.3390/foods12091786