Porcine Intestinal Mucosal Peptides Target Macrophage-Modulated Inflammation and Alleviate Intestinal Homeostasis in Dextrose Sodium Sulfate-Induced Colitis in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Porcine Intestinal Mucosal Peptides (PIMP)
2.2. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.3. Cell Culture
2.4. Cell Viability Assay
2.5. Detection of Nitric Oxide (NO)
2.6. Experimental Animals
2.7. Experimental Design and Animal Grouping
2.8. Disease Activity Index (DAI) and Histological Evaluation
2.9. Myeloperoxidase (MPO) Activity Detection
2.10. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.11. Western Blot Analysis
2.12. Short-Chain Fatty Acids (SCFAs)
2.13. Intestinal Microbiota
2.14. Data Analysis
3. Results
3.1. Molecular Weight
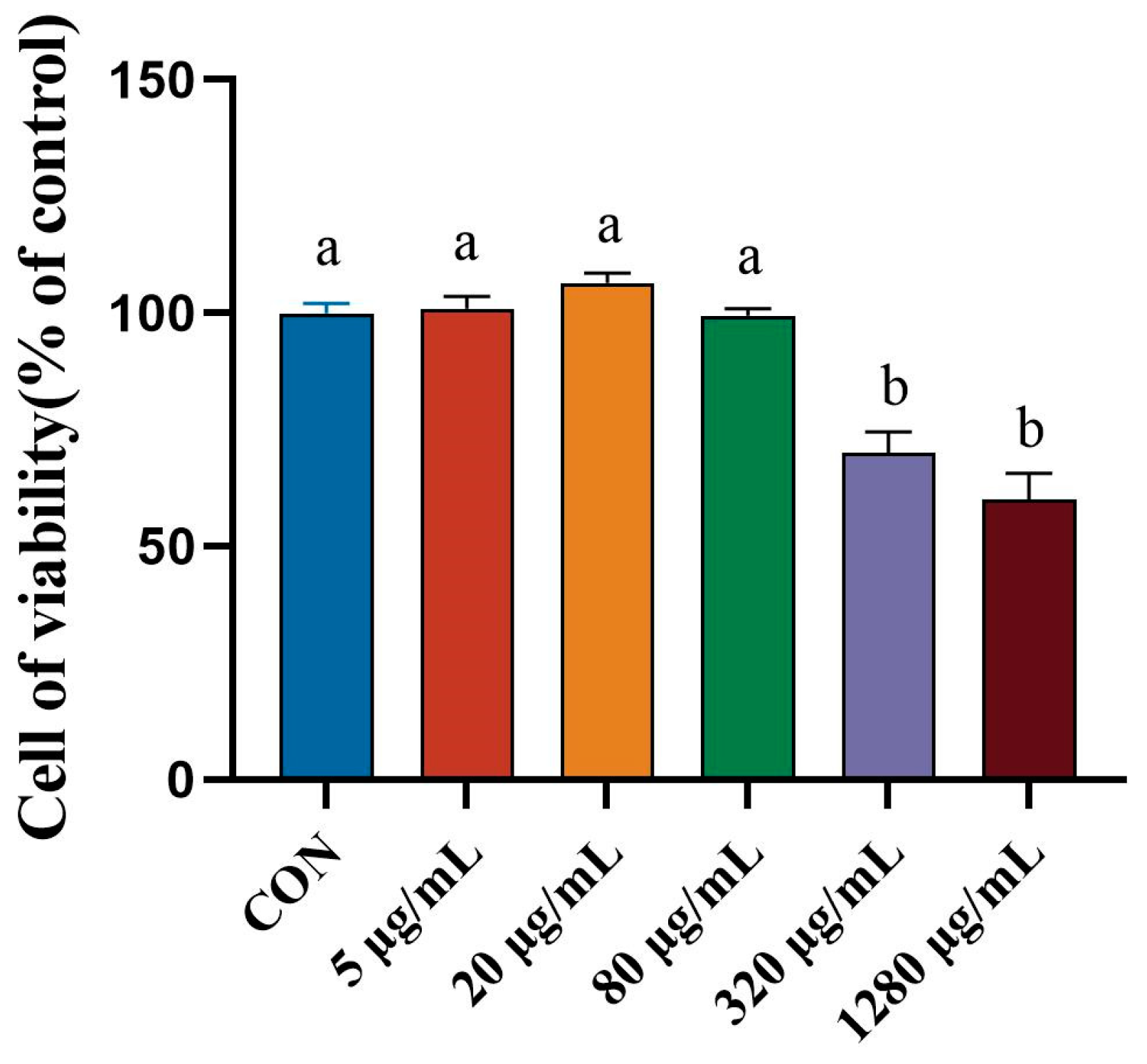
3.2. Effect of Porcine Intestinal Mucosal Peptide on Cell Viability of Lipopolysaccharide Induced Macrophages
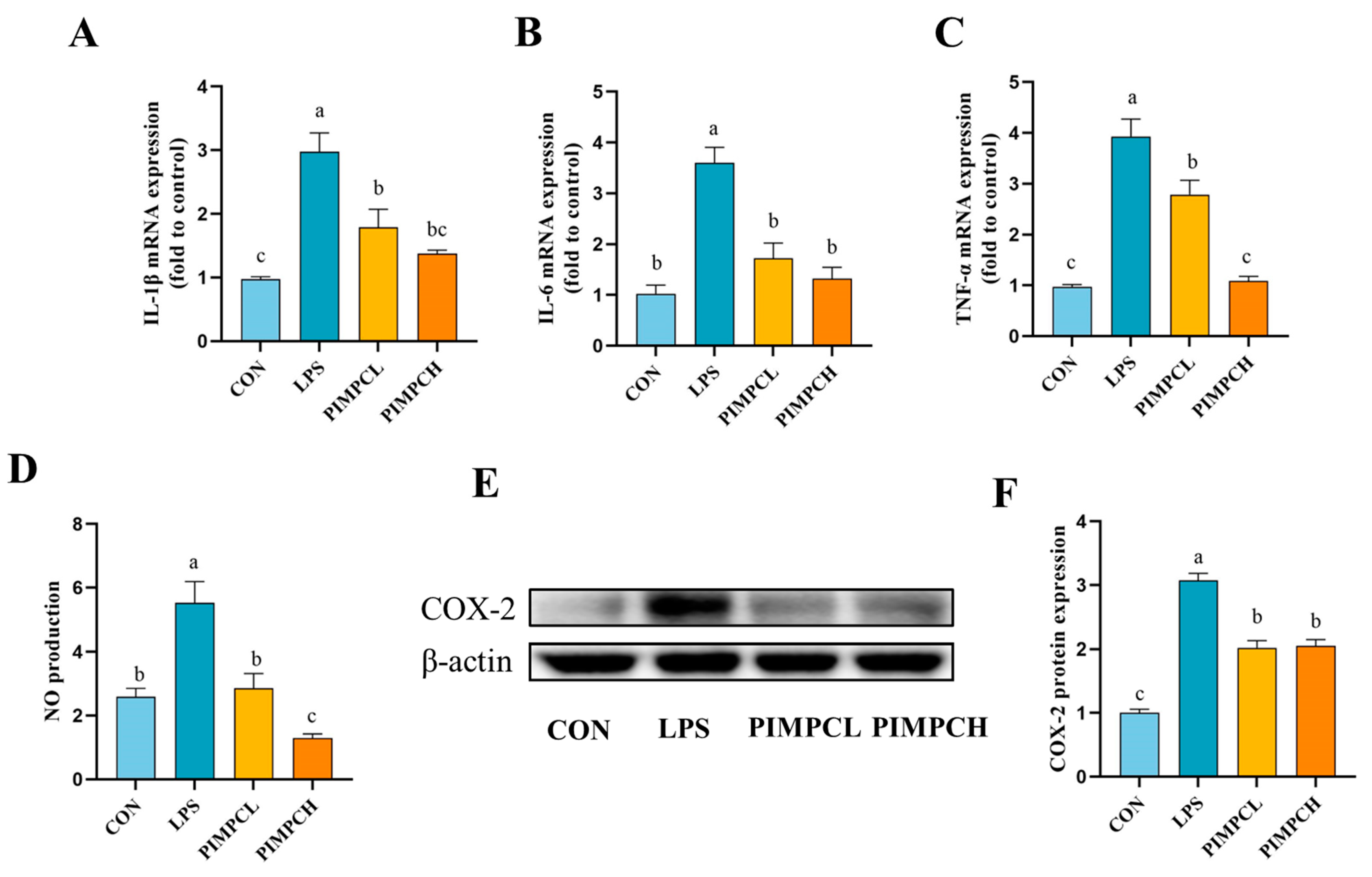
3.3. Inhibition of Inflammatory Responses by Porcine Intestinal Mucosal Peptide
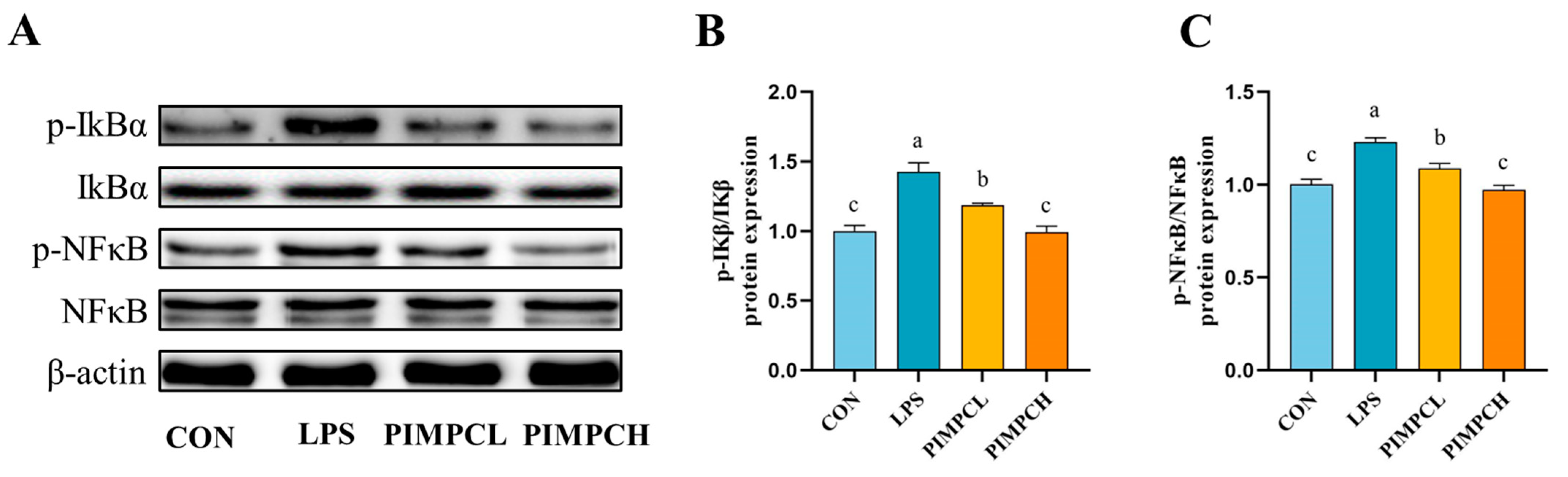
3.4. Porcine Intestinal Mucosal Peptide Inhibits Macrophage Inflammatory Response Induced by Lipopolysaccharide Via the NF-κB Signaling Pathway
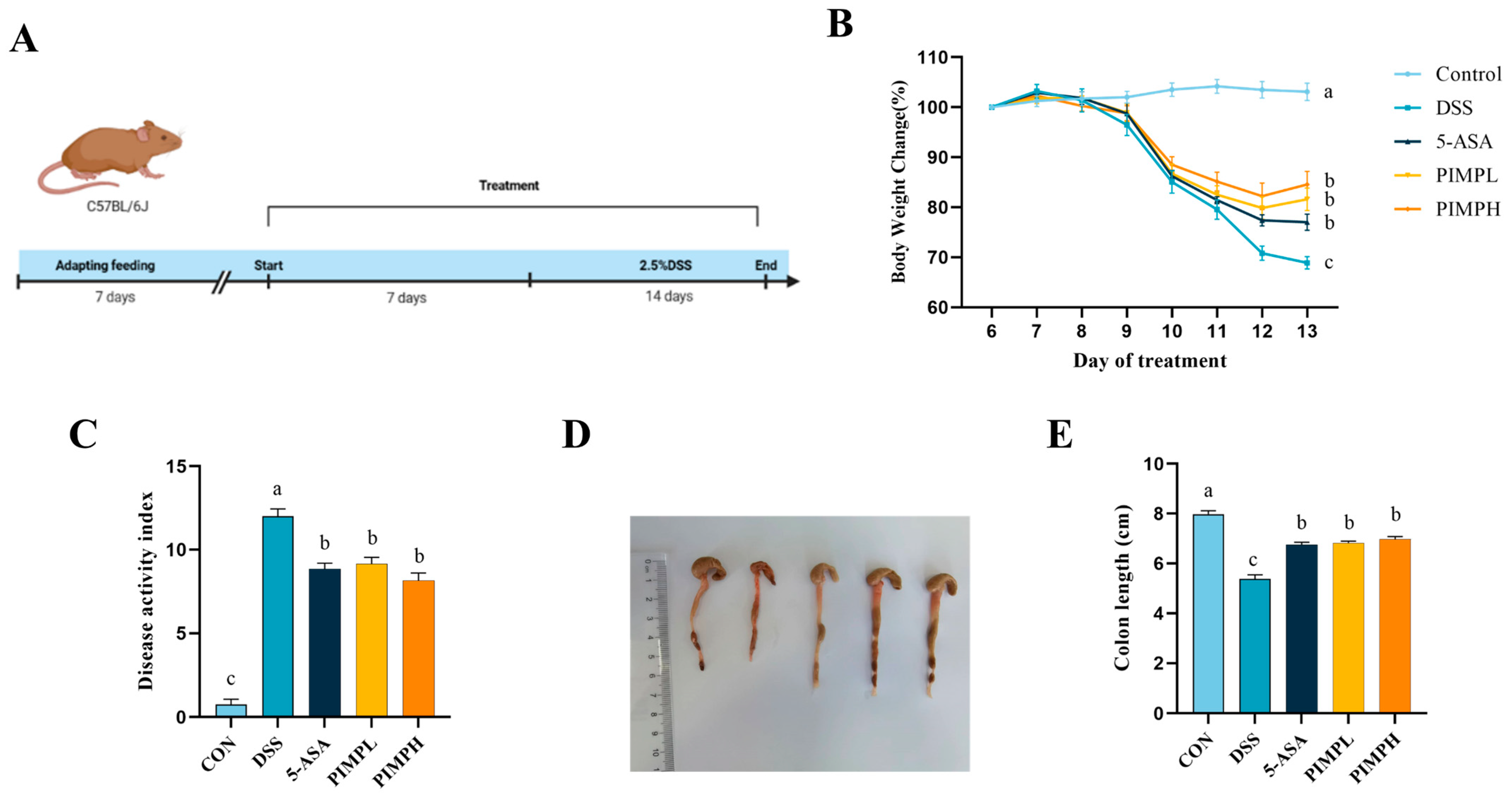
3.5. Porcine Intestinal Mucosal Peptide Alleviates Symptoms of Acute Colitis in Mice
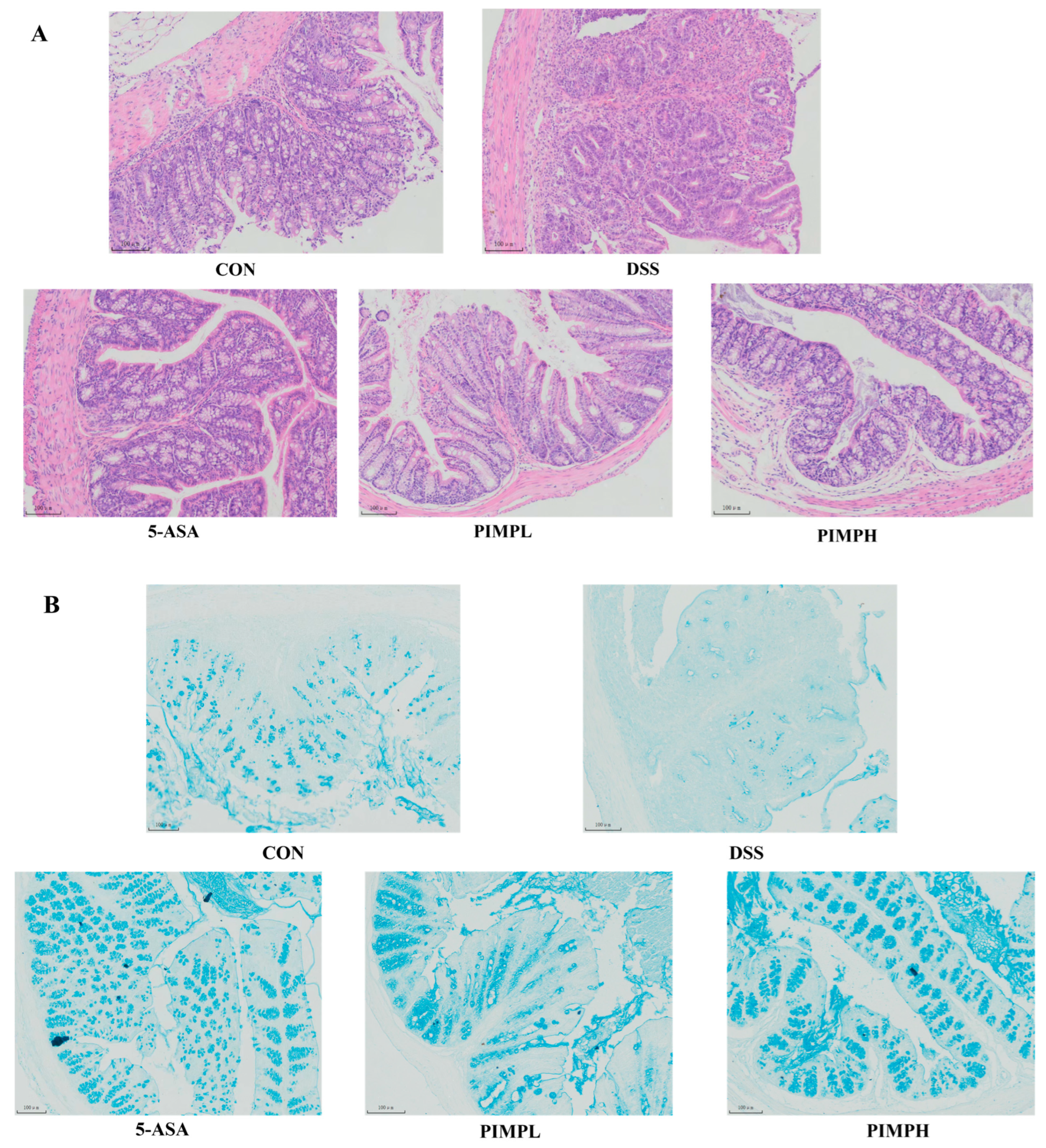
3.6. Porcine Intestinal Mucosal Peptide Alleviates Tissue Damage in Dextrose Sodium Sulfate Induced Colitis
3.7. Porcine Intestinal Mucosal Peptide Restores Intestinal Barrier Damage in Dextrose Sodium Sulfate Induced Colitis Mice
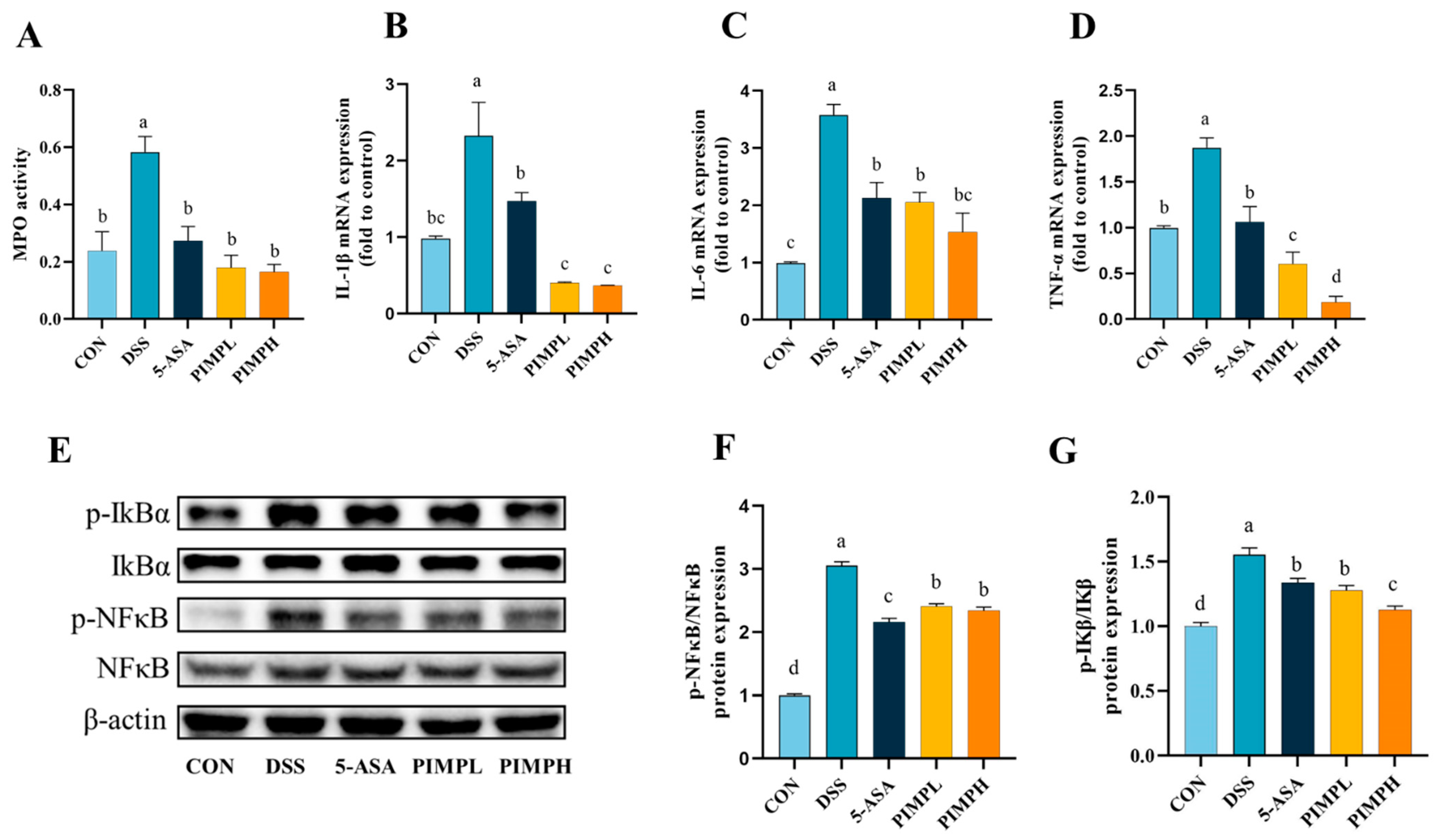
3.8. Porcine Intestinal Mucosal Peptide Suppress Inflammatory Response in Dextrose Sodium Sulfate Induced Colitis
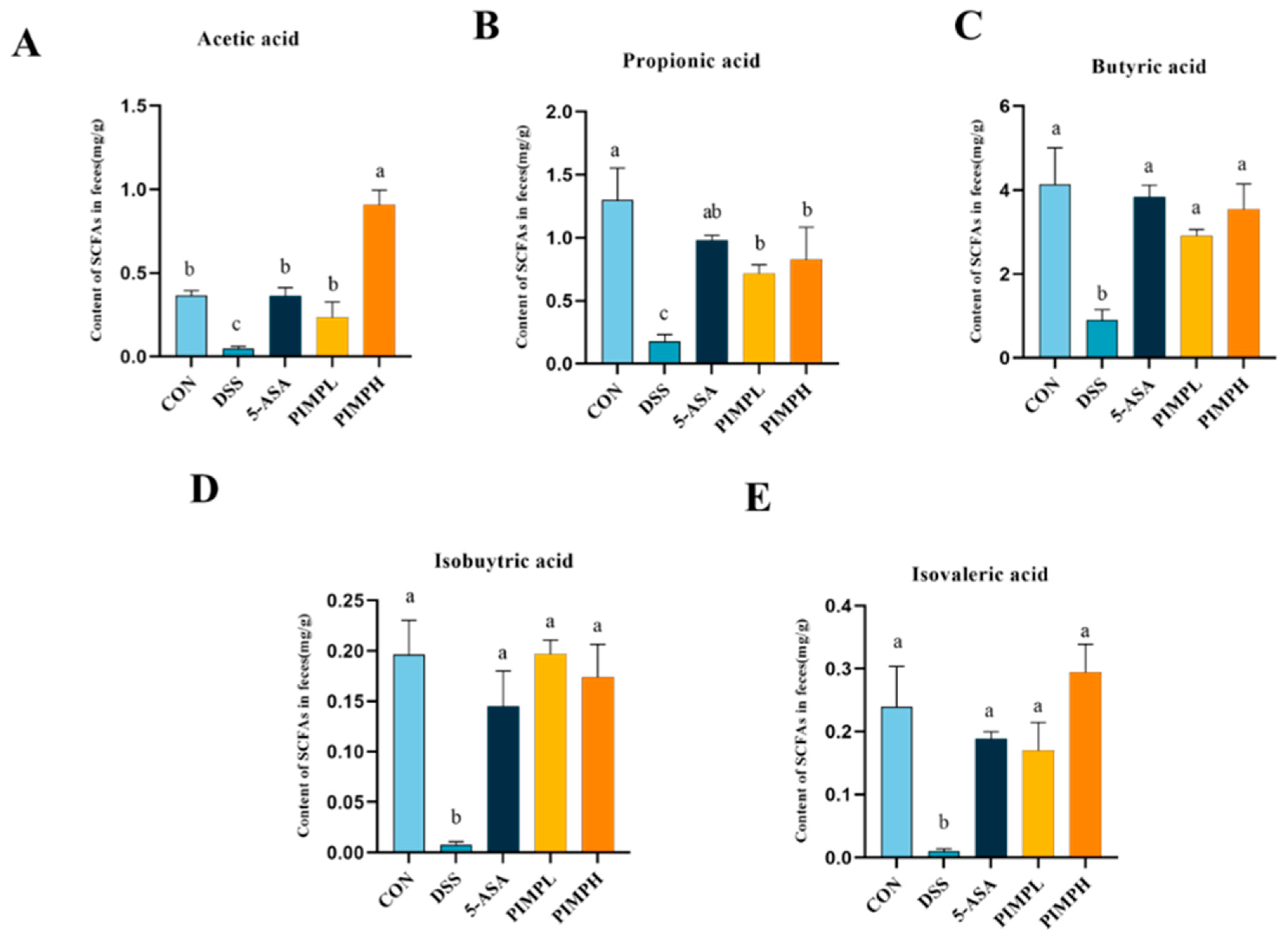
3.9. Porcine Intestinal Mucosal Peptide Adjusts Short-Chain Fatty Acids (SCFAs) in Dextrose Sodium Sulfate Induced Colitis
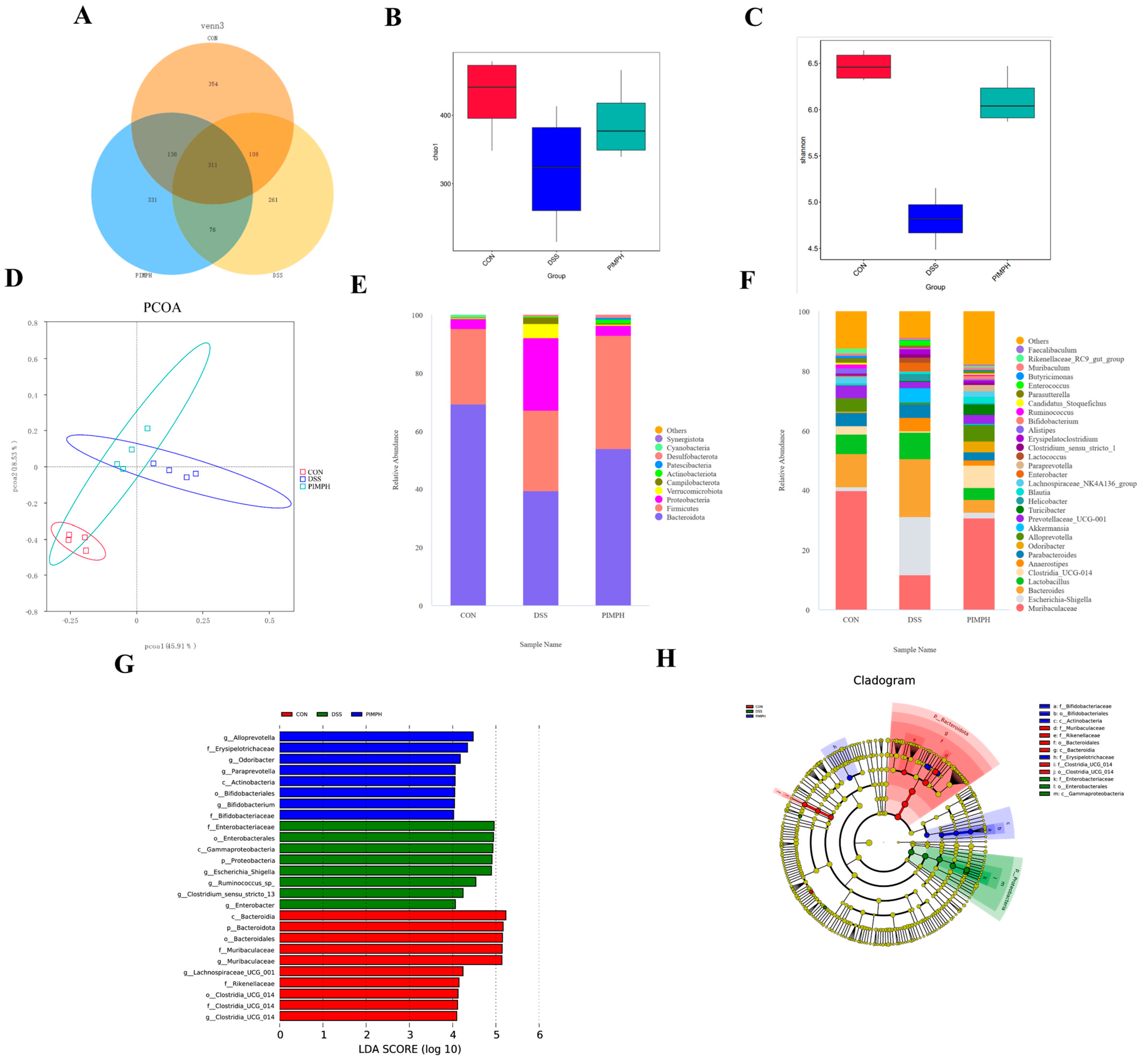
3.10. Porcine Intestinal Mucosal Peptide Regulates the Intestinal Microbiota in Mice with Dextrose Sodium Sulfate Induced Colitis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef] [PubMed]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.-F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef] [PubMed]
- Atreya, I.; Atreya, R.; Neurath, M.F. NF-κB in inflammatory bowel disease. J. Intern. Med. 2008, 263, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Fei, L.; Xu, K. Zhikang Capsule ameliorates dextran sodium sulfate-induced colitis by inhibition of inflammation, apoptosis, oxidative stress and MyD88-dependent TLR4 signaling pathway. J. Ethnopharmacol. 2016, 192, 236–247. [Google Scholar] [CrossRef]
- Kåhrström, C.T.; Pariente, N.; Weiss, U. Intestinal microbiota in health and disease. Nature 2016, 535, 47. [Google Scholar] [CrossRef]
- Rufino, M.N.; da Costa, A.L.; Jorge, E.N.; Paiano, V.F.; Camparoto, M.L.; Keller, R.; Bremer-Neto, H. Synbiotics improve clinical indicators of ulcerative colitis: Systematic review with meta-analysis. Nutr. Rev. 2022, 80, 157–164. [Google Scholar] [CrossRef]
- Weingarden, A.R.; Vaughn, B.P. Intestinal microbiota, fecal microbiota transplantation, and inflammatory bowel disease. Gut Microbes 2017, 8, 238–252. [Google Scholar] [CrossRef]
- Taglieri, I.; Sanmartin, C.; Venturi, F.; Macaluso, M.; Bianchi, A.; Sgherri, C.; Quartacci, M.F.; De Leo, M.; Pistelli, L.; Palla, F.; et al. Bread Fortified with Cooked Purple Potato Flour and Citrus Albedo: An Evaluation of Its Compositional and Sensorial Properties. Foods 2021, 10, 942. [Google Scholar] [CrossRef]
- Sun, C.; Tang, X.; Shao, X.; Han, D.; Zhang, H.; Shan, Y.; Gooneratne, R.; Shi, L.; Wu, X.; Hosseininezhad, M. Mulberry (Morus atropurpurea Roxb.) leaf protein hydrolysates ameliorate dextran sodium sulfate-induced colitis via integrated modulation of gut microbiota and immunity. J. Funct. Foods 2021, 84, 104575. [Google Scholar] [CrossRef]
- Joshi, I.; Mohideen, H.S.; Nazeer, R.A. A Meretrix meretrix visceral mass derived peptide inhibits lipopolysaccharide-stimulated responses in RAW264.7 cells and adult zebrafish model. Int. Immunopharmacol. 2021, 90, 107140. [Google Scholar] [CrossRef]
- Peng, Z.; Chen, B.; Zheng, Q.; Zhu, G.; Cao, W.; Qin, X.; Zhang, C. Ameliorative Effects of Peptides from the Oyster (Crassostrea hongkongensis) Protein Hydrolysates against UVB-Induced Skin Photodamage in Mice. Mar. Drugs 2020, 18, 288. [Google Scholar] [CrossRef] [PubMed]
- Agyei, D.; Danquah, M.K. Rethinking food-derived bioactive peptides for antimicrobial and immunomodulatory activities. Trends Food Sci. Technol. 2012, 23, 62–69. [Google Scholar] [CrossRef]
- Li, Y.; Sadiq, F.A.; Fu, L.; Zhu, H.; Zhong, M.; Sohail, M. Identification of Angiotensin I-Converting Enzyme Inhibitory Peptides Derived from Enzymatic Hydrolysates of Razor Clam Sinonovacula constricta. Mar. Drugs 2016, 14, 110. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Upadhyay, S.K. Enzymes and their production strategies. In Biomass, Biofuels, Biochemicals; Elsevier: Amsterdam, The Netherlands, 2020; pp. 31–48. [Google Scholar]
- Chakrabarti, S.; Guha, S.; Majumder, K. Food-Derived Bioactive Peptides in Human Health: Challenges and Opportunities. Nutrients 2018, 10, 1738. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Li, H.; Huang, Z.; Huang, J.; Sun, J. Chinese Consumers’ Heterogeneous Preferences for the Front-of-Package Labeling on Fresh Pork: A Choice Experiment Approach. Foods 2022, 11, 2929. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, Z.; Wang, Z.; Zhang, Z.; Wang, Q.; Pan, Y. Heterozygosity and homozygosity regions affect reproductive success and the loss of reproduction: A case study with litter traits in pigs. Comput. Struct. Biotechnol. J. 2022, 20, 4060–4071. [Google Scholar] [CrossRef] [PubMed]
- Traspov, A.; Deng, W.; Kostyunina, O.; Ji, J.; Shatokhin, K.; Lugovoy, S.; Zinovieva, N.; Yang, B.; Huang, L. Population structure and genome characterization of local pig breeds in Russia, Belorussia, Kazakhstan and Ukraine. Genet. Sel. Evol. GSE 2016, 48, 16. [Google Scholar] [CrossRef]
- Gale, F.; Hu, D.; Marti, D. China’s Volatile Pork Industry; United States Department of Agriculture: Washington, DC, USA, 2012. [Google Scholar]
- Xiong, X.; Tan, B.; Song, M.; Ji, P.; Kim, K.; Yin, Y.; Liu, Y. Nutritional intervention for the intestinal development and health of weaned pigs. Front. Vet. Sci. 2019, 6, 46. [Google Scholar] [CrossRef]
- Kim, J.D.; Hyun, Y.; Sohn, K.S.; Kim, T.J.; Woo, H.J.; Han, I.K. Optimal Dietary Ratio of Spray Dried Plasma Protein (SDPP) and Dried Porcine Solubles (DPS) in Improving Growth Performance and Immune Status in Pigs Weaned at 21 Days of Age. Asian-Australas J Anim Sci 2001, 14, 338–345. [Google Scholar] [CrossRef]
- Myers, A.J.; Goodband, R.D.; Tokach, M.D.; Dritz, S.S.; DeRouchey, J.M.; Nelssen, J.L. The effects of porcine intestinal mucosa protein sources on nursery pig growth performance1. J. Anim. Sci. 2014, 92, 783–792. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, C.; Han, Q.; Chen, X.; Li, G.; Yu, G. Highly sialylated mucin-type glycopeptide from porcine intestinal mucosa after heparin extraction: O-glycan profiling and immunological activity evaluation. Glycoconj. J. 2021, 38, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xu, C.; Zhang, C.; Cao, W.; Qin, X.; Gao, J.; Zheng, H. The purification and identification of immunoregulatory peptides from oyster (Crassostrea hongkongensis) enzymatic hydrolysate. RSC Adv. 2019, 9, 32854–32863. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Schägger, H. Tricine–SDS-PAGE. Nat. Protoc. 2006, 1, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.-J.; Park, J.M.; Kwon, Y.J.; Kim, K.; Park, S.Y.; Kim, I.; Lim, J.H.; Kim, B.K.; Kim, B.-Y. Immunostimulatory Effect of Heat-Killed Probiotics on RAW264.7 Macrophages. J. Microbiol. Biotechnol. 2022, 32, 638–644. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, S.; Popp, V.; Kindermann, M.; Gerlach, K.; Weigmann, B.; Fichtner-Feigl, S.; Neurath, M.F. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat. Protoc. 2017, 12, 1295–1309. [Google Scholar] [CrossRef]
- Jin, C.; Liu, J.; Jin, R.; Yao, Y.; He, S.; Lei, M.; Peng, X. Linarin ameliorates dextran sulfate sodium-induced colitis in C57BL/6J mice via the improvement of intestinal barrier, suppression of inflammatory responses and modulation of gut microbiota. Food Funct. 2022, 13, 10574–10586. [Google Scholar] [CrossRef]
- Xie, Z.; Zhang, G.; Liu, R.; Wang, Y.; Tsapieva, A.N.; Zhang, L.; Han, J. Heat-Killed Lacticaseibacillus paracasei Repairs Lipopolysaccharide-Induced Intestinal Epithelial Barrier Damage via MLCK/MLC Pathway Activation. Nutrients 2023, 15, 1758. [Google Scholar] [CrossRef]
- Da Silva, M.; Jaggers, G.K.; Verstraeten, S.V.; Erlejman, A.G.; Fraga, C.G.; Oteiza, P.I. Large procyanidins prevent bile-acid-induced oxidant production and membrane-initiated ERK1/2, p38, and Akt activation in Caco-2 cells. Free Radic. Biol. Med. 2012, 52, 151–159. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, L.; Wang, P.; Liu, Y.; Wang, G.; Shan, Y.; Yi, Y.; Zhou, Y.; Liu, B.; Wang, X.; et al. Lactobacillus coryniformis MXJ32 administration ameliorates azoxymethane/dextran sulfate sodium-induced colitis-associated colorectal cancer via reshaping intestinal microenvironment and alleviating inflammatory response. Eur. J. Nutr. 2022, 61, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Meehan, B.; Fu, Z.; Wang, X.Q.D.; Fiset, P.O.; Rieker, R.; Levins, C.; Kong, T.; Zhu, X.; Morin, G.; et al. SMARCA4 loss is synthetic lethal with CDK4/6 inhibition in non-small cell lung cancer. Nat. Commun. 2019, 10, 557. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Cai, X.; Fei, W.; Ye, Y.; Zhao, M.; Zheng, C. The role of short-chain fatty acids in immunity, inflammation and metabolism. Crit. Rev. Food Sci. Nutr. 2022, 62, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Luo, X.; Tang, J.; Mo, Q.; Zhong, H.; Zhang, H.; Feng, F. A bridge for short-chain fatty acids to affect inflammatory bowel disease, type 1 diabetes, and non-alcoholic fatty liver disease positively: By changing gut barrier. Eur. J. Nutr. 2021, 60, 2317–2330. [Google Scholar] [CrossRef] [PubMed]
- Chateau, T.; Feakins, R.; Marchal-Bressenot, A.; Magro, F.; Danese, S.; Peyrin-Biroulet, L. Histological Remission in Ulcerative Colitis: Under the Microscope Is the Cure. Am. J. Gastroenterol. 2020, 115, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Liu, X.; Wang, Y.; Dong, J.; Wu, F.; Mackenzie, G.G.; Su, Z. (−)-Epigallocatechin-3-gallate mitigates cyclophosphamide-induced intestinal injury by modulating the tight junctions, inflammation and dysbiosis in mice. Food Funct. 2021, 12, 11671–11685. [Google Scholar] [CrossRef] [PubMed]
- Patankar, J.V.; Becker, C. Cell death in the gut epithelium and implications for chronic inflammation. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 543–556. [Google Scholar] [CrossRef]
- Schmalensee, R. From “Green Growth” to sound policies: An overview. Energy Econ. 2012, 34, S2–S6. [Google Scholar] [CrossRef]
- Fujiwara, N.; Kobayashi, K. Macrophages in inflammation. Curr. Drug Targets. Inflamm. Allergy 2005, 4, 281–286. [Google Scholar] [CrossRef]
- Raetz, C.R.; Whitfield, C. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 2002, 71, 635–700. [Google Scholar] [CrossRef]
- Yao, L.; Yang, P.; Luo, W.; Li, S.; Wu, Y.; Cai, N.; Bi, D.; Li, H.; Han, Q.; Xu, X. Macrophage-stimulating activity of European eel (Anguilla anguilla) peptides in RAW264.7 cells mediated via NF-κB and MAPK signaling pathways. Food Funct. 2020, 11, 10968–10978. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Shu, W.; Shen, Y.; Sun, Q.; Bai, F.; Wang, J.; Li, D.; Li, Y.; Jin, W.; Yuan, L. Sturgeon protein-derived peptides exert anti-inflammatory effects in LPS-stimulated RAW264.7 macrophages via the MAPK pathway. J. Funct. Foods 2020, 72, 104044. [Google Scholar] [CrossRef]
- Ji, Z.; Mao, J.; Chen, S.; Mao, J. Antioxidant and anti-inflammatory activity of peptides from foxtail millet (Setaria italica) prolamins in HaCaT cells and RAW264.7 murine macrophages. Food Biosci. 2020, 36, 100636. [Google Scholar] [CrossRef]
- Okumura, R.; Takeda, K. Maintenance of intestinal homeostasis by mucosal barriers. Inflamm. Regen. 2018, 38, 5. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, X.; Yuan, J.N.; Lin, R.; Tian, Y.Y.; Li, Y.X.; Zhang, Y.; Wang, X.F.; Xie, Y.H.; Wang, S.W.; et al. Ilex rotunda Thunb Protects Against Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice by Restoring the Intestinal Mucosal Barrier and Modulating the Oncostatin M/Oncostatin M Receptor Pathway. Front. Pharmacol. 2022, 13, 819826. [Google Scholar] [CrossRef] [PubMed]
- Giriwono, P.E.; Shirakawa, H.; Ohsaki, Y.; Sato, S.; Aoyama, Y.; Ho, H.J.; Goto, T.; Komai, M. Geranylgeraniol Suppresses the Expression of IRAK1 and TRAF6 to Inhibit NFκB Activation in Lipopolysaccharide-Induced Inflammatory Responses in Human Macrophage-Like Cells. Int. J. Mol. Sci. 2019, 20, 2320. [Google Scholar] [CrossRef]
- Moraes, T.J.; Zurawska, J.H.; Downey, G.P. Neutrophil granule contents in the pathogenesis of lung injury. Curr. Opin. Hematol. 2006, 13, 21–27. [Google Scholar] [CrossRef]
- Kang, Y.; Xue, Y.; Du, M.; Zhu, M.-J. Preventive effects of Goji berry on dextran-sulfate-sodium-induced colitis in mice. J. Nutr. Biochem. 2017, 40, 70–76. [Google Scholar] [CrossRef]
- Mayangsari, Y.; Suzuki, T. Resveratrol Ameliorates Intestinal Barrier Defects and Inflammation in Colitic Mice and Intestinal Cells. J. Agric. Food Chem. 2018, 66, 12666–12674. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, B.; Ross, R.P.; Jin, Y.; Stanton, C.; Zhao, J.; Zhang, H.; Chen, W. Orally Administered CLA Ameliorates DSS-Induced Colitis in Mice via Intestinal Barrier Improvement, Oxidative Stress Reduction, and Inflammatory Cytokine and Gut Microbiota Modulation. J. Agric. Food Chem. 2019, 67, 13282–13298. [Google Scholar] [CrossRef]
- Peng, Y.; Yan, Y.; Wan, P.; Chen, D.; Ding, Y.; Ran, L.; Mi, J.; Lu, L.; Zhang, Z.; Li, X.; et al. Gut microbiota modulation and anti-inflammatory properties of anthocyanins from the fruits of Lycium ruthenicum Murray in dextran sodium sulfate-induced colitis in mice. Free Radic. Biol. Med. 2019, 136, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Conlin, V.S.; Wu, X.; Nguyen, C.; Dai, C.; Vallance, B.A.; Buchan, A.M.J.; Boyer, L.; Jacobson, K. Vasoactive intestinal peptide ameliorates intestinal barrier disruption associated with Citrobacter rodentium-induced colitis. Am. J. Physiol.-Gastrointest. Liver Physiol. 2009, 297, G735–G750. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [PubMed]
- Anhê, F.F.; Pilon, G.; Roy, D.; Desjardins, Y.; Levy, E.; Marette, A. Triggering Akkermansia with dietary polyphenols: A new weapon to combat the metabolic syndrome? Gut Microbes 2016, 7, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhya, I.; Hansen, R.; El-Omar, E.M.; Hold, G.L. IBD—What role do Proteobacteria play? Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yang, S.; Zhang, Y.; Huang, K.; Liang, T.; Chen, Y.; Guan, Y.; Shang, R.; Guan, T.; Wu, J.; et al. Amino acid-balanced diets improved DSS-induced colitis by alleviating inflammation and regulating gut microbiota. Eur. J. Nutr. 2022, 61, 3531–3543. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, T.E.; Morton, J.M. The Human Gut Microbiome: A Review of the Effect of Obesity and Surgically Induced Weight Loss. JAMA Surg. 2013, 148, 563–569. [Google Scholar] [CrossRef]
- Zhang, S.-L.; Wang, S.-N.; Miao, C.-Y. Influence of Microbiota on Intestinal Immune System in Ulcerative Colitis and Its Intervention. Front. Immunol. 2017, 8, 1674. [Google Scholar] [CrossRef]
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vázquez-Baeza, Y.; Van Treuren, W.; Ren, B.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M.; et al. The Treatment-Naive Microbiome in New-Onset Crohn’s Disease. Cell Host Microbe 2014, 15, 382–392. [Google Scholar] [CrossRef]
- Schirmer, M.; Garner, A.; Vlamakis, H.; Xavier, R.J. Microbial genes and pathways in inflammatory bowel disease. Nat. Rev. Microbiol. 2019, 17, 497–511. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Xie, Z.; Wu, X.; Du, L.; Chong, Z.; Liu, R.; Han, J. Porcine Intestinal Mucosal Peptides Target Macrophage-Modulated Inflammation and Alleviate Intestinal Homeostasis in Dextrose Sodium Sulfate-Induced Colitis in Mice. Foods 2024, 13, 162. https://doi.org/10.3390/foods13010162
Wang Y, Xie Z, Wu X, Du L, Chong Z, Liu R, Han J. Porcine Intestinal Mucosal Peptides Target Macrophage-Modulated Inflammation and Alleviate Intestinal Homeostasis in Dextrose Sodium Sulfate-Induced Colitis in Mice. Foods. 2024; 13(1):162. https://doi.org/10.3390/foods13010162
Chicago/Turabian StyleWang, Yucong, Zhixin Xie, Xiaolong Wu, Lei Du, Zhengchen Chong, Rongxu Liu, and Jianchun Han. 2024. "Porcine Intestinal Mucosal Peptides Target Macrophage-Modulated Inflammation and Alleviate Intestinal Homeostasis in Dextrose Sodium Sulfate-Induced Colitis in Mice" Foods 13, no. 1: 162. https://doi.org/10.3390/foods13010162




