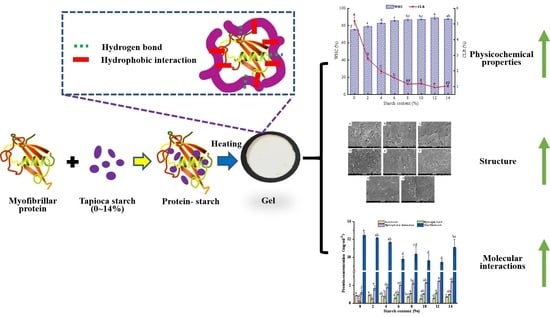
Tapioca Starch Improves the Quality of Virgatus nemipterus Surimi Gel by Enhancing Molecular Interaction in the Gel System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Surimi Gel
2.3. Determination of Gel Texture Properties
2.4. Determination of the Water-Holding Capacity (WHC)
2.5. Determination of the Cooking Loss Rate (CLR)
2.6. Determination of Whiteness
2.7. Determination of Low-Field Nuclear Magnetic Resonance (LF-NMR)
2.8. Light Microscopic Observation
2.9. Scanning Electron Microscopy (SEM) Observation
2.10. Determination of Molecular Forces
2.11. Fourier Transform Infrared (FT-IR) Spectroscopy
2.12. Statistical Analysis
3. Results
3.1. Effect of Starch on the Physicochemical Properties of Surimi Gel
3.1.1. Texture Profile Analysis
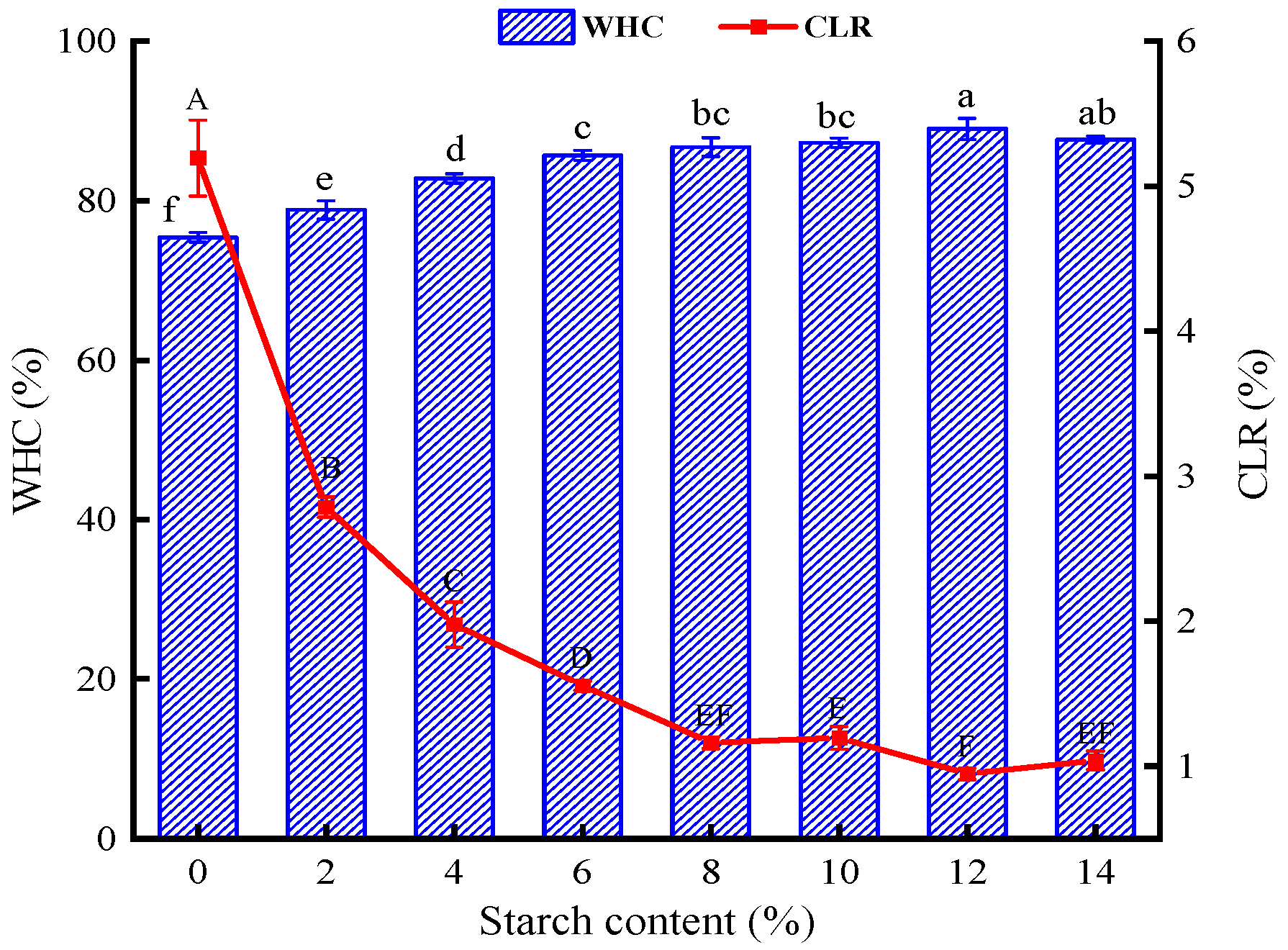
3.1.2. WHC and CLR
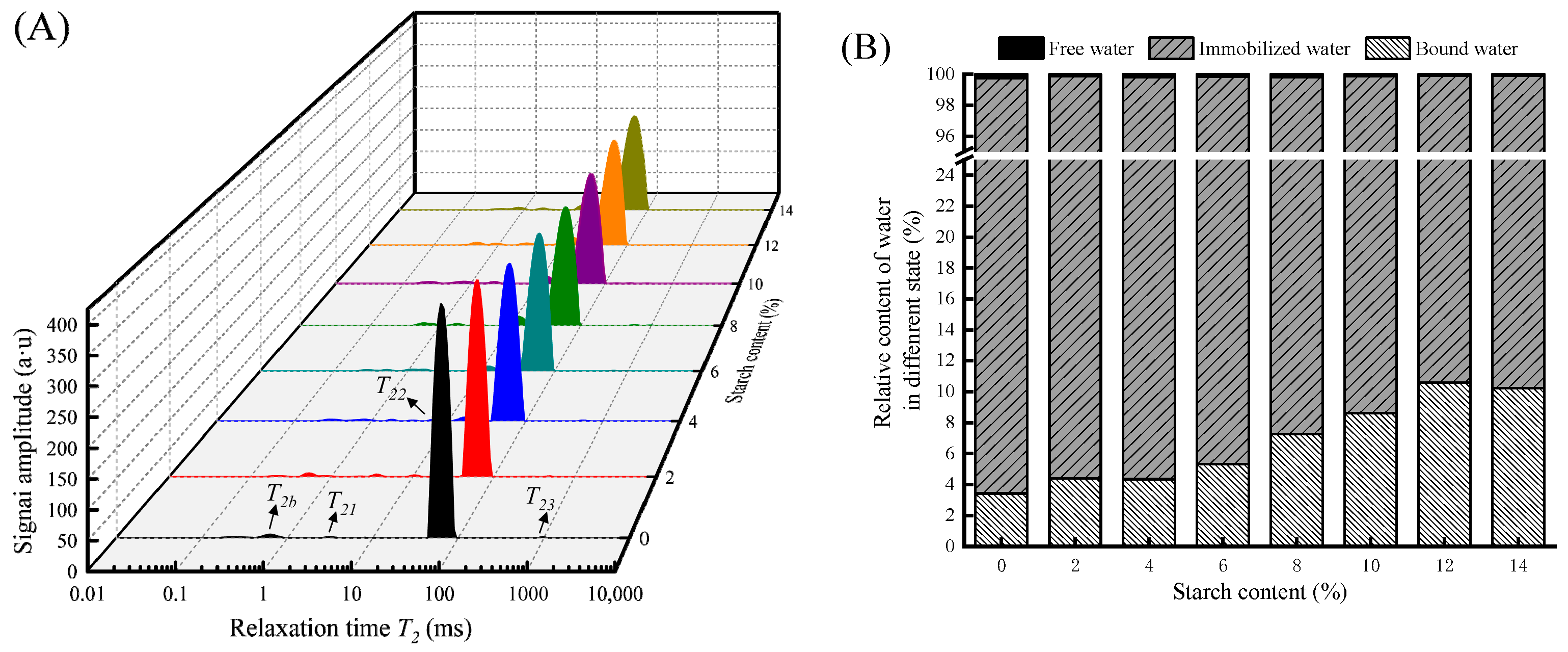
3.1.3. Water Distribution
3.1.4. Whiteness
3.2. Effect of Starch on the Microstructure of Surimi Gel
3.2.1. Light Microscopic Observation
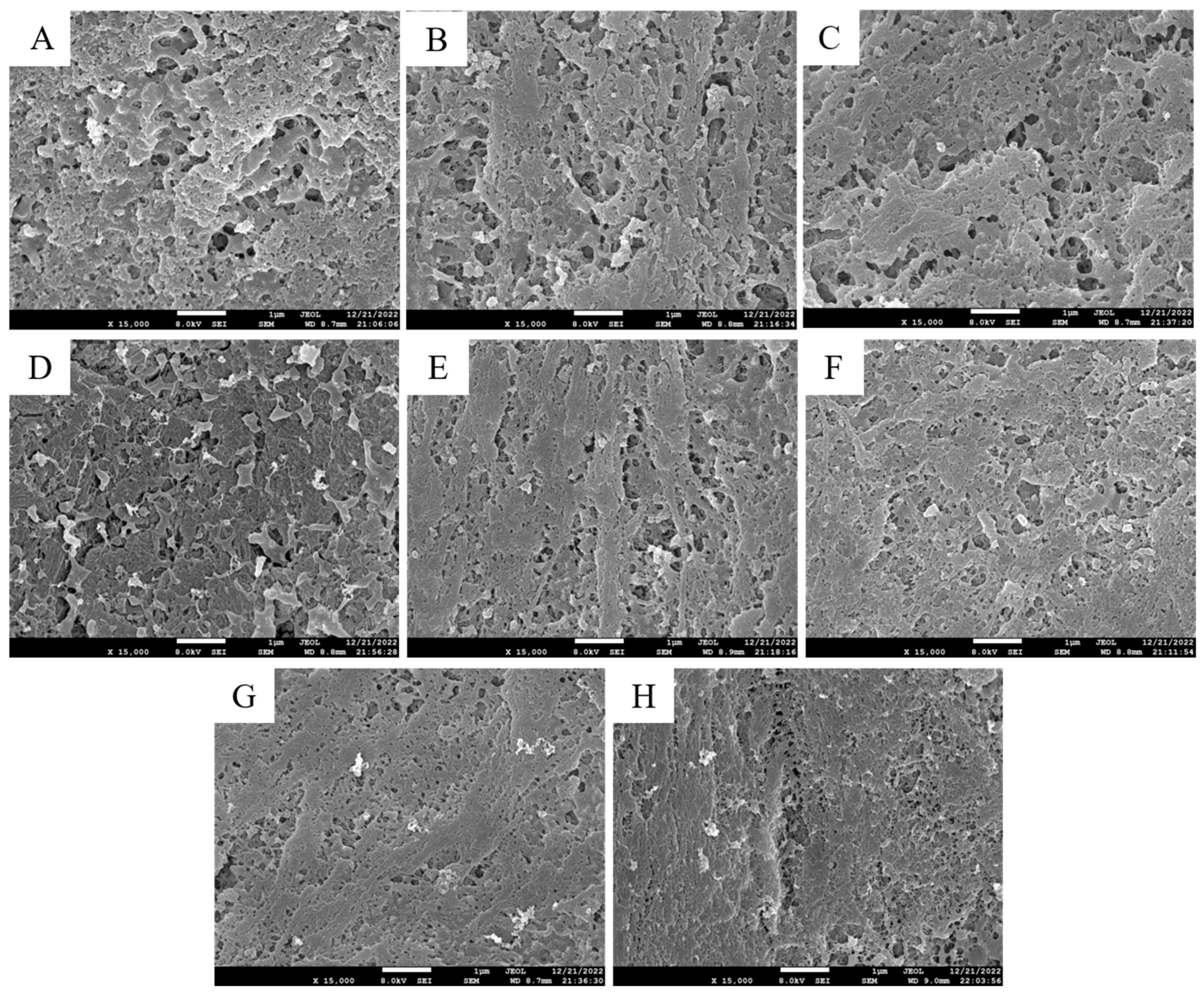
3.2.2. SEM Observation
3.3. Analysis of the Molecular Interactions
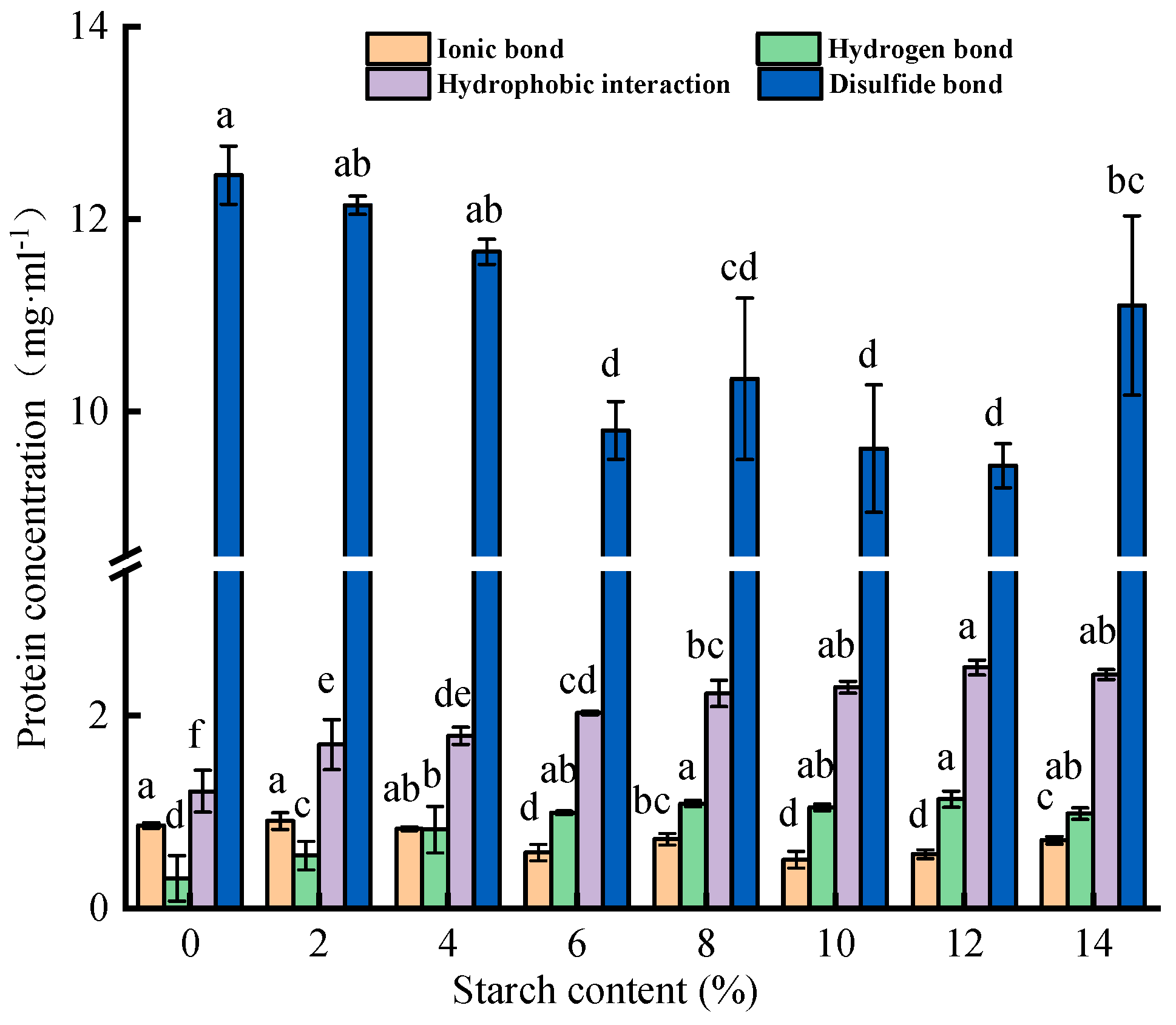
3.3.1. Chemical Interaction Forces
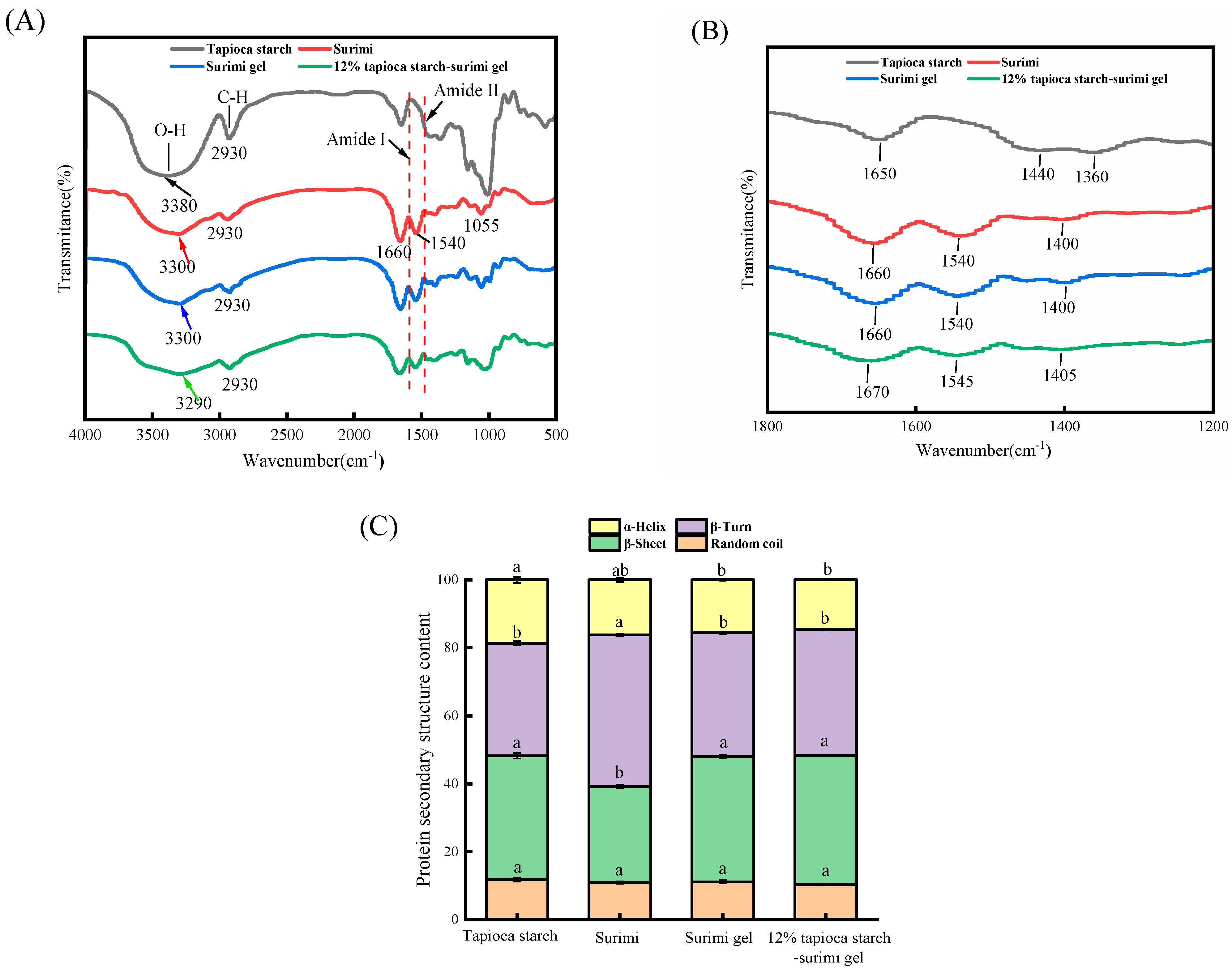
3.3.2. FT-IR Spectroscopy
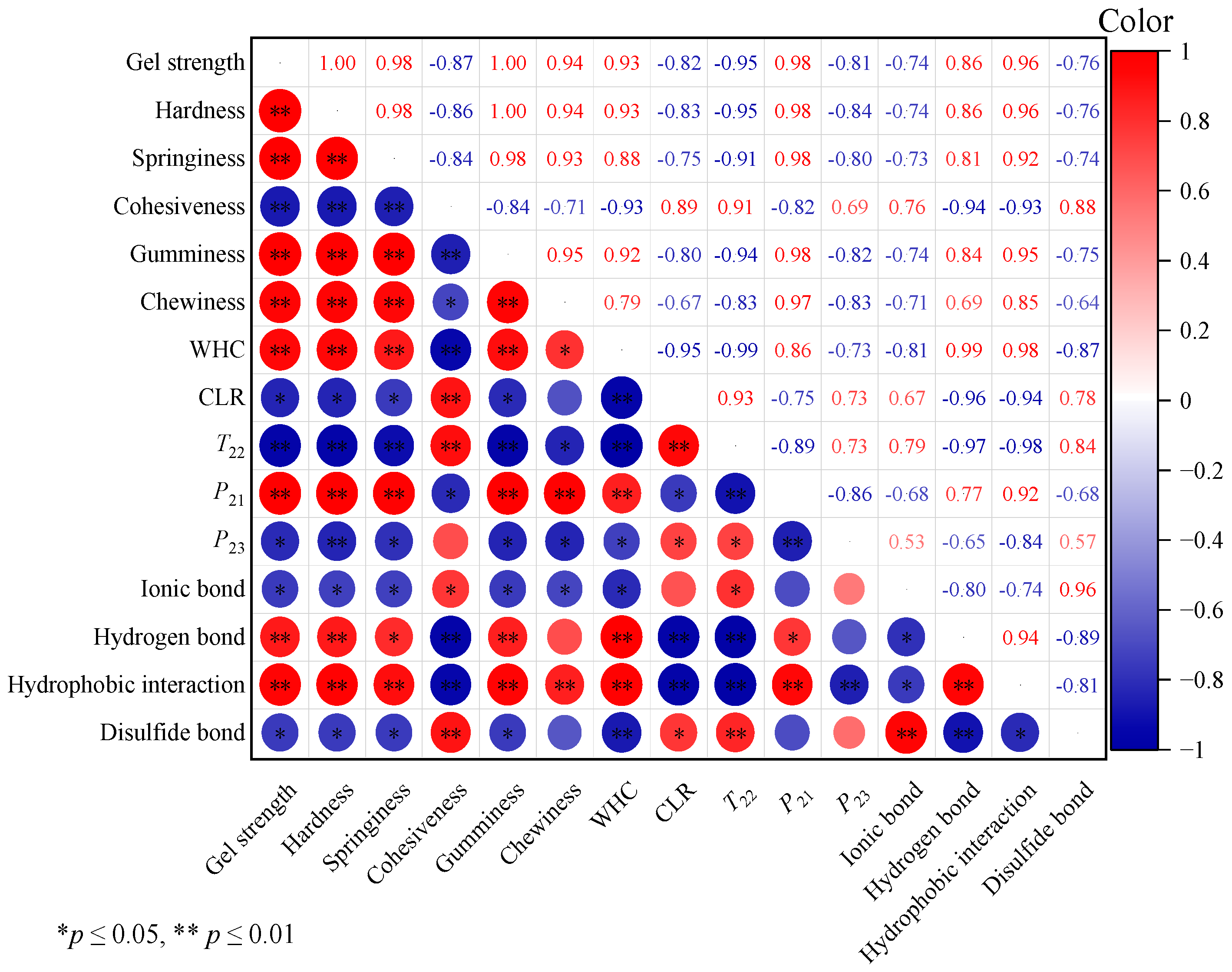
3.4. Correlation Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yi, M.; Hsu, K.; Wang, J.; Feng, B.; Lin, H.; Yan, Y. Genetic structure and diversity of the yellowbelly threadfin bream nemipterus bathybius in the northern south china sea. Diversity 2021, 13, 324. [Google Scholar] [CrossRef]
- Mi, J.; Zhao, X.; Huang, P.; Hong, J.; Jia, R.; Deng, S.; Yu, X.; Wei, H.; Yang, W. Effect of hydroxypropyl distarch phosphate on the physicochemical characteristics and structure of shrimp myofibrillar protein. Food Hydrocoll. 2022, 125, 107417. [Google Scholar] [CrossRef]
- Liu, Z.; Yuan, Y.; Qin, Y.; Feng, A.; He, Y.; Zhou, D.; Dong, X.; Shen, X.; Cao, J.; Li, C. Sweet potato starch addition together with partial substitution of tilapia flesh effectively improved the golden pompano (Trachinotus blochii) surimi quality. J. Texture Stud. 2021, 52, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Mi, H.; Li, Y.; Wang, C.; Yi, S.; Li, X.; Li, J. The interaction of starch-gums and their effect on gel properties and protein conformation of silver carp surimi. Food Hydrocoll. 2021, 112, 106290. [Google Scholar] [CrossRef]
- Luo, H.; Guo, C.; Lin, L.; Si, Y.; Gao, X.; Xu, D.; Jia, R.; Yang, W. Combined use of rheology, LF-NMR, and MRI for characterizing the gel properties of hairtail surimi with potato starch. Food Bioprocess Technol. 2020, 13, 637–647. [Google Scholar] [CrossRef]
- Jia, R.; Katano, T.; Yoshimoto, Y.; Gao, Y.; Watanabe, Y.; Nakazawa, N.; Osako, K.; Okazaki, E. Sweet potato starch with low pasting temperature to improve the gelling quality of surimi gels after freezing. Food Hydrocoll. 2018, 81, 467–473. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, Q.; Xiao, N.; Du, Y.; Feng, Q.; Shi, W. Changes in gel structure and chemical interactions of hypophthalmichthys molitrix surimi gels: Effect of setting process and different starch addition. Foods 2022, 11, 9. [Google Scholar] [CrossRef]
- Mi, H.; Wang, C.; Su, Q.; Li, X.; Yi, S.; Li, J. The effect of modified starches on the gel properties and protein conformation of nemipterus virgatus surimi. J. Texture Stud. 2019, 50, 571–581. [Google Scholar] [CrossRef]
- Dong, X.; Huang, Y.; Pan, Y.; Wang, K.; Prakash, S.; Zhu, B. Investigation of sweet potato starch as a structural enhancer for three-dimensional printing of Scomberomorus niphonius surimi. J. Texture Stud. 2019, 50, 316–324. [Google Scholar] [CrossRef]
- Li, T.; Zhao, J.; Huang, J.; Zhang, W.; Huang, J.; Fan, D.; Zhang, H. Improvement of the quality of surimi products with overdrying potato starches. J. Food Qual. 2017, 2017, 1417856. [Google Scholar] [CrossRef]
- Zhao, H.; Kang, X.; Zhou, X.; Tong, L.; Yu, W.; Zhang, J.; Yang, W.; Lou, Q.; Huang, T. Glycosylation fish gelatin with gum Arabic: Functional and structural properties. LWT 2021, 139, 110634. [Google Scholar] [CrossRef]
- Chisenga, S.M.; Workneh, T.S.; Bultosa, G.; Alimi, B.A. Progress in research and applications of cassava flour and starch: A review. J. Food Sci. Technol. 2019, 56, 2799–2813. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Fang, L.; Wang, C.; Shi, L.; Chang, T.; Yang, H.; Cui, M. Effects of starches on the textural, rheological, and color properties of surimi-beef gels with microbial tranglutaminase. Meat Sci. 2013, 93, 533–537. [Google Scholar] [CrossRef]
- Kong, W.; Zhang, T.; Feng, D.; Xue, Y.; Wang, Y.; Li, Z.; Yang, W.; Xue, C. Effects of modified starches on the gel properties of alaska pollock surimi subjected to different temperature treatments. Food Hydrocoll. 2016, 56, 20–28. [Google Scholar] [CrossRef]
- Mao, M.; Jia, R.; Gao, Y.; Yang, W.; Tong, J.; Xia, G. Effects of innovative gelation and modified tapioca starches on the physicochemical properties of surimi gel during frozen storage. Int. J. Food Sci. Technol. 2022, 57, 6591–6601. [Google Scholar] [CrossRef]
- Song, C.; Lin, Y.; Hong, P.; Liu, H.; Zhou, C. Compare with different vegetable oils on the quality of the Nemipterus virgatus surimi gel. Food Sci. Nutr. 2022, 10, 2935–2946. [Google Scholar] [CrossRef]
- Jiang, Q.; Chen, N.; Gao, P.; Yu, D.; Yang, F.; Xu, Y.; Xia, W. Influence of L-arginine addition on the gel properties of reduced-salt white leg shrimp (Litopenaeus vannamei) surimi gel treated with microbial transglutaminase. LWT 2023, 173, 114310. [Google Scholar] [CrossRef]
- Chen, N.; Jiang, Q.; Gao, P.; Yu, D.; Yang, F.; Xu, Y.; Xia, W. Gel properties, physicochemical properties, and sensory attributes of white leg shrimp (Litopenaeus vannamei) surimi gel treated with sodium chloride (NaCl) substitutes. Int. J. Food Sci. Technol. 2023, 58, 22–36. [Google Scholar] [CrossRef]
- Jia, R.; Katano, T.; Yoshimoto, Y.; Gao, Y.; Nakazawa, N.; Osako, K.; Okazaki, E. Effect of small granules in potato starch and wheat starch on quality changes of direct heated surimi gels after freezing. Food Hydrocoll. 2020, 104, 105732. [Google Scholar] [CrossRef]
- Rodríguez Patino, J.M.; Pilosof, A.M.R. Protein-polysaccharide interactions at fluid interfaces. Food Hydrocoll. 2011, 25, 1925–1937. [Google Scholar] [CrossRef]
- Eom, S.; Kim, J.; Son, B.; You, D.H.; Han, J.M.; Oh, J.; Kim, B.; Kong, C. Effects of carrageenan on the gelatinization of salt-based surimi gels. Fish. Aquat. Sci. 2013, 16, 143–147. [Google Scholar] [CrossRef]
- Zhang, C.; He, Y.; Zheng, Y.; Ai, C.; Cao, H.; Xiao, J.; El-Seedi, H.; Chen, L.; Teng, H. Effect of carboxymethyl cellulose (CMC) on some physico-chemical and mechanical properties of unrinsed surimi gels. LWT 2023, 180, 114653. [Google Scholar] [CrossRef]
- Zhang, F.L.; Liang, Y.; Tan, C.P.; Lu, Y.M.; Cui, B. Research on the water-holding capacity of pork sausage with acetate cassava starch. Starch-Stärke 2014, 66, 1033–1040. [Google Scholar] [CrossRef]
- Zhang, B.; Guo, X.; Lin, J.; Sun, P.; Ren, X.; Xu, W.; Tong, Y.; Li, D. Effect and synergy of different exogenous additives on gel properties of the mixed shrimp surimi (Antarctic krill and white shrimp). Int. J. Food Sci. Technol. 2022, 57, 5338–5348. [Google Scholar] [CrossRef]
- Song, C.; Lin, Y.; Hong, P.; Liu, H.; Zhou, C. Low-content pre-emulsified safflower seed oil enhances the quality and flavor of the nemipterus virgatus surimi gel. Gels 2022, 8, 106. [Google Scholar] [CrossRef]
- Zhuang, X.; Han, M.; Bai, Y.; Liu, Y.; Xing, L.; Xu, X.; Zhou, G. Insight into the mechanism of myofibrillar protein gel improved by insoluble dietary fiber. Food Hydrocoll. 2018, 74, 219–226. [Google Scholar] [CrossRef]
- Zhuang, X.; Han, M.; Jiang, X.; Bai, Y.; Zhou, H.; Li, C.; Xu, X.L.; Zhou, G.H. The effects of insoluble dietary fiber on myofibrillar protein gelation: Microstructure and molecular conformations. Food Chem. 2019, 275, 770–777. [Google Scholar] [CrossRef]
- Gravelle, A.J.; Marangoni, A.G.; Barbut, S. Insight into the mechanism of myofibrillar protein gel stability: Influencing texture and microstructure using a model hydrophilic filler. Food Hydrocoll. 2016, 60, 415–424. [Google Scholar] [CrossRef]
- Wu, S. Effect of pullulan on gel properties of Scomberomorus niphonius surimi. Int. J. Biol. Macromol. 2016, 93, 1118–1120. [Google Scholar] [CrossRef]
- Li, S.; Lin, S.; Jiang, P.; Bao, Z.; Li, S.; Sun, N. Insight into the gel properties of antarctic krill and pacific white shrimp surimi gels and the feasibility of polysaccharides as texture enhancers of antarctic krill surimi gels. Foods 2022, 11, 2517. [Google Scholar] [CrossRef]
- Chen, L.; Yang, R.; Fan, X.; He, G.; Zhao, Z.; Wang, F.; Liu, Y.; Wang, M.; Han, M.; Ullah, N.; et al. Changes in the quality of myofibrillar protein gel damaged by high doses of epigallocatechin-3-gallate as affected by the addition of amylopectin. Foods 2023, 12, 1790. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhou, T.; Wang, X.; Zou, Y.; Wang, D.; Xu, W. Effects of the structure and gel properties of myofibrillar protein on chicken breast quality treated with ultrasound-assisted potassium alginate. Food Chem. 2021, 358, 129873. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Wu, D.; Wang, L.; Song, G.; Chi, R.; Ma, J.; Li, Z.; Wang, L.; Sun, W. Properties of pork myofibrillar protein: A water distribution, microstructure and intermolecular interactions study. Food Chem. 2023, 411, 135386. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhao, J.; Guo, J.; Lian, X.; Wang, H. Effects of amylopectins from five different sources on disulfide bond formation in alkali-soluble glutenin. Foods 2023, 12, 414. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, R.; Xu, X.; Zhou, G.; Liu, D. Structural modification by high-pressure homogenization for improved functional properties of freeze-dried myofibrillar proteins powder. Food Res. Int. 2017, 100, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, Z.; Wang, Y.; Xue, Y.; Xue, C. Effects of konjac glucomannan on heat-induced changes of physicochemical and structural properties of surimi gels. Food Res. Int. 2016, 83, 152–161. [Google Scholar] [CrossRef]
- López-Barón, N.; Gu, Y.; Vasanthan, T.; Hoover, R. Plant proteins mitigate in vitro wheat starch digestibility. Food Hydrocoll. 2017, 69, 19–27. [Google Scholar] [CrossRef]
- Lian, W.; Hu, Q.; Qu, M.; Sun, B.; Liu, L.; Zhu, Y.; Xia, X.; Huang, Y.; Zhu, X. Impact of Insoluble dietary fiber and CaCl2 on structural properties of soybean protein isolate-wheat gluten composite gel. Foods 2023, 12, 1890. [Google Scholar] [CrossRef]
- Zhou, X.; Jiang, S.; Zhao, D.; Zhang, J.; Gu, S.; Pan, Z.; Ding, Y. Changes in physicochemical properties and protein structure of surimi enhanced with camellia tea oil. LWT-Food Sci. Technol. 2017, 84, 562–571. [Google Scholar] [CrossRef]

| Tapioca Starch (g per 100 g) | Gel Strength/N | Hardness/N | Springiness | Cohesiveness | Gumminess | Chewiness |
|---|---|---|---|---|---|---|
| 0 | 7.59 ± 0.02 g | 12.61 ± 0.58 g | 0.77 ± 0.00 c | 0.66 ± 0.00 a | 8.64 ± 0.11 f | 6.66 ± 0.08 d |
| 2 | 8.51 ± 0.11 f | 13.95 ± 0.21 f | 0.78 ± 0.00 c | 0.65 ± 0.00 ab | 9.07 ± 0.43 f | 6.99 ± 0.42 d |
| 4 | 10.13 ± 0.22 e | 15.48 ± 0.15 e | 0.77 ± 0.01 c | 0.65 ± 0.01 abc | 10.19 ± 0.09 e | 7.90 ± 0.17 d |
| 6 | 11.75 ± 0.19 d | 17.64 ± 0.36 d | 1.01 ± 0.34 bc | 0.65 ± 0.00 bcd | 11.47 ± 0.41 d | 9.97 ± 0.69 c |
| 8 | 14.06 ± 0.20 c | 19.69 ± 0.69 c | 1.20 ± 0.33 ab | 0.64 ± 0.00 d | 12.65 ± 0.45 c | 10.77 ± 0.38 c |
| 10 | 15.17 ± 0.31 b | 20.69 ± 0.29 b | 1.21 ± 0.32 ab | 0.64 ± 0.01 cd | 13.53 ± 0.22 b | 18.79 ± 0.40 b |
| 12 | 17.48 ± 0.23 a | 24.00 ± 0.55 a | 1.41 ± 0.01 a | 0.64 ± 0.00 d | 15.42 ± 0.33 a | 21.36 ± 1.13 a |
| 14 | 17.27 ± 0.10 a | 23.47 ± 0.03 a | 1.40 ± 0.00 ab | 0.65 ± 0.00 bcd | 15.26 ± 0.09 a | 21.54 ± 0.23 a |
| Tapioca Starch (g per 100 g) | T2B | T22 | T23 |
|---|---|---|---|
| 0 | 2.36 ± 0.24 ab | 44.49 ± 0.00 a | 784.70 ± 31.81 a |
| 2 | 2.97 ± 0.21 a | 41.50 ± 0.00 b | 309.09 ± 73.66 c |
| 4 | 2.12 ± 0.64 bc | 36.12 ± 0.00 c | 459.16 ± 16.99 b |
| 6 | 1.48 ± 0.10 c | 33.70 ± 0.00 d | 472.95 ± 65.86 b |
| 8 | 1.66 ± 0.65 bc | 31.44 ± 0.00 e | 483.43 ± 37.82 b |
| 10 | 1.64 ± 0.06 bc | 30.74 ± 1.22 e | 322.37 ± 18.63 c |
| 12 | 1.82 ± 0.43 bc | 28.67 ± 1.13 f | 460.15 ± 39.17 b |
| 14 | 1.81 ± 0.39 bc | 29.33 ± 0.00 f | 465.42 ± 43.12 b |
| Tapioca Starch (g per 100 g) | L* | a* | b* | Whiteness |
|---|---|---|---|---|
| 0 | 71.68 ± 0.17 a | −2.51 ± 0.03 a | 5.17 ± 0.19 a | 71.10 ± 0.20 a |
| 2 | 70.21 ± 0.33 b | −2.73 ± 0.02 bc | 4.24 ± 0.11 b | 69.78 ± 0.34 b |
| 4 | 68.57 ± 0.11 c | −2.81 ± 0.05 cd | 4.04 ± 0.18 bc | 68.18 ± 0.12 c |
| 6 | 66.59 ± 0.06 d | −2.85 ± 0.05 d | 3.79 ± 0.14 cd | 66.25 ± 0.06 d |
| 8 | 65.31 ± 0.06 e | −2.83 ± 0.06 d | 3.49 ± 0.14 e | 65.01 ± 0.05 e |
| 10 | 64.01 ± 0.07 f | −2.79 ± 0.05 cd | 3.38 ± 0.03 e | 63.74 ± 0.06 f |
| 12 | 62.59 ± 0.45 g | −2.81 ± 0.01 cd | 3.53 ± 0.12 de | 62.32 ± 0.44 g |
| 14 | 62.00 ± 0.16 h | −2.66 ± 0.06 b | 3.48 ± 0.27 e | 61.75 ± 0.18 h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Liu, Q.; Wang, P.; Song, C.; Ma, H.; Hong, P.; Zhou, C. Tapioca Starch Improves the Quality of Virgatus nemipterus Surimi Gel by Enhancing Molecular Interaction in the Gel System. Foods 2024, 13, 169. https://doi.org/10.3390/foods13010169
Huang X, Liu Q, Wang P, Song C, Ma H, Hong P, Zhou C. Tapioca Starch Improves the Quality of Virgatus nemipterus Surimi Gel by Enhancing Molecular Interaction in the Gel System. Foods. 2024; 13(1):169. https://doi.org/10.3390/foods13010169
Chicago/Turabian StyleHuang, Xiaobing, Qingguan Liu, Pengkai Wang, Chunyong Song, Huanta Ma, Pengzhi Hong, and Chunxia Zhou. 2024. "Tapioca Starch Improves the Quality of Virgatus nemipterus Surimi Gel by Enhancing Molecular Interaction in the Gel System" Foods 13, no. 1: 169. https://doi.org/10.3390/foods13010169