Biofortification and Valorization of Celery byproducts Using Selenium and PGPB under Reduced Nitrogen Regimes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design and Treatments
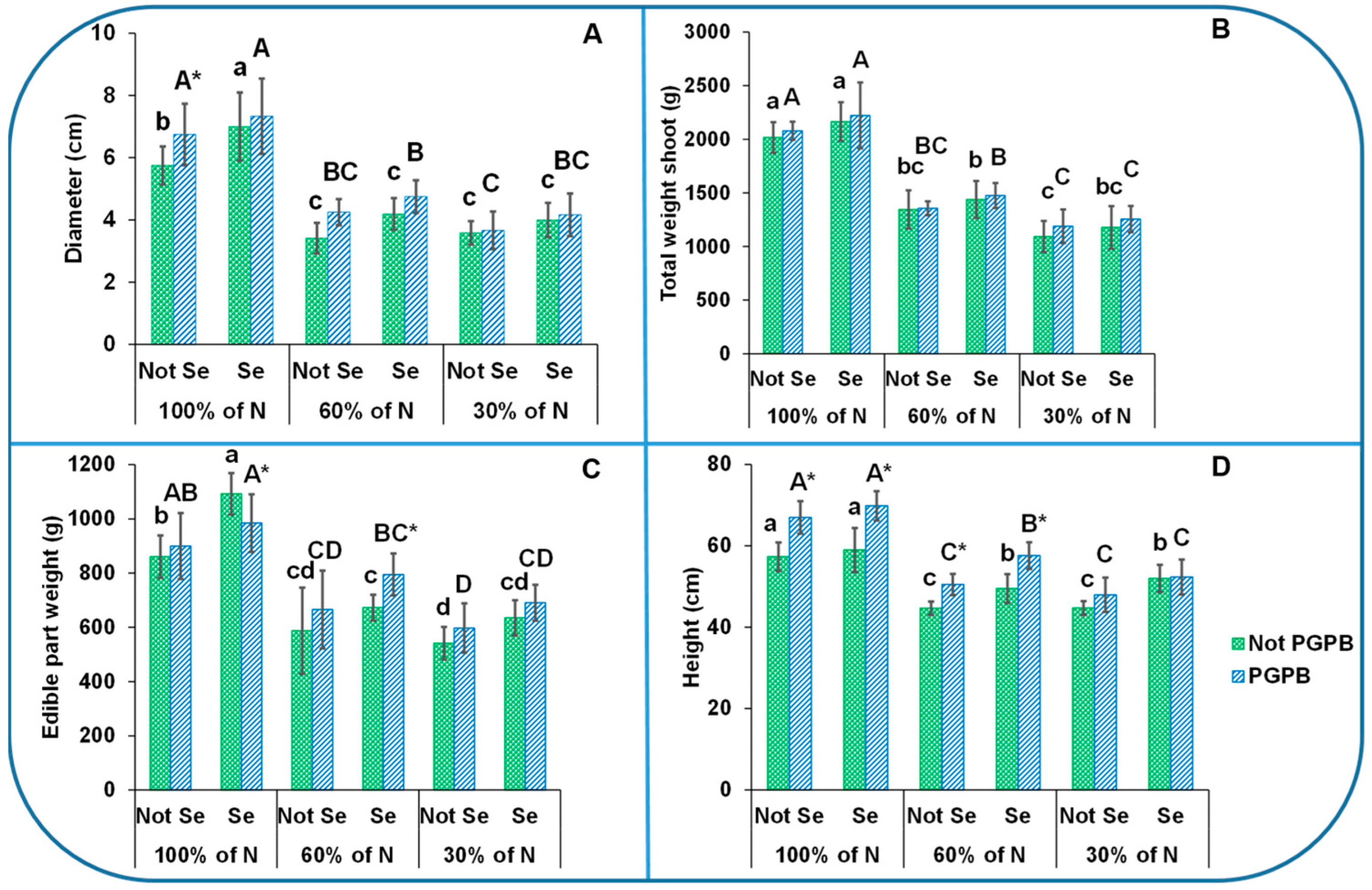
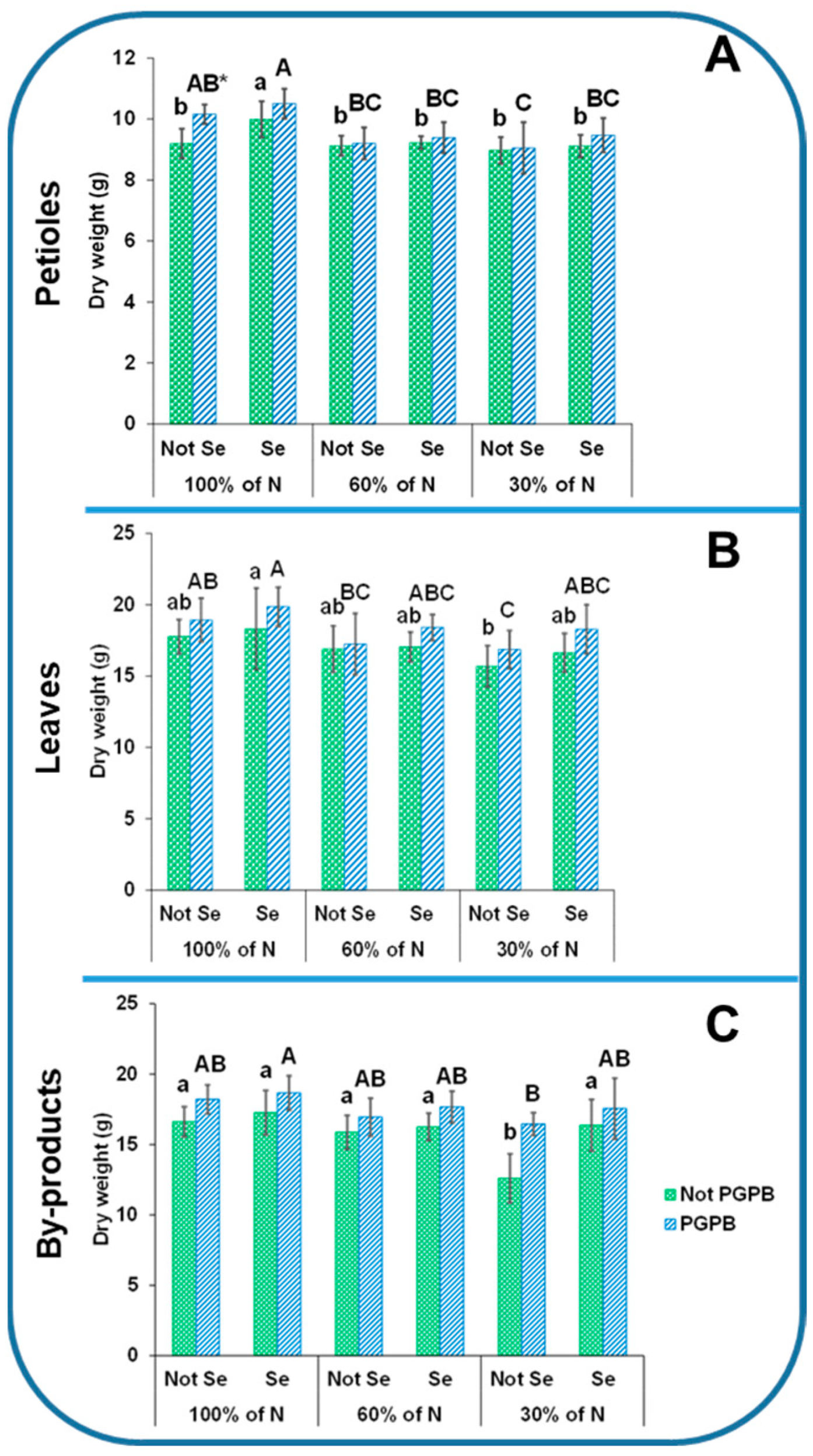
2.2. Plant Biomass Measurements
2.3. Chemicals and Reagents
2.4. Determination of Cations
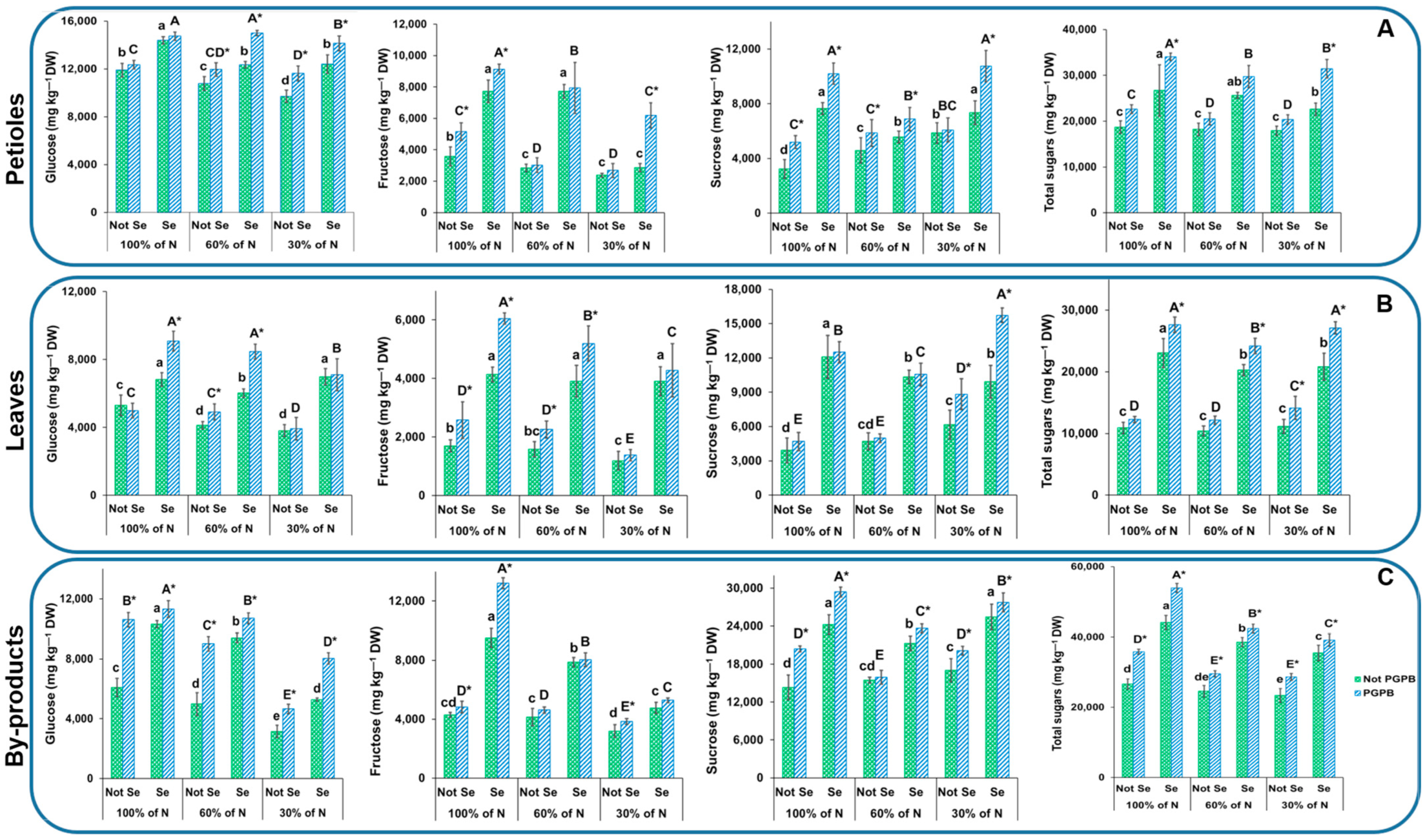
2.5. Determination of Sugar Contents
2.6. Determination of Protein Content
2.7. Determination of Lipid Peroxidation
2.8. Statistical Analysis
3. Results and Discussion
3.1. Determination of Celery Biomass
3.2. Determination of Cations
3.3. Determination of Sugar Contents
3.4. Determination of Protein Contents
3.5. Determination of Lipid Peroxidation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al Aboody, M.S. Cytotoxic, antioxidant, and antimicrobial activities of Celery (Apium graveolens L.). Bioinformation 2021, 17, 147. [Google Scholar] [CrossRef] [PubMed]
- Beltrán Sanahuja, A.; Ponce Landete, M.; Domingo Martínez, M.I.; Prats Moya, M.S.; Valdés García, A. Optimization of volatile compounds extraction from industrial celery (Apium graveolens) by-products by using response surface methodology and study of their potential as antioxidant sources. Foods 2021, 10, 2664. [Google Scholar] [CrossRef] [PubMed]
- Golubkina, N.A.; Kharchenko, V.A.; Moldovan, A.I.; Koshevarov, A.A.; Zamana, S.; Nadezhkin, S.; Soldatenko, A.; Sekara, A.; Tallarita, A.; Caruso, G. Yield, Growth, Quality, Biochemical Characteristics and Elemental Composition of Plant Parts of Celery Leafy, Stalk and Root Types Grown in the Northern Hemisphere. Plants 2020, 9, 484. [Google Scholar] [CrossRef]
- Haque, F.; Fan, C.; Lee, Y.Y. From waste to value: Addressing the relevance of waste recovery to agricultural sector in line with circular economy. J. Clean. Prod. 2023, 415, 137873. [Google Scholar] [CrossRef]
- Munene, R.; Changamu, E.; Korir, N.; Joseph, G.-O. Effects of Different Nitrogen Forms on growth, phenolics, flavonoids and antioxidant Activity in Amaranth Species. Trop. Plant Res. 2017, 4, 81–89. [Google Scholar] [CrossRef]
- Stefaniak, J.; Przybył, J.L.; Latocha, P.; Łata, B. Bioactive Compounds, Total Antioxidant Capacity and Yield of Kiwiberry Fruit under Different Nitrogen Regimes in Field Conditions. J. Sci. Food Agric. 2020, 100, 3832–3840. [Google Scholar] [CrossRef] [PubMed]
- Collado-González, J.; Piñero, M.C.; Otalora, G.; López-Marín, J.; del Amor, F.M. Plant Growth-Promoting Bacteria as affected by N availability as a suitable strategy to enhance the nutritional composition of lamb’s lettuce affected by global warming. Food Chem. 2023, 426, 136559. [Google Scholar] [CrossRef]
- Consentino, B.B.; Aprile, S.; Rouphael, Y.; Ntatsi, G.; De Pasquale, C.; Iapichino, G.; Alibrandi, P.; Sabatino, L. Application of PGPB Combined with Variable N Doses Affects Growth, Yield-Related Traits, N-Fertilizer Efficiency and Nutritional Status of Lettuce Grown under Controlled Condition. Agronomy 2022, 12, 236. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, S.; Liang, Y.; Li, B.; Ma, S.; Wang, Z.; Ma, B.; Li, M. Nitrogen Levels Regulate Sugar Metabolism and Transport in the Shoot Tips of Crabapple Plants. Front. Plant Sci. 2021, 12, 626149. [Google Scholar] [CrossRef]
- Razmjooei, Z.; Etemadi, M.; Eshghi, S.; Ramezanian, A.; Abarghuei, F.M.; Alizargar, J. Potential role of foliar application of Azotobacter on growth, nutritional value and quality of Lettuce under different nitrogen levels. Plants 2022, 11, 406. [Google Scholar] [CrossRef]
- Schiavon, M.; Nardi, S.; dalla Vecchia, F.; Ertani, A. Selenium Biofortification in the 21st Century: Status and Challenges for Healthy Human Nutrition. Plant Soil 2020, 453, 245–270. [Google Scholar] [CrossRef]
- Genchi, G.; Lauria, G.; Catalano, A.; Sinicropi, M.S.; Carocci, A. Biological activity of selenium and its impact on human health. Int. J. Mol. Sci. 2023, 24, 2633. [Google Scholar] [CrossRef]
- De Oliveira Maia, M.; Batista, B.; Sousa, M.P.; de Souza, L.M.; Maia, C.S.C. Selenium and thyroid cancer: A systematic review. Nutr. Cancer 2020, 72, 1255–1263. [Google Scholar] [CrossRef]
- Ganjouii, F.A.; Nasibi, F.; Kalantari, K.M.; Mousavi, E.A. Effect of Seed Priming with Selenium Nanoparticles and Plant Growth Promoting Rhizobacteria on Improving Quinoa Seedling Growth under Salinity Stress. J. Plant Process Funct. 2023, 11, 65–74. [Google Scholar]
- Cunha, M.; Oliveira, L.C.A.; Mendes, N.A.C.; Silva, V.M.; Vicente, E.F.; dos Reis, A.R. Selenium Increases Photosynthetic Pigments, Flavonoid Biosynthesis, Nodulation, and Growth of Soybean Plants (Glycine max L.). J. Soil Sci. Plant Nutr. 2023, 23, 1397–1407. [Google Scholar] [CrossRef]
- Piñero, M.C.; Otálora, G.; Collado-González, J.; López-Marín, J.; del Amor, F.M. Effects of selenium on the chlorophylls, gas exchange, antioxidant activity and amino acid composition of lettuce grown under an aquaponics system. Horticulturae 2022, 8, 30. [Google Scholar] [CrossRef]
- Balibrea, M.E.; Cuartero, J.; Bolarín, M.C.; Pérez-Alfocea, F. Sucrolytic Activities during Fruit Development of Lycopersicon Genotypes Differing in Tolerance to Salinity. Physiol. Plant. 2003, 118, 38–46. [Google Scholar] [CrossRef]
- Balestrasse, K.B.; Gallego, S.M.; Tomaro, M.L. Aluminium Stress Affects Nitrogen Fixation and Assimilation in Soybean (Glycine max L.). Plant Growth Regul. 2006, 48, 271–281. [Google Scholar]
- Guerra, N.; Carrozzi, L.; Goñi, G.; Roura, S.; Yommi, A. Quality characterization of celery (Apium graveolens L.) by plant zones and two harvest dates. J. Food Sci. 2010, 75, S327–S332. [Google Scholar] [CrossRef]
- Consentino, B.B.; Virga, G.; Giuseppe, G.; Placa, L.; Sabatino, L.; Rouphael, Y.; Ntatsi, G.; Iapichino, G.; La Bella, S.; Mauro, R.P.; et al. Celery (Apium graveolens L.) Performances as Subjected to Different Sources of Protein Hydrolysates. Plants 2020, 9, 1633. [Google Scholar] [CrossRef]
- Jarquín-Rosales, D.; Raymundo Enríquez-Del Valle, J.; Alpuche, J.; Rodríguez-Ortiz, G. The Effects of Fertirrigation and ‘Azospirillum brasilense’ inoculation on Photosynthetic Compounds of ‘Agave angustifolia’. Aust. J. Crop Sci. 2022, 16, 162–168. [Google Scholar] [CrossRef]
- Hong, J.; Xu, F.; Chen, G.; Huang, X.; Wang, S.; Du, L.; Ding, G. Evaluation of the Effects of Nitrogen, Phosphorus, and Potassium Applications on the Growth, Yield, and Quality of Lettuce (Lactuca sativa L.). Agronomy 2022, 12, 2477. [Google Scholar] [CrossRef]
- Kalembasa, S.; Malinowska, E.; Kalembasa, D.; Symanowicz, B.; Pakula, K. Effect of foliar fertilization with Tytanit on the content of selected macroelements and sodium in celery. J. Elem. 2014, 19, 683–696. [Google Scholar] [CrossRef]
- Siddiqui, H.; Sami, F.; Faizan, M.; Faraz, A.; Hayat, S. Brassinosteroid Mediated Regulation of Photosynthesis in Plants. In Brassinosteroids: Plant Growth and Development; Springer: Singapore, 2019; pp. 185–217. [Google Scholar]
- Ikiz, B.; Dasgan, H.; Gruda, N.S. Utilizing the power of plant growth promoting rhizobacteria on reducing mineral fertilizer, improved yield, and nutritional quality of Batavia lettuce in a floating culture. Sci. Rep. 2024, 14, 1616. [Google Scholar] [CrossRef]
- Hungria, M.; Ribeiro, R.A.; Nogueira, M.A. Draft Genome Sequences of Azospirillum brasilense Strains Ab-V5 and Ab-V6, Commercially Used in Inoculants for Grasses and Legumes in Brazil. Genome Announc. 2018, 6, 10–1128. [Google Scholar] [CrossRef]
- Puccinelli, M.; Malorgio, F.; Pintimalli, L.; Rosellini, I.; Pezzarossa, B. Biofortification of Lettuce and Basil Seedlings to Produce Selenium Enriched Leafy Vegetables. Horticulturae 2022, 8, 801. [Google Scholar] [CrossRef]
- Bian, Z.H.; Bo, L.E.I.; Cheng, R.F.; Yu, W.A.N.G.; Tao, L.I.; Yang, Q.C. Selenium distribution and nitrate metabolism in hydroponic lettuce (Lactuca sativa L.): Effects of selenium forms and light spectra. J. Integr. Agric. 2020, 19, 133–144. [Google Scholar]
- Pannico, A.; El-Nakhel, C.; Graziani, G.; Kyriacou, M.C.; Giordano, M.; Soteriou, G.A.; Zarrelli, A.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Selenium Biofortification Impacts the Nutritive Value, Polyphenolic Content, and Bioactive Constitution of Variable Microgreens Genotypes. Antioxidants 2020, 9, 272. [Google Scholar] [CrossRef]
- Teixeira, L.S.; Pimenta, T.M.; Brito, F.A.L.; Malheiros, R.S.P.; Arruda, R.S.; Araújo, W.L.; Ribeiro, D.M. Selenium Uptake and Grain Nutritional Quality Are Affected by Nitrogen Fertilization in Rice (Oryza sativa L.). Plant Cell Rep. 2021, 40, 871–880. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, S.; Jiang, Z.; Wang, Y.; Zhang, Z. Selenium Biofortification Modulates Plant Growth, Microelement and Heavy Metal Concentrations, Selenium Uptake, and Accumulation in Black-Grained Wheat. Front. Plant Sci. 2021, 12, 748523. [Google Scholar] [CrossRef]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Katrine Knutsen, H.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; et al. Dietary reference values for sodium. EFSA J. 2019, 17, e05778. [Google Scholar]
- Muiesan, M.L.; Buso, G.; Agabiti Rosei, C. Less Sodium and More Potassium to Reduce Cardiovascular Risk. Eur. Heart J. Suppl. 2023, 25, B108–B110. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA); Turck, D.; Bresson, J.L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.; et al. Dietary reference values for potassium. EFSA J. 2016, 14, e04592. [Google Scholar]
- Zhao, M.; Lin, Y.; Chen, H. Improving Nutritional Quality of Rice for Human Health. Theor. Appl. Genet. 2020, 133, 1397–1413. [Google Scholar] [CrossRef]
- Silva, C.S.; Moutinho, C.; Ferreira da Vinha, A.; Matos, C. Trace Minerals in Human Health: Iron, Zinc, Copper, Manganese and Fluorine. Int. J. Sci. Res. Methodol. 2019, 13, 57–80. [Google Scholar]
- Li, M.; Wang, Y.; Wei, X.; Wang, Z.; Wang, C.; Du, X.; Lin, Y.; Zhang, Y.; Wang, Y.; He, W.; et al. Effects of pretreatment and freezing storage on the bioactive components and antioxidant activity of two kinds of celery after postharvest. Food Chem. 2023, 18, 100655. [Google Scholar] [CrossRef]
- Mezeyova, I.; Hegedűsová, A.; Mezey, J.; Šlosár, M. Evaluation of Quantitative and Qualitative Characteristics of Selected Celery (Apium graveolens var. dulce) Varieties in the Context of Juices Production. Potravinarstvo 2018, 12, 173–179. [Google Scholar] [CrossRef]
- Al-Din, A.; Pill Baek, J.; Mady, E.; Eldekashy, M.; Craker, L. Phytochemical Analysis of Some Celery Accessions. J. Med. Act. Plants 2015, 4, 1–7. [Google Scholar]
- Becker, C.; Urlić, B.; Špika, M.J.; Kläring, H.P.; Krumbein, A.; Baldermann, S.; Ban, S.G.; Perica, S.; Schwarz, D. Nitrogen Limited Red and Green Leaf Lettuce Accumulate Flavonoid Glycosides, Caffeic Acid Derivatives, and Sucrose While Losing Chlorophylls, β-Carotene and Xanthophylls. PLoS ONE 2015, 10, e0142867. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Fernandes, Â.; Calhelha, R.C.; Di Gioia, F.; Kolovou, P.; Barros, L.; Ferreira, I.C.F.R. Chemical Composition and Bioactive Properties of Cichorium Spinosum L. in Relation to Nitrate/Ammonium Nitrogen Ratio. J. Sci. Food Agric. 2019, 99, 6741–6750. [Google Scholar] [CrossRef] [PubMed]
- Abdel Latef, A.A.H.; Abu Alhmad, M.F.; Kordrostami, M.; Abo–Baker, A.B.A.E.; Zakir, A. Inoculation with Azospirillum Lipoferum or Azotobacter Chroococcum Reinforces Maize Growth by Improving Physiological Activities Under Saline Conditions. J. Plant Growth Regul. 2020, 39, 1293–1306. [Google Scholar] [CrossRef]
- Khalofah, A.; Migdadi, H.; El-Harty, E. Antioxidant Enzymatic Activities and Growth Response of Quinoa (Chenopodium Quinoa Willd) to Exogenous Selenium Application. Plants 2021, 10, 719. [Google Scholar] [CrossRef]
- Afzal, S.; Chaudhary, N.; Singh, N.K. Role of Soluble Sugars in Metabolism and Sensing under Abiotic Stress. In Plant Growth Regulators: Signalling under Stress Conditions; Springer: Cham, Switzerland, 2021; pp. 305–334. [Google Scholar]
- Gad, D.A.M.; Abd-Elrahman, H.A.; Aboud, F.S.; Mark, C. Response Growth, Yield, and Quality of Celery Plants to Foliar Spray with some Organic Extracts. J. Plant Prod. 2023, 14, 13–20. [Google Scholar] [CrossRef]
- Tewari, R.K.; Yadav, N.; Gupta, R.; Kumar, P. Oxidative Stress under Macronutrient Deficiency in Plants. J. Soil Sci. Plant Nutr. 2021, 21, 832–859. [Google Scholar] [CrossRef]
- Pardo-Díaz, S.; Romero-Perdomo, F.; Mendoza-Labrador, J.; Delgadillo-Duran, D.; Castro-Rincon, E.; Silva, A.M.M.; Rojas-Tapias, D.F.; Cardoso, E.J.B.N.; Estrada-Bonilla, G.A. Endophytic PGPB Improves Plant Growth and Quality, and Modulates the Bacterial Community of an Intercropping System. Front. Sustain. Food Syst. 2021, 5, 715270. [Google Scholar] [CrossRef]
- Rajput, V.D.; Minkina, T.; Kumari, A.; Kumar Singh, V.; Verma, K.K.; Mandzhieva, S.; Sushkova, S.; Srivastava, S.; Keswani, C. Coping with the Challenges of Abiotic Stress in Plants: New Dimensions in the Field Application of Nanoparticles. Plants 2021, 10, 1221. [Google Scholar] [CrossRef]
- Zhang, Z.; Lynch, J.P.; Zhang, B.; Wang, Q. NPK deficiency modulates oxidative stress in plants. In Plant Macronutrient Use Efficiency; Academic Press: Cambridge, MA, USA, 2017; pp. 245–265. [Google Scholar] [CrossRef]
- de Dios Alché, J. A concise appraisal of lipid oxidation and lipoxidation in higher plants. Redox Biol. 2019, 23, 101136. [Google Scholar]
- García-Caparrós, P.; De Filippis, L.; Gul, A.; Hasanuzzaman, M.; Ozturk, M.; Altay, V.; Lao, M.T. Oxidative Stress and Antioxidant Metabolism under Adverse Environmental Conditions: A Review. Bot. Rev. 2021, 87, 421–466. [Google Scholar] [CrossRef]

| Macroelements | 100% N | 60% N | 30% N | |||||
|---|---|---|---|---|---|---|---|---|
| Not Se | Se | Not Se | Se | Not Se | Se | |||
| Na | Pet | Not B | 8.6 ± 0.4 a | 7.6 ± 0.7 b | 7.3 ± 0.7 b | 7.3 ± 0.3 b | 6.5 ± 0.4 c | 5.7 ± 0.3 d |
| PGPB | 8.3 ± 0.8 A | 7.1 ± 0.7 B | 6.8 ± 0.5 B | 5.7 ± 0.2 C* | 5.5 ± 0.3 C* | 4.7 ± 0.3 D* | ||
| L | Not B | 7.5 ± 0.5 a | 5.7 ± 0.7 c | 6.3 ± 0.3 b | 5.4 ± 0.3 c | 4.1 ± 0.4 d | 3.6 ± 0.2 d | |
| PGPB | 5.0 ± 0.6 A* | 4.7 ± 0.5 AB | 4.3 ± 0.5 B* | 3.7 ± 0.3 C* | 3.2 ± 0.4 CD | 3.1 ± 0.3 D | ||
| B–P | Not B | 10.1 ± 0.8 ab | 9.8 ± 0.9 ab | 10.7± 0.4 a | 9.3 ± 0.9 b | 7.3 ± 0.5 c | 6.9 ± 0.4 c | |
| PGPB | 10.0 ± 1.2 A | 9.1 ± 1.5 AB | 8.7 ± 0.8 B* | 8.4 ± 0.3 B | 6.2 ± 0.3 C* | 5.3 ± 0.5 C* | ||
| K | Pet | Not B | 55.2 ± 4.4 a | 57.7 ± 2.8 a | 54.2 ± 2.1 a | 55.0 ± 2.3 a | 59.4 ± 6.2 a | 61.3 ± 4.6 a |
| PGPB | 60.2 ± 5.1 A | 63.8 ± 4.3 A* | 58.0± 4.4 A | 61.5 ± 3.4 A* | 61.8 ± 5.7 A | 63.2 ± 3.2 A | ||
| L | Not B | 41.8 ± 3.7 a | 42.3 ± 4.4 a | 42.6 ± 3.1 a | 43.0 ± 1.9 a | 43.1 ± 3.7 a | 46.3 ± 1.8 a | |
| PGPB | 43.8 ± 3.3 A | 44.6 ± 2.6 A | 43.8 ± 2.9 A | 44.0 ± 1.4 A | 44.6 ± 4.2 A | 48.2 ± 3.5 A | ||
| B–P | Not B | 35.9 ± 2.3 c | 48.0 ± 3.3 a | 35.9 ± 3.6 c | 35.9 ± 3.0 c | 43.1 ± 1.7 b | 43.2 ± 2.3 b | |
| PGPB | 48.6 ± 3.8 B* | 48.8 ± 5.2 B | 36.2 ± 2.1 C | 53.3 ± 1.7 A* | 46.3 ± 2.6 B | 55.9 ± 4.2 A* | ||
| Ca | Pet | Not B | 5.4 ± 0.5 a | 3.8 ± 0.3 b | 5.9 ± 0.7 a | 4.3 ± 0.2 b | 5.9 ± 0.6 a | 5.6 ± 0.9 a |
| PGPB | 5.0 ± 0.4 B | 3.6 ± 0.4 D | 5.3 ± 0.6 AB | 4.4 ± 0.3 C | 5.8 ± 0.5 A | 5.0 ± 0.5 B | ||
| L | Not B | 12.5 ± 2.1 ab | 9.8 ± 0.5 b | 12.3 ± 2.5 ab | 11.5 ± 1.2 ab | 13.7 ± 1.1 a | 10.4 ± 0.9 ab | |
| PGPB | 12.8 ± 0.7 A | 8.8 ± 0.6 C | 13.4 ± 1.3 A | 10.0 ± 0.1 B | 12.5 ± 0.9 A | 10.1 ± 0.6 B | ||
| B–P | Not B | 25.1 ± 1.8 ab | 23.7 ± 1.0 ab | 26.6 ± 1.6 a | 22.6 ± 0.9 ab | 24.3 ± 4.0 ab | 21.2 ± 2.4 b | |
| PGPB | 23.5 ± 2.9 A | 21.5 ± 1.8 A | 23.7 ± 2.5 A* | 18.3 ± 0.2 B* | 22.6 ± 1.6 A | 15.2 ± 1.3 A* | ||
| Mg | Pet | Not B | 2.2 ± 0.2 b | 1.8 ± 0.1 c | 2.6 ± 0.4 a | 1.9 ±0.1 c | 2.5 ± 0.4 a | 1.7 ± 0.1 c |
| PGPB | 2.8 ± 0.2 A | 2.2 ± 0.1 B | 2.6 ±0.2 A | 2.4 ± 0.3 AB | 2.6 ± 0.1 A | 2.4 ± 0.4 AB* | ||
| L | Not B | 3.4 ± 0.2 a | 2.8 ± 0.2 b | 3.4 ± 0.2 a | 2.7 ± 0.2 b | 3.4 ± 0.2 a | 2.4 ± 0.2 c | |
| PGPB | 4.0 ± 0.6 A | 3.3 ± 0.1 C | 3.8 ± 0.5 AB | 3.1 ± 0.3 C | 3.4 ± 0.3 BC | 2.9 ± 0.3 C | ||
| B–P | Not B | 4.9 ± 0.3 a | 4.3 ± 0.3 b | 4.7 ± 0.2 a | 4.1 ± 0.4 b | 4.8 ± 0.2 a | 3.6 ± 0.2 c | |
| PGPB | 5.6 ± 0.3 A* | 4.7 ± 0.2 BC | 5.0 ± 0.3 B | 4.7 ± 0.4 BC | 5.0 ± 0.3 B | 4.6 ± 0.4 C* | ||
| P | Pet | Not B | 5.9 ± 0.1 b | 6.4 ± 0.2 a | 5.2 ± 0.1 c | 5.4 ± 0.3 c | 4.6 ± 0.3 d | 4.8 ± 0.2 a |
| PGPB | 5.5 ± 0.3 B | 6.2 ± 0.4 A | 4.7 ± 0.1 D* | 5.0 ± 0.3 CD | 4.7 ± 0.4 D | 5.1 ± 0.3 C | ||
| L | Not B | 7.7 ± 0.2 b | 8.6 ± 0.1 a | 6.8 ± 0.4 d | 7.2 ± 0.5 c | 5.4 ± 0.3 e | 6.7 ± 0.3 d | |
| PGPB | 7.2 ± 0.2 B | 8.7 ± 0.5 A | 6.3 ± 1.1 C | 7.2 ± 0.4 B | 5.8 ± 0.4 C | 5.9 ± 0.2 C* | ||
| B–P | Not B | 7.4 ± 0.3 b | 8.6 ± 1.1 a | 5.3 ± 0.3 c | 5.6 ± 0.2 c | 4.6 ± 0.2 d | 3.9 ± 0.2 e | |
| PGPB | 6.6 ± 0.7 B | 8.2 ± 0.1 A | 4.2 ± 0.1 D* | 6.3 ± 0.2 B* | 5.2 ± 0.2 C* | 4.6 ± 0.3 D* | ||
| Microelements | 100% N | 60% N | 30% N | |||||
|---|---|---|---|---|---|---|---|---|
| Not Se | Se | Not Se | Se | Not Se | Se | |||
| Fe | Pet | Not B | 21.0 ± 1.9 b | 13.9 ± 0.9 d | 21.8 ± 1.3 b | 15.0 ± 0.8 d | 29.5 ± 2.5 a | 17.0 ± 0.8 c |
| PGPB | 17.8 ± 0.5 E* | 20.9 ± 2.6 D* | 35.3 ± 2.9 B* | 24.3 ± 1.2 C* | 56.4 ± 2.9 A* | 25.8 ± 1.1 C* | ||
| L | Not B | 48.9 ± 0.7 c | 45.0 ± 1.0 d | 54.0 ± 1.8 b | 46.4 ± 1.8 d | 59.8 ± 1.8 a | 46.4 ± 2.6 d | |
| PGPB | 47.8 ± 1.0 E | 52.6 ± 1.6 D* | 60.0 ± 2.3 B* | 54.2 ± 2.5 D* | 81.4 ± 1.1 A* | 57.7 ± 1.8 C* | ||
| B–P | Not B | 56.1 ± 4.0 c | 51.0 ± 4.1 d | 62.8 ± 1.9 b | 51.0 ± 0.9 d | 71.9 ± 2.0 a | 53.5 ± 3.8 cd | |
| PGPB | 53.4 ± 1.7 D | 60.2 ± 2.4 BC* | 72.6 ± 4.7 A* | 63.7 ± 2.2 B* | 73.2 ± 4.0 A | 65.6 ± 2.1 B* | ||
| Cu | Pet | Not B | 2.2 ± 0.2 a | 1.6 ± 0.2 b | 2.1 ± 0.1 a | 1.7 ± 0.1 b | 2.1 ± 0.1 a | 1.3 ± 0.1 c |
| PGPB | 1.2 ± 0.2 D* | 1.8 ± 0.1 C | 2.2 ± 0.2 A | 2.1 ± 0.1 AB* | 2.2 ± 0.2 A | 1.9 ± 0.1 BC* | ||
| L | Not B | 2.8 ± 0.1 a | 2.6 ± 0.4 a | 2.8 ± 0.1 a | 2.7 ± 0.2 a | 2.8 ± 0.2 a | 2.7 ± 0.4 a | |
| PGPB | 2.4 ± 0.1 D* | 2.7 ± 0.2 C | 3.2 ± 0.1 A | 3.1 ± 0.1 AB | 3.0 ± 0.2 AB | 2.9 ± 0.1 B | ||
| B–P | Not B | 4.5 ± 0.3 b | 3.1 ± 0.1 c | 4.8 ± 0.4 ab | 2.8 ± 0.1 c | 4.9 ± 0.3 a | 3.0 ± 0.2 c | |
| PGPB | 1.6 ± 0.2 C* | 2.2 ± 0.4 C* | 7.3 ± 1.0 A* | 4.1 ± 0.1 B* | 8.0 ± 0.6 A* | 4.2 ± 0.3 B* | ||
| Mn | Pet | Not B | 35.7 ± 1.0 bc | 44.0 ± 2.3 a | 33.0 ± 1.7 cd | 41.3 ± 2.2 a | 32.2 ± 2.0 d | 37.7 ± 0.3 b |
| PGPB | 28.4 ± 1.5 B* | 30.7 ± 2.3 A* | 24.3 ± 1.6 C* | 29.3 ± 0.4 AB* | 20.0 ± 1.8 D* | 29.2 ± 2.4 AB* | ||
| L | Not B | 62.8 ± 2.0 c | 75.1 ± 2.9 a | 59.7 ± 2.5 d | 66.1 ± 2.9 e | 56. 3 ± 1.9 e | 63.4 ± 1.9 bc | |
| PGPB | 47.4 ± 2.3 BC* | 53.0 ± 2.2 A* | 44.8 ± 1.8 C* | 49.8 ± 5.7 AB* | 36.9 ± 2.8 D* | 47.6 ± 2.1 BC* | ||
| B–P | Not B | 114.2 ± 3.4 bc | 143.2 ± 13.0 a | 107.8 ± 7.2 c | 126.1 ± 2.8 b | 102.0 ± 7.5 c | 124.4 ± 5.6 b | |
| PGPB | 77.0 ± 2.2 C* | 97.6 ± 1.3 A* | 62.8 ± 5.2 D* | 86.1 ± 2.9B * | 58.3 ± 3.9 D* | 83.8 ± 2.1 B* | ||
| Zn | Pet | Not B | 15.8 ± 0.7 ab | 17.3 ± 0.7 a | 15.4 ± 0.7 ab | 16.8 ± 0.9 a | 14.5 ± 2.4 b | 15.8 ± 0.5 ab |
| PGPB | 14.1 ± 0.9 AB | 14.4 ± 0.5 A* | 13.9 ± 0.4 AB | 14.9 ± 0.4 A | 13.0 ± 1.6 B | 14.4 ± 0.9 A | ||
| L | Not B | 37.2 ± 0.9 bc | 41.8 ± 1.8 a | 36.7 ± 2.4 c | 39.4 ± 3.5 bc | 36.2 ± 2.4 c | 39.2 ± 4.0 bc | |
| PGPB | 33.7 ± 2.5 A | 35.8 ± 1.7 A* | 33.4 ± 3.0 A | 34.7 ± 3.0 A | 33.2 ± 1.8 A | 34.3 ± 2.9 A | ||
| B–P | Not B | 35.8 ± 2.5 ab | 39.9 ± 1.5 a | 35.0 ± 3.1 ab | 38.4 ± 5.1 ab | 33.9 ± 1.9 b | 36.6 ± 3.5 ab | |
| PGPB | 29.5 ± 0.8 AB* | 33.3 ± 1.6 A* | 26.8 ± 1.8 BC* | 31.7 ± 2.7 A* | 24.7 ± 2.2 C* | 30.7 ± 3.9 AB | ||
| B | Pet | Not B | 34.4 ± 2.8 b | 41.5 ± 1.6 a | 31.1 ± 3.6 b | 32.7 ± 3.2 b | 30.8 ± 2.0 b | 31.6 ± 2.8 b |
| PGPB | 22.2 ± 2.0 BC* | 27.3 ± 1.0 A* | 22.1 ± 2.4 BC* | 23.4 ± 1.3 BC* | 20.8 ± 0.7 C* | 23.7 ± 2.0 B* | ||
| L | Not B | 32.1 ± 2.7 a | 37.7 ± 5.1 a | 31.7 ± 1.4 a | 36.5 ± 3.3 a | 31.8 ± 4.4 a | 37.5 ± 3.7 a | |
| PGPB | 27.4 ± 1.5 A* | 30.5 ± 4.3 A | 27.7 ± 3.2 A* | 30.6 ± 2.1 A* | 26.5 ± 1.7 A* | 30.3 ± 3.1 A* | ||
| B–P | Not B | 20.5 ± 1.2 ab | 22.5 ± 1.7 a | 18.1 ± 1.6 bc | 22.6 ± 1.5 a | 17.5 ± 1.4 c | 22.0 ± 1.5 a | |
| PGPB | 15.7 ± 0.8 ABC* | 17.4 ± 1.1 AB* | 15.7 ± 1.0 BC* | 17.4 ± 1.0 A* | 14.9 ± 0.7 C* | 17.0 ± 1.2 AB* | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Collado-González, J.; Piñero, M.C.; Otálora Alcón, G.; López-Marín, J.; del Amor, F.M. Biofortification and Valorization of Celery byproducts Using Selenium and PGPB under Reduced Nitrogen Regimes. Foods 2024, 13, 1437. https://doi.org/10.3390/foods13101437
Collado-González J, Piñero MC, Otálora Alcón G, López-Marín J, del Amor FM. Biofortification and Valorization of Celery byproducts Using Selenium and PGPB under Reduced Nitrogen Regimes. Foods. 2024; 13(10):1437. https://doi.org/10.3390/foods13101437
Chicago/Turabian StyleCollado-González, Jacinta, María Carmen Piñero, Ginés Otálora Alcón, Josefa López-Marín, and Francisco M. del Amor. 2024. "Biofortification and Valorization of Celery byproducts Using Selenium and PGPB under Reduced Nitrogen Regimes" Foods 13, no. 10: 1437. https://doi.org/10.3390/foods13101437
APA StyleCollado-González, J., Piñero, M. C., Otálora Alcón, G., López-Marín, J., & del Amor, F. M. (2024). Biofortification and Valorization of Celery byproducts Using Selenium and PGPB under Reduced Nitrogen Regimes. Foods, 13(10), 1437. https://doi.org/10.3390/foods13101437













