Variations in Cold Resistance and Contents of Bioactive Compounds among Dendrobium officinale Kimura et Migo Strains
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Culture Conditions
2.2. Determination of Agronomic Traits
2.3. Transplanting of the Tissue-Culture-Raised Seedlings
2.4. Determination of Water and Chlorophyll Contents
2.5. Determination of Electrical Conductivity
2.6. Determination of MDA Content and CAT and POD Activities
2.7. Analysis of Expression Patterns of Cold Resistance-Related Genes
2.8. Determination of Polysaccharide Content
2.9. Determination of Flavonoid Content
2.10. Determination of Phenol Content
2.11. Statistical Analysis
3. Results
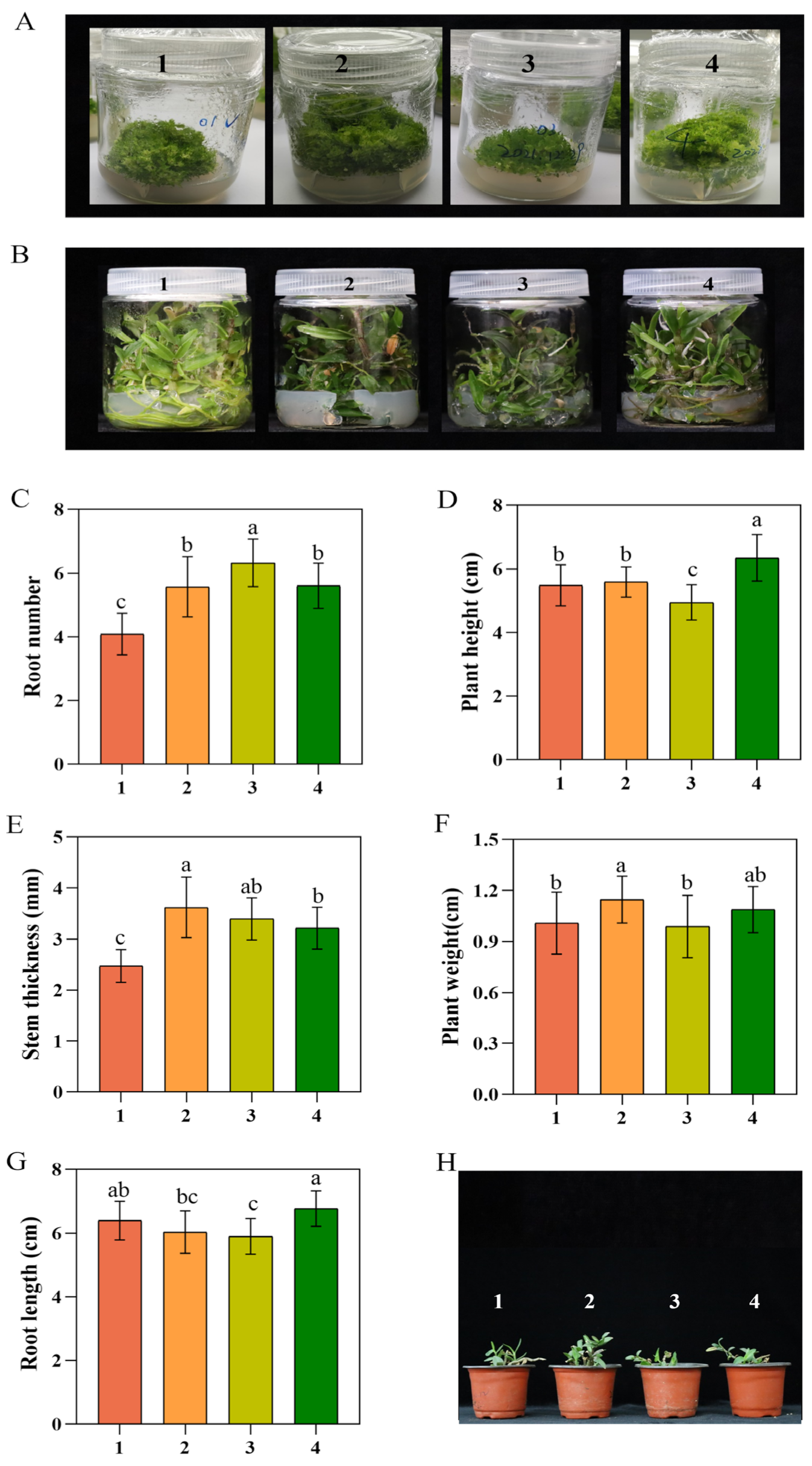
3.1. A Comparison of the Growth Abilities of the Four Strains of D. officinale
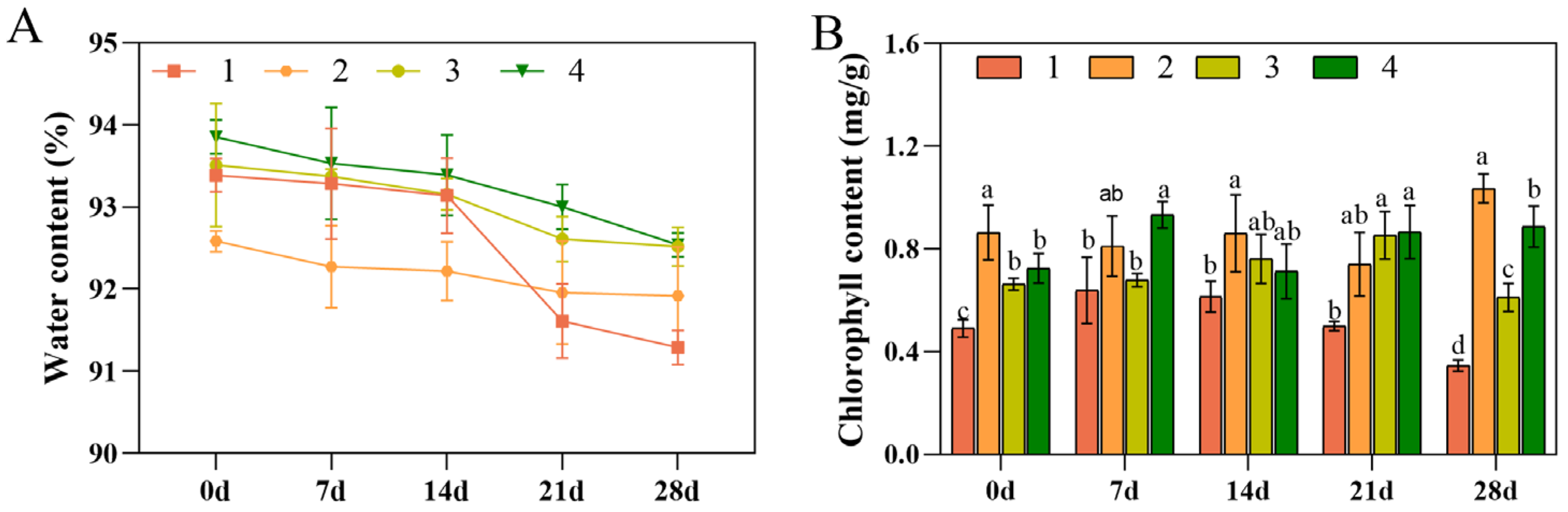
3.2. Water and Chlorophyll Contents
3.3. Conductivity and MDA Content
3.4. Determination of CAT and POD Activities
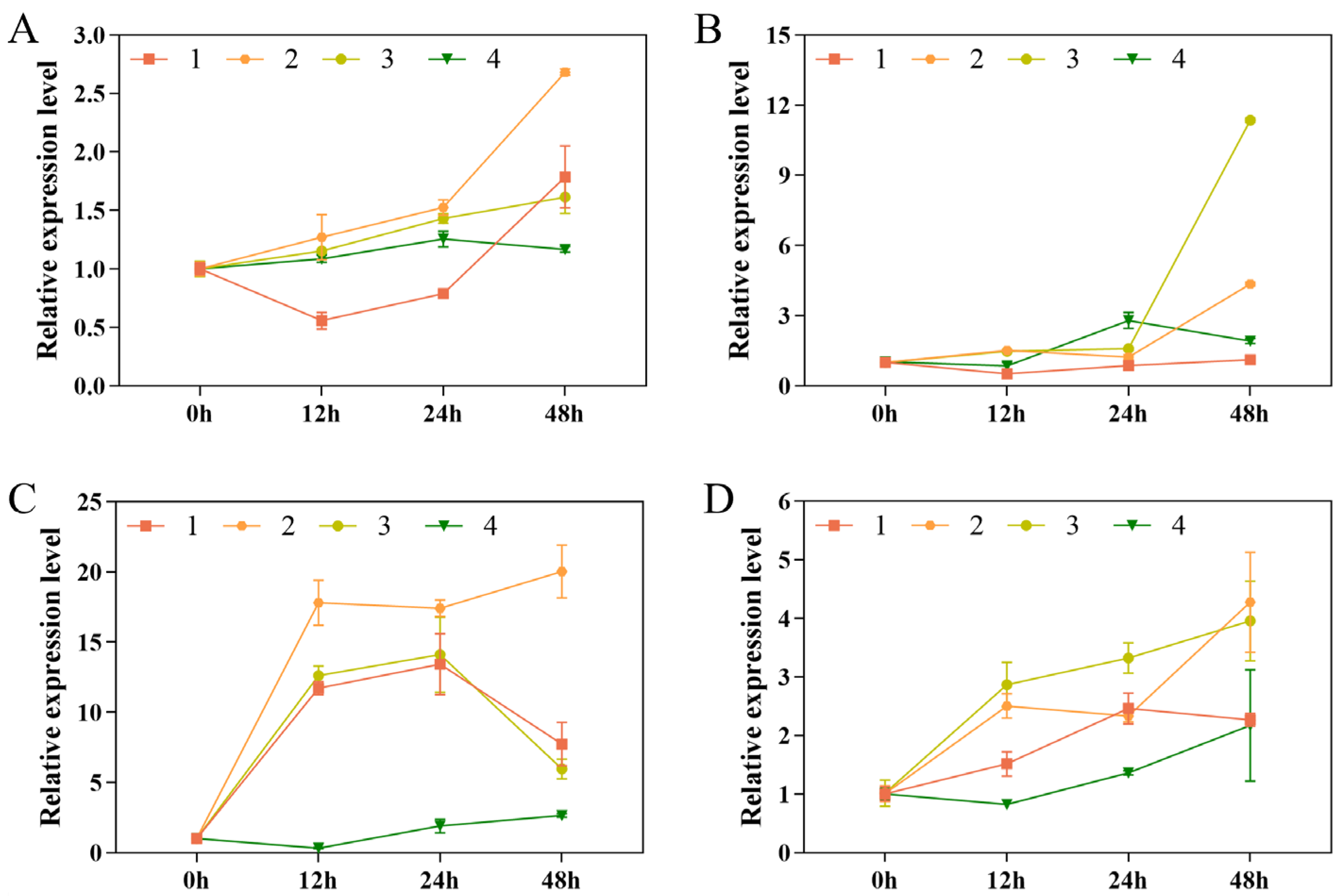
3.5. Expression Analysis of Cold Resistance-Related Genes
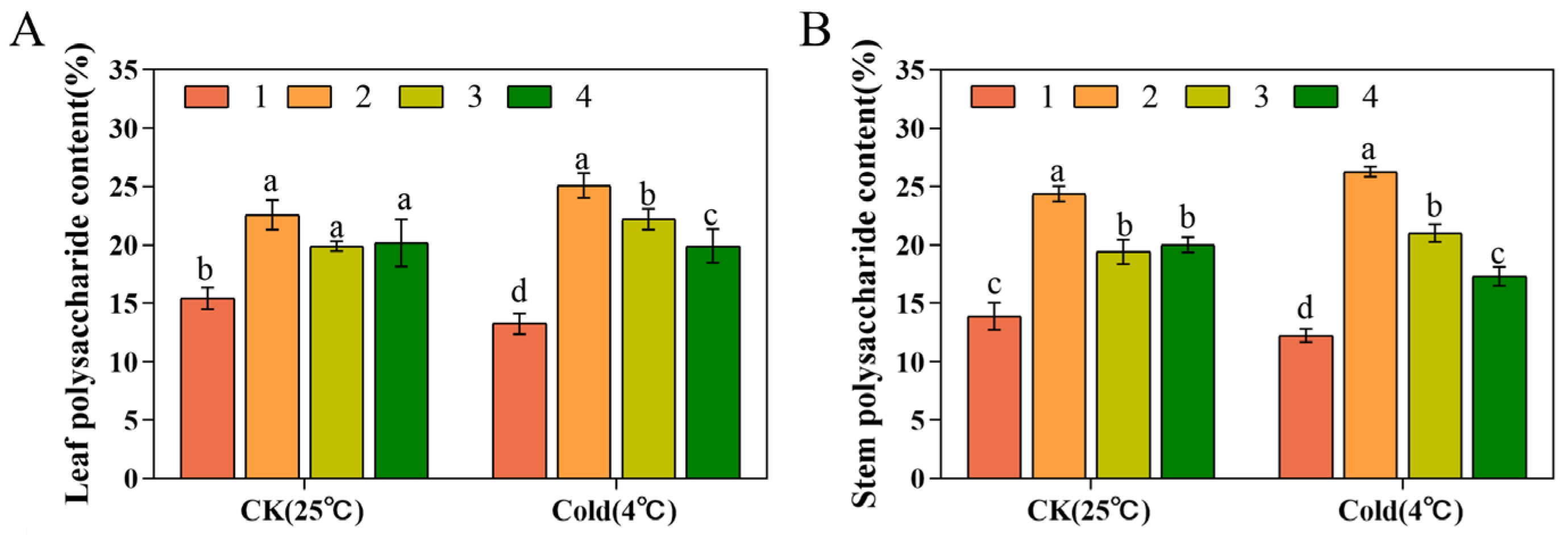
3.6. Polysaccharide Contents
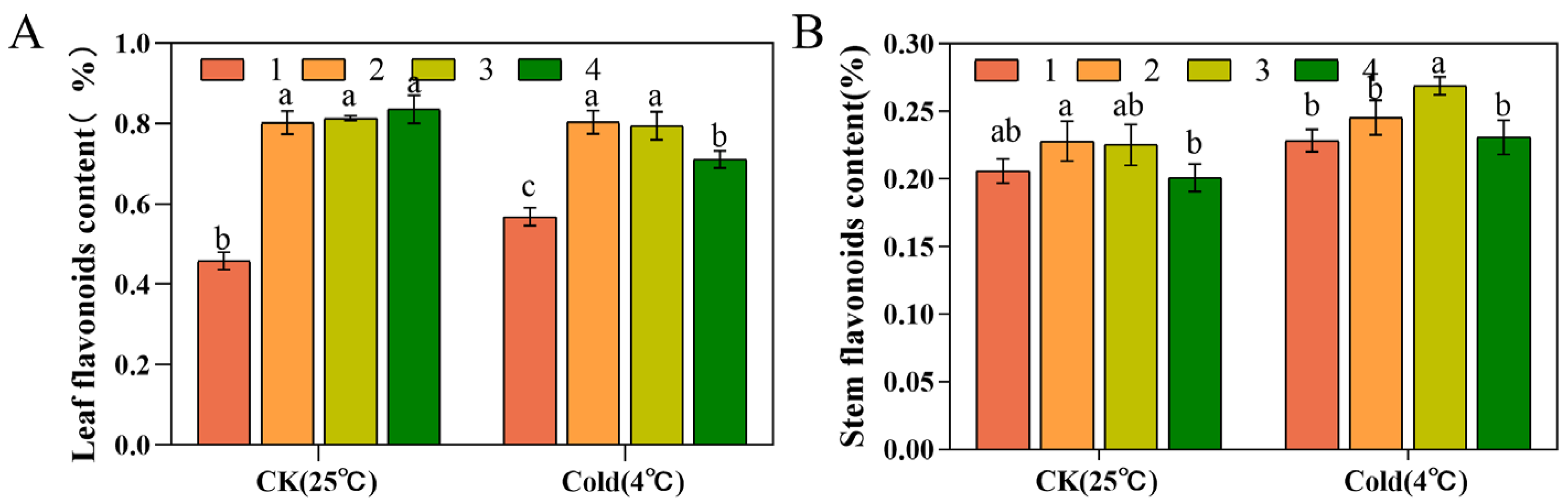
3.7. Flavonoid Contents
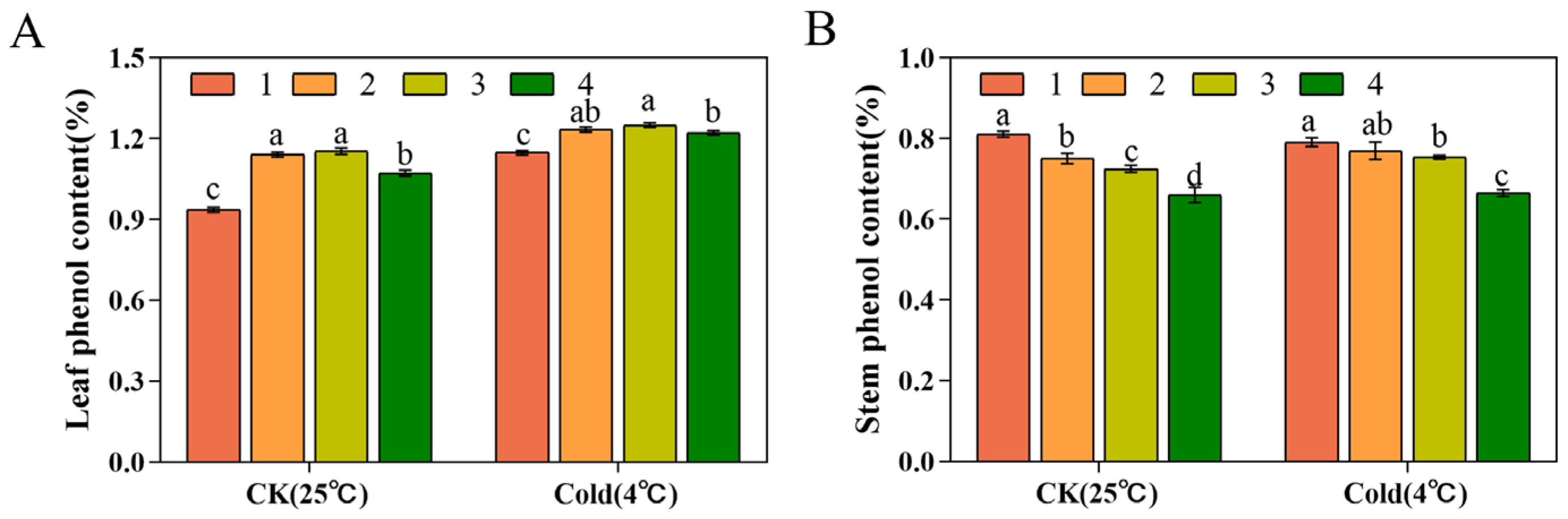
3.8. Dendrophenol Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, Y.Y.; Zhang, J.; Fu, J.H.; Zhang, P.; Qin, N.; Li, G.Q. Artificial Propagation Technology of Dendrobium officinale. North Hortic. 2021, 473, 115–123. [Google Scholar]
- Chen, H.H.; Nie, Q.X.; Hu, J.L.; Huang, X.J.; Huang, W.Q.; Nie, S.P. Metabolism amelioration of Dendrobium officinale polysaccharide on type II diabetic rats. Food Hydrocoll. 2019, 102, 105582. [Google Scholar] [CrossRef]
- Guo, Z.B.; Zhou, Y.M.; Yang, J.P.; Shao, X.M. Dendrobium candidum extract inhibits proliferation and induces apoptosis of liver cancer cells by inactivating Wnt/β-catenin signaling pathway. Biomed. Pharmacother. 2019, 110, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.Z.; Liu, B.; Zhang, D.D.; Zha, X.Q.; Pan, L.H.; Luo, J.P. Intestinal immunomodulating activity and structural characterization of a new polysaccharide from stems of Dendrobium officinale. Food Funct. 2016, 7, 2789–2799. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jo, K.; Byun, S.B.; Han, S.H.; Yu, K.W.; Suh, H.J.; Hong, K.B. Chemical and biological properties of puffed Dendrobium officinale extracts: Evaluation of antioxidant and anti-fatigue activities. J. Funct. Foods 2020, 73, 104144. [Google Scholar] [CrossRef]
- Yang, K.; Lu, T.T.; Zhan, L.H.; Zhou, C.; Zhang, N.Y.; Lei, S.S.; Wang, Y.Z.; Yang, J.S.; Yan, M.Q.; Lv, G.Y. Physicochemical characterization of polysaccharide from the leaf of Dendrobium officinale and effect on LPS induced damage in GES-1 cell. Int. J. Biol. Macromol. 2020, 149, 320–330. [Google Scholar] [CrossRef]
- Liu, C.Z.; Chen, W.; Wang, M.X.; Wang, Y.; Chen, L.Q.; Zhao, F.; Shi, Y.; Liu, H.J.; Dou, X.B.; Liu, C.; et al. Dendrobium officinale Kimura et Migo and American ginseng mixture: A Chinese herbal formulation for gut microbiota modulation. Chin. J. Nat. Med. 2020, 18, 446–459. [Google Scholar] [CrossRef]
- Yang, J.; Kuang, M.T.; Yang, L.; Huang, W.; Hu, J.M. Modern interpretation of the traditional application of Shihu—A comprehensive review on phytochemistry and pharmacology progress of Dendrobium officinale. J. Ethnopharmacol. 2022, 302, 115912. [Google Scholar] [CrossRef]
- Kim, H.E.; Han, J.E.; Lee, H.; Murthy, H.N.; Kwon, H.; Lee, G.M.; Park, S.Y. Establishment of an Efficient In Vitro Propagation of Cnidium officinale Makino and Selection of Superior Clones through Flow Cytometric Assessment of DNA Content. Genes 2022, 13, 1815. [Google Scholar] [CrossRef]
- Ming, X.J.; Feng, T.T. Research Progress on the Seedling Breeding Techniques of Dendrobium in China. J. Anhui Agric. Sci. 2010, 38, 2899–2902. [Google Scholar]
- Longchar, T.B.; Deb, C.R. Optimization of in vitro propagation protocol of Dendrobium heterocarpum Wall. ex. Lindl. and clonal genetic fidelity assessment of the regenerates: An orchid of horticultural and medicinal importance. S. Afr. J. Bot. 2022, 149, 67–78. [Google Scholar] [CrossRef]
- Chen, B.; Trueman, J.S.; Li, J.; Li, Q.Z.; Fan, H.H.; Zhang, J. Micropropagation of the Endangered Medicinal Orchid, Dendrobium officinale. Life Sci. J. 2014, 11, 9. [Google Scholar]
- Tang, H.X.; Zhao, T.W.; Sheng, Y.G.; Zheng, T.; Fu, L.Z.; Zhang, Y.S. Dendrobium officinale Kimura et Migo: A Review on Its Ethnopharmacology, Phytochemistry, Pharmacology, and Industrialization. Evid.-Based Complement. Altern. Med. 2017, 2017, 7436259. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Y.; Dou, M.M.; Zhang, Z.H.; Zhang, D.D.; Huang, C.Z. Protective effect of Dendrobium officinale polysaccharides on H2O2-induced injury in H9c2 cardiomyocytes. Biomed. Pharmacother. 2017, 94, 72–78. [Google Scholar] [CrossRef]
- Chakraborty, I.; Sen, K.I.; Mondal, S.; Rout, D.; Bhanja, S.K.; Maity, G.N.; Mondal, S.; Maity, P. Bioactive polysaccharides from natural sources: A review on the antitumor and immunomodulating activities. Biocatal. Agric. Biotechnol. 2019, 22, 101425. [Google Scholar] [CrossRef]
- Sun, C.B.; Zhang, N.; Xu, G.P.; Jiang, P.; Huang, S.P.; Zhao, Q.; He, Y.F. Anti-tumor and immunomodulation activity of polysaccharides from Dendrobium officinale in S180 tumor-bearing mice. J. Funct. Foods 2022, 94, 105105. [Google Scholar] [CrossRef]
- Zeng, X.J.; Shen, T.; Hu, J.X.; Wang, Y.F.; Mu, B.Q.; Zong, X.L.; Li, T. Characteristics of Biomass Allocation and Polysaccharide Content in Stem and Leaf of Different Dendrobium officinale Strains. Southwest China J. Agric. Sci. 2018, 31, 57–61. [Google Scholar]
- Ke, Y.; Zhan, L.; Lu, T.; Zhou, C.; Chen, X.; Dong, Y.; Lv, G.; Chen, S. Polysaccharides of Dendrobium officinale Kimura & Migo Leaves Protect Against Ethanol-Induced Gastric Mucosal Injuryviathe AMPK/mTOR Signaling Pathwayin Vitroandvivo. Front. Pharmacol. 2020, 11, 526349. [Google Scholar]
- Lv, M.; Liang, Q.Q.; He, X.F.; Du, X.C.; Liu, Y.H.; Liu, Y.; Fang, C.Y. Hypoglycemic effects of Dendrobium officinale leaves. Front. Pharmacol. 2023, 14, 1163028. [Google Scholar] [CrossRef]
- Li, Y.B.; Yang, X.Q.; Ren, G.X.; Feng, Y.Z.; Zhang, Q.; Li, P. Changes analysis in physiological properties of several gramineous grass species and cold-resistance comparison on under cold stress. Acta Ecol. Sin. 2009, 29, 1341–1347. [Google Scholar]
- Li, R.X. Resistance Mechanism Study Screening of Cold Resistant Evergreen Magnolaceae Plant and Cold. Ph.D. Thesis, Central South University of Forestry and Technology, Changsha, China, 2018. [Google Scholar]
- Shen, Y.C. Research on Cold Resistance Characterisitics of Dendrobium officinale and Related Bacteria. Master’s Thesis, Zhejiang Sci-Tech University, Hangzhou, China, 2019. [Google Scholar]
- Jiang, Y.; Zhu, Y.Q.; Gao, Y.H.; Si, J.P. Cloning and expression analysis of WRKY5 gene in Dendrobium officinale. Chin. Tradi. Herbal Drugs 2016, 47, 301–308. [Google Scholar]
- Li, Y.; Liu, B.T.; Lai, S.H.; Luo, W.H.; Yu, B.Y.; Liu, Y.J. Cloning and Expression of DcbHLH14 from Dendrobium catenatum Lindl. Fujian J. Agric. Sci. 2022, 37, 1145–1155. [Google Scholar]
- Zhang, X.Y. The Tissue Culture Optimization of Dendrobium officinale and DNA Barcode Identification System Construction. Master’s Thesis, China Jiliang University, Hangzhou, China, 2016. [Google Scholar]
- Knudson, L.L.; Tibbitts, T.W.; Edwards, G.E. Measurement of Ozone Injury by Determination of Leaf Chlorophyll Concentration. Plant. Physiol. 1977, 60, 606–608. [Google Scholar] [CrossRef] [PubMed]
- Li, D.B. Cloning And Expression of Cold Resistance Related Genes in Dendrobium officinale Under Low Temperature Stress. Master’s Thesis, Zhejiang A&F University, Hangzhou, China, 2013. [Google Scholar]
- Zhang, T.T.; Li, Y.X.; Zhang, D.Y.; Kang, Y.Y.; Wang, J.; Song, X.Q.; Zhou, Y. Genome-Wide Identification and Expression Analyses of PP2C Gene Family in Dendrobium catenatum. Acta Hortic. Sin. 2021, 48, 2458–2470. [Google Scholar]
- Sheng, K.; Gao, Y.H.; Si, J.P.; Zhu, Y.Q. Cloning and Expression Analysis of DoCDPK Genes in Dendrobium officinale. Acta Hortic. Sin. 2016, 43, 2412–2422. [Google Scholar]
- Sheng, K.; Gao, Y.H.; Si, J.P.; Zhu, Y.Q.; Liu, J.J. Cloning and Expression Analysis of DoCDPK6 and Promoter in Dendrobium officinale. J. Agric. Biotechnol. 2017, 25, 588–598. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F.J.A.C. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Li, Z.Q. A Study on the Main Medicinal Components and Hepatoprotective and Anti-Inflammatory Effects of Huoshan Dendrobium with Different Growth Years. Master’s Thesis, Jiangsu University, Zhenjiang, China, 2020. [Google Scholar]
- Li, J.; Ma, X.X.; Li, X.X.; Zhang, J.Z. Determination of Total Phenols in Dendrobii Officinalis Caulis. Chin. J. ETMF 2013, 19, 60–62. [Google Scholar]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- McWilliam, J.R.; Naylor, A.W. Temperature and plant adaptation. I. Interaction of temperature and light in the synthesis of chlorophyll in corn. Plant Physiol. 1967, 42, 1711–1715. [Google Scholar] [CrossRef]
- Li, S.; Yang, Y.; Zhang, Q.; Liu, N.; Xu, Q.; Hu, L. Differential physiological and metabolic response to low temperature in two zoysiagrass genotypes native to high and low latitude. PLoS ONE 2018, 13, e0198885. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Jia, D.; Gao, Z.; Dong, Q.; He, L. Physiological responses to low temperature in spring and winter wheat varieties. J. Sci. Food Agric. 2016, 96, 1967–1973. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.B.; Cape, J.N.; Fowler, D. Quantification of frost damage in plant-tissues by rates of electrolyte leakage. New Phytol. 1989, 113, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Santanoo, S.; Vongcharoen, K.; Banterng, P.; Vorasoot, N.; Jogloy, S.; Roytrakul, S.; Theerakulpisut, P. Physiological and Proteomic Responses of Cassava to Short-Term Extreme Cool and Hot Temperature. Plants 2022, 11, 2307. [Google Scholar] [CrossRef]
- Prasad, T.K.; Anderson, M.D.; Martin, B.A.; Stewart, C.R. Evidence for Chilling-Induced Oxidative Stress in Maize Seedlings and a Regulatory Role for Hydrogen Peroxide. Plant Cell 1994, 6, 65–74. [Google Scholar] [CrossRef]
- Rajput, V.D.; Harish; Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; et al. Recent Developments in Enzymatic Antioxidant Defence Mechanism in Plants with Special Reference to Abiotic Stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef]
- Shao, W.P. Study on Cold Ressistance of Evergreen Broad-Leaved Plants. Master’s Thesis, Shandong Agricultural University, Tai’an, China, 2010. [Google Scholar]
- Sun, Y.L. Study on the Effects of Low Temperature on Proline Content and Electrical Conductivity of Leaves of 2 Koelreuteria Species. Anhui For. Sci. Technol. 2022, 48, 41–42. [Google Scholar]
- Campos, P.S.; Quartin, V.; Ramalho, J.C.; Nunes, M.A. Electrolyte leake and lipiddegradation account for cold sensitivity in leaves of Coffea sp, plants. Plant Physiol. 2003, 160, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.L. Effects of Low Temperature on Physiological Characterristics of Different Genotypes Seedling of Dendrobium Candidum. Master’s Thesis, Zhejiang University, Hangzhou, China, 2012. [Google Scholar]
- Li, J. Salicylic Acid Mediated Cold Resistance Mechanism of Dendrobium Officinale by Sphingomonas Paucimobilis ZJSH1. Master’s Thesis, Zhejiang Sci-Tech University, Hangzhou, China, 2021. [Google Scholar]
- Li, H.C.; Guo, X.L.; Wang, D.M.; Li, G.L. Responses of HSP70 gene expression to temperature stresses in maize (Zea mays L.). J. Agric. Univ. Hebei 2010, 33, 12–15+25. [Google Scholar]
- Wu, Y.H. Molecular Marker Assisted Selection for Cold Resistance Dendrobium Candida. Master’s Thesis, Huaqiao University, Quanzhou, China, 2018. [Google Scholar]
- Zhang, B.H.; Chen, N.Z.; Peng, X.J.; Shen, S.H. Identification of the PP2C gene family in paper mulberry (Broussonetia papyrifera) and its roles in the regulation mechanism of the response to cold stress. Biotechnol. Lett. 2021, 243, 1089–1102. [Google Scholar] [CrossRef]
- Zuo, R.; Hu, R.B.; Chai, G.H.; Xu, M.; Qi, G.; Kong, Y.; Zhou, G. Genome-wide identification, classification, and expression analysis of CDPK and its closely related gene families in poplar (Populous trichocarpa). Mol. Biol. Rep. 2012, 40, 2645–2662. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, X.; Zhu, X.; Hua, Y. Chemical Constituents, Bioactivities, and Pharmacological Mechanisms of Dendrobium officinale: A Review of the Past Decade. J. Agric. Food Chem. 2023, 71, 14870–14889. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.H.; Wu, J.J.; Li, X.F.; Lu, J.M.; Wu, W.; Sun, Y.Q.; Zhu, B.; Qin, L.P. Isolation, structural properties, bioactivities of polysaccharides from Dendrobium officinale Kimura et. Migo: A review. Int. J. Biol. Macromol. 2021, 84, 1000–1013. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.W.; Shen, Y.H. Analysis of Main Functional Components and Antioxidant Activity of Dendrobium Candidum Leavesin Different Growth Years. Jiangxi Chem. Ind. 2021, 37, 60–62. [Google Scholar]
- Lu, L.P.; Wang, H.; Xiong, L.; Yang, T.; Pan, T.; Tang, J.W.; Pan, W. Toxicological Evaluation of Dendrobium Officinale Leaves. Chin. J. Ethn. Ethn. 2022, 31, 12–16. [Google Scholar]
- Hu, Y.; Zhao, M.; Qiu, Y.X.; Ye, D.B.; Liu, Y.Q.; Zhang, C.F.; Wang, H.B.; Chen, J.M. Research Progress on Dendrobii Officinale Caulis as Medicinal and Edible Traditional Chinese Medicine. J. Nanjing Univ. Tradit. Chin. Med. 2024, 1, 94–108. [Google Scholar]
- Tang, L.; Li, J.; Long, H.; Li, G. Content differences of total polysaccharides, total alkaloids and total flavonoids from stems and leaves of Dendrobium candidum at different growth ages. Guangdong Agric. Sci. 2015, 42, 17–21. [Google Scholar]
- Li, C.L. Molecular Cloning Flavoniod-Related Genes from Tartary Buckwheat and Their Respponses under UV-B, Cold, and Drought Stresses in Sprouts, and Enzymatic Activity Analysis of the Recombinant FtFLS Proteins. Ph.D. Thesis, Sichuan Agricultural University, Ya’an, China, 2013. [Google Scholar]



| Primer Name | Sequence (5′-3′) | References |
|---|---|---|
| β-actin-F | TTGTGTTGGATTCTGGTGATGGTGT | [27] |
| β-actin-R | TTTCCCGTTCTGCTGTTGTTGTGAA | |
| HSP70-F | TCTTACGACCTCTTTCTCAAGCCCT | [27] |
| HSP70-R | AATACCTATCGCTGGACCCTCTCCC | |
| DcPP2C5-F | CTTTGAAGATGTTGAGTTCCCAC | [28] |
| DcPP2C5-R | TGCTTTCCGCACTGAATCCT | |
| DoCDPK1-F | GAAAGATGCCGCTGTTGTAGTAAGA | [29] |
| DoCDPK1-R | CAATGTCGTGAAATCTCTTCTCTGG | |
| DoCDPK6-F | AAAGCGGTATGGTATTGAGGCAGAT | [30] |
| DoCDPK6-R | AAAATACCTTGTTCGGACTCTGCCC |
| Strain | 1 Taizhou | 2 Xianju | 3 Fujian | 4 Wenzhou |
|---|---|---|---|---|
| Survival rate (%) | 56 | 77 | 68 | 70 |
| Strain | Plant Height (cm) | Stem Thickness (mm) | Leaf Length (cm) |
|---|---|---|---|
| 1 Taizhou | 5.03 ± 0.70 b | 2.25 ± 0.24 b | 3.31 ± 0.30 c |
| 2 Xianju | 6.21 ± 0.59 a | 3.46 ± 0.31 a | 4.55 ± 0.38 a |
| 3 Fujian | 5.28 ± 0.76 a,b | 3.17 ± 0.38 a | 3.32 ± 0.33 c |
| 4 Wenzhou | 6.11 ± 0.74 a | 3.28 ± 0.40 a | 3.75 ± 0.30 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, H.; Bao, H.; Wang, Y.; Wang, F.; Jiang, Q.; Li, H.; Ding, Y.; Zhu, C. Variations in Cold Resistance and Contents of Bioactive Compounds among Dendrobium officinale Kimura et Migo Strains. Foods 2024, 13, 1467. https://doi.org/10.3390/foods13101467
Bao H, Bao H, Wang Y, Wang F, Jiang Q, Li H, Ding Y, Zhu C. Variations in Cold Resistance and Contents of Bioactive Compounds among Dendrobium officinale Kimura et Migo Strains. Foods. 2024; 13(10):1467. https://doi.org/10.3390/foods13101467
Chicago/Turabian StyleBao, Hexigeduleng, Hainan Bao, Yu Wang, Feijuan Wang, Qiong Jiang, Hua Li, Yanfei Ding, and Cheng Zhu. 2024. "Variations in Cold Resistance and Contents of Bioactive Compounds among Dendrobium officinale Kimura et Migo Strains" Foods 13, no. 10: 1467. https://doi.org/10.3390/foods13101467





