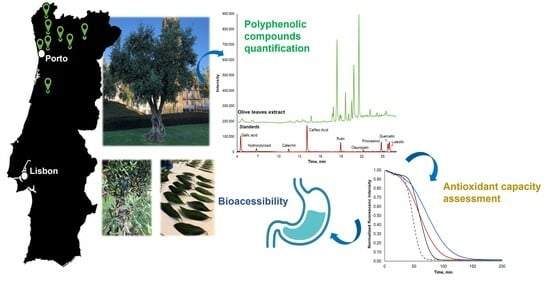
Olive Oil Waste as a Source of Functional Food Ingredients: Assessing Polyphenolic Content and Antioxidant Activity in Olive Leaves
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Solutions
2.2. Olive Leaves Samples
2.3. Preparation of Olive Leaves Extracts
2.4. Chromatographic Conditions
2.5. Antioxidant Assays
2.5.1. CUPRAC: Cupric-Reducing Antioxidant Capacity Assay
2.5.2. Folin–Ciocalteu-Reducing Assay
2.5.3. DPPH: 2,2-Diphenyl-1-picrylhydrazyl Radical Scavenging Capacity Assay
2.5.4. ABTS: 2,2′-Azinobis-(3-ethylbenzothiazoline-6-sulphonate) Radical Cation Scavenging Capacity Assay
2.5.5. ORAC: Oxygen Radical Absorbance Capacity Assay
2.5.6. Samples Theoretical Trolox Equivalent Antioxidant Capacity (TEAC) Values
2.6. Bioaccessibility Assays
2.7. Statistical Analysis
3. Results and Discussion
3.1. Selection of the Solid–liquid Extraction Solvent
3.2. Sample Analysis
3.2.1. Quantification of Polyphenols
3.2.2. Antioxidant Activity
3.3. Stability of Lyophilization Process and Bioaccessibility Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Bruno, A.; Romeo, R.; Piscopo, A.; Poiana, M. Antioxidant quantification in different portions obtained during olive oil extraction process in an olive oil press mill. J. Sci. Food Agric. 2021, 101, 1119–1126. [Google Scholar] [CrossRef]
- Abdallah, M.; Marzocco, S.; Adesso, S.; Zarrouk, M.; Guerfel, M. Olive oil polyphenols extracts inhibit inflammatory markers in J774A.1 murine macrophages and scavenge free radicals. Acta Histochem. 2018, 120, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bozzetto, L.; Alderisio, A.; Clemente, G.; Giorgini, M.; Barone, F.; Griffo, E.; Costabile, G.; Vetrani, C.; Cipriano, P.; Giacco, A.; et al. Gastrointestinal effects of extra-virgin olive oil associated with lower postprandial glycemia in type 1 diabetes. Clin. Nutr. 2019, 38, 2645–2651. [Google Scholar] [CrossRef] [PubMed]
- Bilal, R.M.; Liu, C.; Zhao, H.; Wang, Y.; Farag, M.R.; Alagawany, M.; Hassan, F.-u.; Elnesr, S.S.; Elwan, H.A.; Qiu, H. Olive oil: Nutritional applications, beneficial health aspects and its prospective application in poultry production. Front. Pharmacol. 2021, 12, 723040. [Google Scholar] [CrossRef] [PubMed]
- Kaddoumi, A.; Denney, T.S.; Deshpande, G.; Robinson, J.L.; Beyers, R.J.; Redden, D.T.; Praticò, D.; Kyriakides, T.C.; Lu, B.N.; Kirby, A.N.; et al. Extra-Virgin Olive Oil Enhances the Blood-Brain Barrier Function in Mild Cognitive Impairment: A Randomized Controlled Trial. Nutrients 2022, 14, 5102. [Google Scholar] [CrossRef]
- Amel, N.; Wafa, T.; Samia, D.; Yousra, B.; Issam, C.; Cheraif, I.; Attia, N.; Mohamed, H. Extra virgin olive oil modulates brain docosahexaenoic acid level and oxidative damage caused by 2,4-Dichlorophenoxyacetic acid in rats. J. Food Sci. Technol.-Mysore 2016, 53, 1454–1464. [Google Scholar] [CrossRef]
- European Commission. Olives, the Source of “Liquid Gold,” Offer More Riches to Unlock. Available online: https://ec.europa.eu/research-and-innovation/en/horizon-magazine/olives-source-liquid-gold-offer-more-riches-unlock (accessed on 8 December 2023).
- Selim, S.; Albqmi, M.; Al-Sanea, M.M.; Alnusaire, T.S.; Almuhayawi, M.S.; AbdElgawad, H.; Al Jaouni, S.K.; Elkelish, A.; Hussein, S.; Warrad, M.; et al. Valorizing the usage of olive leaves, bioactive compounds, biological activities, and food applications: A comprehensive review. Front. Nutr. 2022, 9, 1008349. [Google Scholar] [CrossRef]
- European Commission. Olive Oil in the EU. Available online: https://agriculture.ec.europa.eu/farming/crop-productions-and-plant-based-products/olive-oil_en (accessed on 30 November 2023).
- Abbattista, R.; Ventura, G.; Calvano, C.D.; Cataldi, T.R.; Losito, I. Bioactive compounds in waste by-products from olive oil production: Applications and structural characterization by mass spectrometry techniques. Foods 2021, 10, 1236. [Google Scholar] [CrossRef]
- Teatro Naturale. Il Portogallo Olivicolo è il Paese Emergente nell’Europa dell’olio di Oliva. Available online: https://www.teatronaturale.it/tracce/mondo/36891-il-portogallo-olivicolo-e-forma-emergente-nell-europa-dell-olio-di-oliva.htm (accessed on 9 December 2023).
- Olive Oil Times. Portugal May Be the Third-Largest Olive Oil Producer by 2030. Available online: https://www.oliveoiltimes.com/production/portugal-may-be-the-third-largest-olive-oil-producer-by-2030/74445 (accessed on 3 July 2023).
- Tapia-Quirós, P.; Montenegro-Landívar, M.F.; Reig, M.; Vecino, X.; Cortina, J.L.; Saurina, J.; Granados, M. Recovery of polyphenols from agri-food by-products: The olive oil and winery industries cases. Foods 2022, 11, 362. [Google Scholar] [CrossRef]
- Olive Oil Times. Record Yields for Portugal in the 2021/22 Crop Year. Available online: https://www.oliveoiltimes.com/production/record-yields-for-portugal/105753 (accessed on 20 November 2023).
- Azaizeh, H.; Abu Tayeh, H.N.; Gerchman, Y. Chapter 2—Valorisation of olive oil industry solid waste and production of ethanol and high value-added biomolecules. In Biovalorisation of Wastes to Renewable Chemicals and Biofuels; Krishnaraj Rathinam, N., Sani, R.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 27–40. [Google Scholar]
- Duque-Soto, C.; Quirantes-Piné, R.; Borrás-Linares, I.; Segura-Carretero, A.; Lozano-Sánchez, J. Characterization and influence of static in vitro digestion on bioaccessibility of bioactive polyphenols from an olive leaf extract. Foods 2022, 11, 743. [Google Scholar] [CrossRef]
- Rahmanian, N.; Jafari, S.M.; Wani, T.A. Bioactive profile, dehydration, extraction and application of the bioactive components of olive leaves. Trends Food Sci. Technol. 2015, 42, 150–172. [Google Scholar] [CrossRef]
- Zhang, C.C.; Xin, X.T.; Zhang, J.M.; Zhu, S.L.; Niu, E.L.; Zhou, Z.J.; Liu, D.Q. Comparative evaluation of the phytochemical profiles and antioxidant potentials of olive leaves from 32 cultivars grown in China. Molecules 2022, 27, 1292. [Google Scholar] [CrossRef] [PubMed]
- Mansour, H.M.M.; Zeitoun, A.A.; Abd-Rabou, H.S.; El Enshasy, H.A.; Dailin, D.J.; Zeitoun, M.A.A.; El-Sohaimy, S.A. Antioxidant and anti-diabetic properties of olive (Olea europaea) leaf extracts: In vitro and in vivo evaluation. Antioxidants 2023, 12, 1275. [Google Scholar] [CrossRef] [PubMed]
- Romero-Márquez, J.M.; Navarro-Hortal, M.D.; Forbes-Hernández, T.Y.; Varela-López, A.; Puentes, J.G.; Del Pino-García, R.; Sánchez-González, C.; Elio, I.; Battino, M.; García, R.; et al. Exploring the antioxidant, neuroprotective, and anti-inflammatory potential of olive leaf extracts from Spain, Portugal, Greece, and Italy. Antioxidants 2023, 12, 1538. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Gutiérrez, M.; Bascón-Villegas, I.; Rodríguez, A.; Pérez-Rodríguez, F.; Fernández-Prior, A.; Rosal, A.; Carrasco, E. Valorisation of Olea europaea L. olive leaves through the evaluation of their extracts: Antioxidant and antimicrobial Activity. Foods 2021, 10, 966. [Google Scholar] [CrossRef]
- Gandhi, G.R.; Vasconcelos, A.B.S.; Wu, D.T.; Li, H.B.; Antony, P.J.; Li, H.; Geng, F.; Gurgel, R.Q.; Narain, N.; Gan, R.Y. Citrus flavonoids as promising phytochemicals targeting diabetes and related complications: A systematic review of in vitro and in vivo studies. Nutrients 2020, 12, 2907. [Google Scholar] [CrossRef] [PubMed]
- Pirkovic, A.; Vilotic, A.; Borozan, S.; Nacka-Aleksic, M.; Bojic-Trbojevic, Ä.; Krivokuca, M.J.; Battino, M.; Giampieri, F.; Dekanski, D. Oleuropein Attenuates Oxidative Stress in Human Trophoblast Cells. Antioxidants 2023, 12, 197. [Google Scholar] [CrossRef]
- Pojero, F.; Aiello, A.; Gervasi, F.; Caruso, C.; Ligotti, M.E.; Calabrò, A.; Procopio, A.; Candore, G.; Accardi, G.; Allegra, M. Effects of Oleuropein and Hydroxytyrosol on Inflammatory Mediators: Consequences on Inflammaging. Int. J. Mol. Sci. 2023, 24, 380. [Google Scholar] [CrossRef]
- Subias-Gusils, A.; Alvarez-Monell, A.; Boqué, N.; Caimari, A.; Mariné-Casadó, R.; Escorihuela, R.M.; Solanas, M. Effects of a Calorie-Restricted Cafeteria Diet and Oleuropein Supplementation on Adiposity and mRNA Expression of Energy Balance Related Genes in Obese Male Rats. Metabolites 2023, 13, 147. [Google Scholar] [CrossRef]
- Carnevale, R.; Silvestri, R.; Loffredo, L.; Novo, M.; Cammisotto, V.; Castellani, V.; Bartimoccia, S.; Nocella, C.; Violi, F. Oleuropein, a component of extra virgin olive oil, lowers postprandial glycaemia in healthy subjects. Br. J. Clin. Pharmacol. 2018, 84, 1566–1574. [Google Scholar] [CrossRef]
- Vezza, T.; Rodríguez-Nogales, A.; Algieri, F.; Garrido-Mesa, J.; Romero, M.; Sánchez, M.; Toral, M.; Martín-García, B.; Gómez-Caravaca, A.M.; Arráez-Román, D.; et al. The metabolic and vascular protective effects of olive (Olea europaea L.) leaf extract in diet-induced obesity in mice are related to the amelioration of gut microbiota dysbiosis and to its immunomodulatory properties. Pharmacol. Res. 2019, 150, 104487. [Google Scholar] [CrossRef] [PubMed]
- Andújar-Tenorio, N.; Cobo, A.; Martínez-Rodríguez, A.M.; Hidalgo, M.; Prieto, I.; Gálvez, A.; Martínez-Cañamero, M. Intestinal microbiota modulation at the strain level by the olive oil polyphenols in the diet. Front. Nutr. 2023, 10, 1272139. [Google Scholar] [CrossRef] [PubMed]
- Bulotta, S.; Celano, M.; Lepore, S.M.; Montalcini, T.; Pujia, A.; Russo, D. Beneficial effects of the olive oil phenolic components oleuropein and hydroxytyrosol: Focus on protection against cardiovascular and metabolic diseases. J. Transl. Med. 2014, 12, 219. [Google Scholar] [CrossRef] [PubMed]
- Sciandra, F.; Bottoni, P.; De Leo, M.; Braca, A.; Brancaccio, A.; Bozzi, M. Verbascoside elicits its beneficial effects by enhancing mitochondrial spare respiratory capacity and the Nrf2/HO-1 mediated antioxidant system in a murine skeletal muscle cell line. Int. J. Mol. Sci. 2023, 24, 15276. [Google Scholar] [CrossRef] [PubMed]
- Mir-Cerdà, A.; Nuñez, O.; Granados, M.; Sentellas, S.; Saurina, J. An overview of the extraction and characterization of bioactive phenolic compounds from agri-food waste within the framework of circular bioeconomy. TrAC-Trends Anal. Chem. 2023, 161, 116994. [Google Scholar] [CrossRef]
- Monteleone, J.I.; Sperlinga, E.; Siracusa, L.; Spagna, G.; Parafati, L.; Todaro, A.; Palmeri, R. Water as a solvent of election for obtaining oleuropein-rich extracts from olive (Olea europaea) leaves. Agronomy 2021, 11, 465. [Google Scholar] [CrossRef]
- da Rosa, G.S.; Vanga, S.K.; Gariepy, Y.; Raghavan, V. Comparison of microwave, ultrasonic and conventional techniques for extraction of bioactive compounds from olive leaves (Olea europaea L.). Innov. Food Sci. Emerg. Technol. 2019, 58, 102234. [Google Scholar] [CrossRef]
- Lama-Munoz, A.; Contreras, M.D.M.; Espinola, F.; Moya, M.; Romero, I.; Castro, E. Content of phenolic compounds and mannitol in olive leaves extracts from six Spanish cultivars: Extraction with the Soxhlet method and pressurized liquids. Food Chem. 2020, 320, 126626. [Google Scholar] [CrossRef]
- Caballero, A.S.; Romero-García, J.M.; Castro, E.; Cardona, C.A. Supercritical fluid extraction for enhancing polyphenolic compounds production from olive waste extracts. J. Chem. Technol. Biotechnol. 2020, 95, 356–362. [Google Scholar] [CrossRef]
- Yusoff, I.M.; Taher, Z.M.; Rahmat, Z.; Chua, L.S. A review of ultrasound-assisted extraction for plant bioactive compounds: Phenolics, flavonoids, thymols, saponins and proteins. Food Res. Int. 2022, 151, 111268. [Google Scholar] [CrossRef]
- Akli, H.; Grigorakis, S.; Kellil, A.; Loupassaki, S.; Makris, D.P.; Calokerinos, A.; Mati, A.; Lydakis-Simantiris, N. Extraction of Polyphenols from Olive Leaves Employing Deep Eutectic Solvents: The Application of Chemometrics to a Quantitative Study on Antioxidant Compounds. Appl. Sci. 2022, 12, 831. [Google Scholar] [CrossRef]
- Jaski, J.M.; da Cruz, R.M.S.; Pimentel, T.C.; Stevanato, N.; da Silva, C.; Barao, C.E.; Cardozo, L. Simultaneous Extraction of Bioactive Compounds from Olea europaea L. Leaves and Healthy Seed Oils Using Pressurized Propane. Foods 2023, 12, 948. [Google Scholar] [CrossRef] [PubMed]
- Vasilopoulou, K.; Papadopoulos, G.A.; Lioliopoulou, S.; Pyrka, I.; Nenadis, N.; Savvidou, S.; Symeon, G.; Dotas, V.; Panitsidis, I.; Arsenos, G.; et al. Effects of Dietary Supplementation of a Resin-Purified Aqueous-Isopropanol Olive Leaf Extract on Meat and Liver Antioxidant Parameters in Broilers. Antioxidants 2023, 12, 1723. [Google Scholar] [CrossRef] [PubMed]
- Clodoveo, M.L.; Crupi, P.; Annunziato, A.; Corbo, F. Innovative Extraction Technologies for Development of Functional Ingredients Based on Polyphenols from Olive Leaves. Foods 2022, 11, 103. [Google Scholar] [CrossRef] [PubMed]
- U.S. Pharmacopeia. U.S. Pharmacopeial Convention. Reagents, indicators, and solutions. Solutions. Buffer Solutions. In United States Pharmacopeia and National Formulary (USP 38–NF 33); United States Pharmacopeial Convention: North Bethesda, ML, USA, 2015; pp. 1884–1885. [Google Scholar]
- Magalhães, L.M.; Ramos, I.I.; Reis, S.; Segundo, M.A. Antioxidant profile of commercial oenological tannins determined by multiple chemical assays. Aust. J. Grape Wine Res. 2014, 20, 72–79. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Magalhães, L.M.; Segundo, M.A.; Reis, S.; Lima, J.L.F.C. Methodological aspects about in vitro evaluation of antioxidant properties. Anal. Chim. Acta 2008, 613, 1–19. [Google Scholar] [CrossRef]
- Carvalho, J.R.; Meireles, A.N.; Marques, S.S.; Gregório, B.J.; Ramos, I.I.; Silva, E.M.; Barreiros, L.; Segundo, M.A. Exploiting kinetic features of ORAC assay for evaluation of radical scavenging capacity. Antioxidants 2023, 12, 505. [Google Scholar] [CrossRef]
- Marques, S.S.; Magalhaes, L.M.; Tóth, I.V.; Segundo, M.A. Insights on antioxidant assays for biological samples based on the reduction of copper complexes-The importance of analytical conditions. Int. J. Mol. Sci. 2014, 15, 11387–11402. [Google Scholar] [CrossRef]
- Baghalabadi, V.; Razmi, H.; Doucette, A. Salt-mediated organic solvent precipitation for enhanced recovery of peptides generated by pepsin digestion. Proteomes 2021, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, Y.X.; Chu, L.; Wei, Y.; Wang, D.; Cai, S.B.; Zhou, F.; Ji, B.P. Relationship between the structures of flavonoids and Oxygen Radical Absorbance Capacity values: A quantum chemical analysis. J. Phys. Chem. A 2013, 117, 1784–1794. [Google Scholar] [CrossRef] [PubMed]
- Nenadis, N.; Wang, L.F.; Tsimidou, M.Z.; Zhang, H.Y. Radical scavenging potential of phenolic compounds encountered in O. europaea products as indicated by calculation of bond dissociation enthalpy and ionization potential values. J. Agric. Food Chem. 2005, 53, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Biela, M.; Rimarcík, J.; Senajová, E.; Kleinová, A.; Klein, E. Antioxidant action of deprotonated flavonoids: Thermodynamics of sequential proton-loss electron-transfer. Phytochemistry 2020, 180, 112528. [Google Scholar] [CrossRef]
- Gregório, B.J.R.; Pereira, A.M.; Fernandes, S.R.; Matos, E.; Castanheira, F.; Almeida, A.A.; Fonseca, A.J.M.; Cabrita, A.R.J.; Segundo, M.A. Flow-based dynamic approach to assess bioaccessible zinc in dry dog food samples. Molecules 2020, 25, 1333. [Google Scholar] [CrossRef]



| S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | S11 | S12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gallic Acid | 3.2 ± 0.1 | 1.6 ± 0.2 | 2.03 ± 0.05 | 2.2 ± 0.3 | 3.3 ± 0.1 | 3.57 ± 0.05 | n/d | 0.5 ± 0.1 | n/d | n/d | n/d | n/d |
| Hydroxytyrosol | 22 ± 3 | 25.1 ± 0.3 | 27 ± 2 | 21 ± 2 | 86 ± 8 | 56 ± 6 | 70 ± 5 | 146 ± 12 | 62 ± 8 | 49 ± 4 | 84 ± 3 | 13 ± 1 |
| Catechin | 1.9 ± 0.1 | 4.8 ± 0.2 | 7 ± 1 | 9 ± 1 | 4.1 ± 0.4 | 6 ± 1 | 22 ± 2 | 25.48 ± 0.01 | 3.7 ± 0.3 | 4.4 ± 0.1 | 15.5 ± 0.1 | 8.1 ± 0.5 |
| Oleuropein | 164 ± 22 | 583 ± 15 | 131 ± 8 | 71 ± 6 | 2894 ± 214 | 103 ± 9 | 1852 ± 141 | 474 ± 56 | 1040 ± 131 | 135 ± 17 | 518 ± 40 | 165 ± 12 |
| Pinoresinol | 8.9 ± 0.4 | 5.3 ± 0.7 | 3.4 ± 0.1 | 29 ± 4 | 22 ± 2 | 10.1 ± 0.1 | 8 ± 1 | 6 ± 1 | 7.4 ± 0.2 | 1.8 ± 0.1 | 3.3 ± 0.2 | n/d |
| Caffeic Acid | n/d | 3.04 ± 0.02 | 2.72 ± 0.01 | n/d | 9 ± 1 | 0.39 ± 0.01 | 8 ± 1 | 0.6 ± 0.1 | 2.1 ± 0.2 | n/d | 0.58 ± 0.05 | n/d |
| Rutin | 12 ± 2 | 35 ± 3 | 52.6 ± 0.2 | 63 ± 3 | 52 ± 5 | 18 ± 1 | 90 ± 7 | 101 ± 12 | 31 ± 4 | 17.3 ± 2.1 | 67 ± 6 | 20 ± 2 |
| Quercetin | 3.2 ± 0.2 | 12.6 ± 0.3 | 10 ± 1 | 35 ± 3 | 12 ± 2 | 10 ± 1 | 19 ± 2 | 26 ± 2 | 10 ± 1 | 10.2 ± 1.1 | 17 ± 2 | 3.8 ± 0.4 |
| Luteolin | 32 ± 2 | 70 ± 2 | 78 ± 3 | 96 ± 6 | 25 ± 1 | 40 ± 2 | 58 ± 8 | 63 ± 4 | 28 ± 2 | 30 ± 3 | 62 ± 5 | 6 ± 1 |
| Verbascoside | 14 ± 2 | 13 ± 1 | 7.8 ± 0.4 | 52 ± 3 | 150 ± 13 | 106 ± 11 | 183 ± 21 | 67 ± 7 | 66 ± 7 | 67 ± 7 | 77 ± 5 | 3.3 ± 0.1 |
| FOLIN a | DPPH b | ABTS b | CUPRAC b | ORAC b | |
|---|---|---|---|---|---|
| S1 | 0.18 ± 0.03 | 0.11 ± 0.01 | 0.28 ± 0.03 | 0.30 ± 0.01 | 0.9 ± 0.1 |
| S2 | 0.31 ± 0.05 | 0.17 ± 0.02 | 0.37 ± 0.05 | 0.43 ± 0.06 | 0.22 ± 0.04 |
| S3 | 0.24 ± 0.03 | 0.14 ± 0.02 | 0.36 ± 0.04 | 0.31 ± 0.02 | 0.53 ± 0.09 |
| S4 | 0.22 ± 0.01 | 0.09 ± 0.01 | 0.25 ± 0.04 | 0.27 ± 0.01 | 1.4 ± 0.2 |
| S5 | 0.38 ± 0.05 | 0.19 ± 0.01 | 0.51 ± 0.05 | 0.52 ± 0.04 | 0.9 ± 0.1 |
| S6 | 0.15 ± 0.01 | 0.06 ± 0.01 | 0.19 ± 0.02 | 0.22 ± 0.01 | 1.0 ± 0.1 |
| S7 | 0.33 ± 0.04 | 0.16 ± 0.02 | 0.46 ± 0.02 | 0.43 ± 0.02 | 0.61 ± 0.07 |
| S8 | 0.22 ± 0.01 | 0.12 ± 0.01 | 0.31 ± 0.04 | 0.36 ± 0.02 | 1.8 ± 0.1 |
| S9 | 0.28 ± 0.01 | 0.15 ± 0.01 | 0.35 ± 0.02 | 0.35 ± 0.01 | 0.78 ± 0.08 |
| S10 | 0.18 ± 0.02 | 0.16 ± 0.02 | 0.23 ± 0.02 | 0.22 ± 0.01 | 0.25 ± 0.05 |
| S11 | 0.31 ± 0.04 | 0.16 ± 0.01 | 0.41 ± 0.04 | 0.42 ± 0.02 | 0.9 ± 0.1 |
| S12 | 0.18 ± 0.01 | 0.142 ± 0.004 | 0.21 ± 0.01 | 0.25 ± 0.01 | 0.27 ± 0.01 |
| Sample 5 | Sample 7 | |
|---|---|---|
| Hydroxytyrosol | 14.4 ± 0.1 | 5.5 ± 0.3 |
| Oleuropein | 837 ± 1 | 135 ± 3 |
| Pinoresinol | 7.2 ± 0.1 | 1.93 ± 0.2 |
| Verbascoside | 21.7 ± 0.1 | 6.4 ± 0.1 |
| Rutin | 15.1± 0.1 | 8.2± 0.3 |
| Quercetin | 3.30 ± 0.01 | 2.0 ± 0.1 |
| Luteolin | 4.1 ± 0.1 | 4.9 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ronca, C.L.; Marques, S.S.; Ritieni, A.; Giménez-Martínez, R.; Barreiros, L.; Segundo, M.A. Olive Oil Waste as a Source of Functional Food Ingredients: Assessing Polyphenolic Content and Antioxidant Activity in Olive Leaves. Foods 2024, 13, 189. https://doi.org/10.3390/foods13020189
Ronca CL, Marques SS, Ritieni A, Giménez-Martínez R, Barreiros L, Segundo MA. Olive Oil Waste as a Source of Functional Food Ingredients: Assessing Polyphenolic Content and Antioxidant Activity in Olive Leaves. Foods. 2024; 13(2):189. https://doi.org/10.3390/foods13020189
Chicago/Turabian StyleRonca, Carolina L., Sara S. Marques, Alberto Ritieni, Rafael Giménez-Martínez, Luisa Barreiros, and Marcela A. Segundo. 2024. "Olive Oil Waste as a Source of Functional Food Ingredients: Assessing Polyphenolic Content and Antioxidant Activity in Olive Leaves" Foods 13, no. 2: 189. https://doi.org/10.3390/foods13020189